Abstract
OBJECTIVE: This review updates the surgeon about the cellular, matrix, and growth factor components of scarless fetal wound repair. SUMMARY BACKGROUND DATA: Fetal skin wound healing is characterized by the absence of scar tissue formation. This unique repair process is not dependent on the sterile, aqueous intrauterine environment. The differences between fetal and adult skin wound healing appear to reflect processes intrinsic to fetal tissue, such as the unique fetal fibroblasts, a more rapid and ordered deposition and turnover of tissue components, and, particularly, a markedly reduced inflammatory infiltrate and cytokine profile. Scarless fetal wounds are relatively deficient in the inflammatory cytokine, transforming growth factor beta (TGF-beta). In contrast, the fibrosis characteristic of adult wound repair may be associated with TGF-beta excess. Recent experimental studies suggest that specific anti-TGF-beta therapeutic strategies can ameliorate scar formation in adult wound repair and fibrotic diseases. Inhibitors of TGF-beta may be important future drugs to control scar. CONCLUSIONS: Based on the scarless fetal wound repair model, a number of ways in which the matrix and cellular response of the healing adult wound might be manipulated to reduce scarring are reviewed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
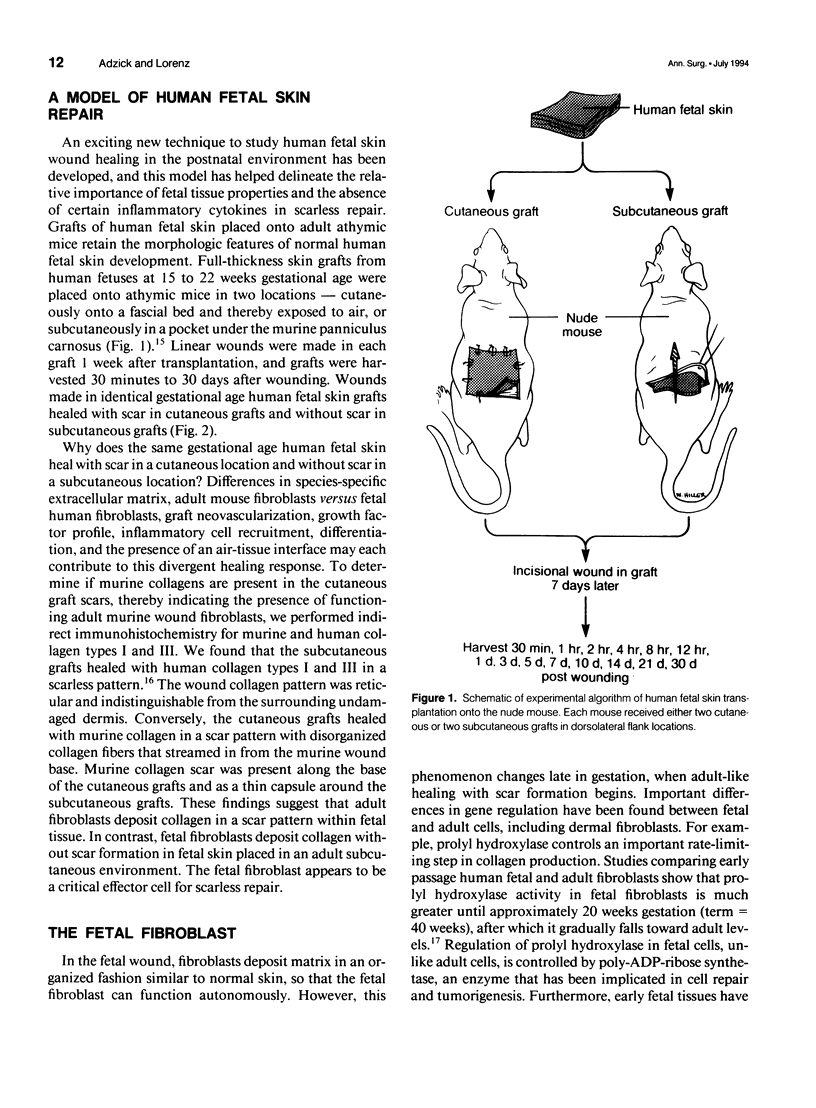
- Adzick N. S., Harrison M. R., Glick P. L., Beckstead J. H., Villa R. L., Scheuenstuhl H., Goodson W. H., 3rd Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985 Aug;20(4):315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- Adzick N. S., Longaker M. T. Scarless wound healing in the fetus: the role of the extracellular matrix. Prog Clin Biol Res. 1991;365:177–192. [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Baue A. E. The horror autotoxicus and multiple-organ failure. Arch Surg. 1992 Dec;127(12):1451–1462. doi: 10.1001/archsurg.1992.01420120085016. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R., Brockes J. P. Identification and expression of a regeneration-specific homeobox gene in the newt limb blastema. Development. 1991 Feb;111(2):489–496. doi: 10.1242/dev.111.2.489. [DOI] [PubMed] [Google Scholar]
- Castilla A., Prieto J., Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med. 1991 Apr 4;324(14):933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
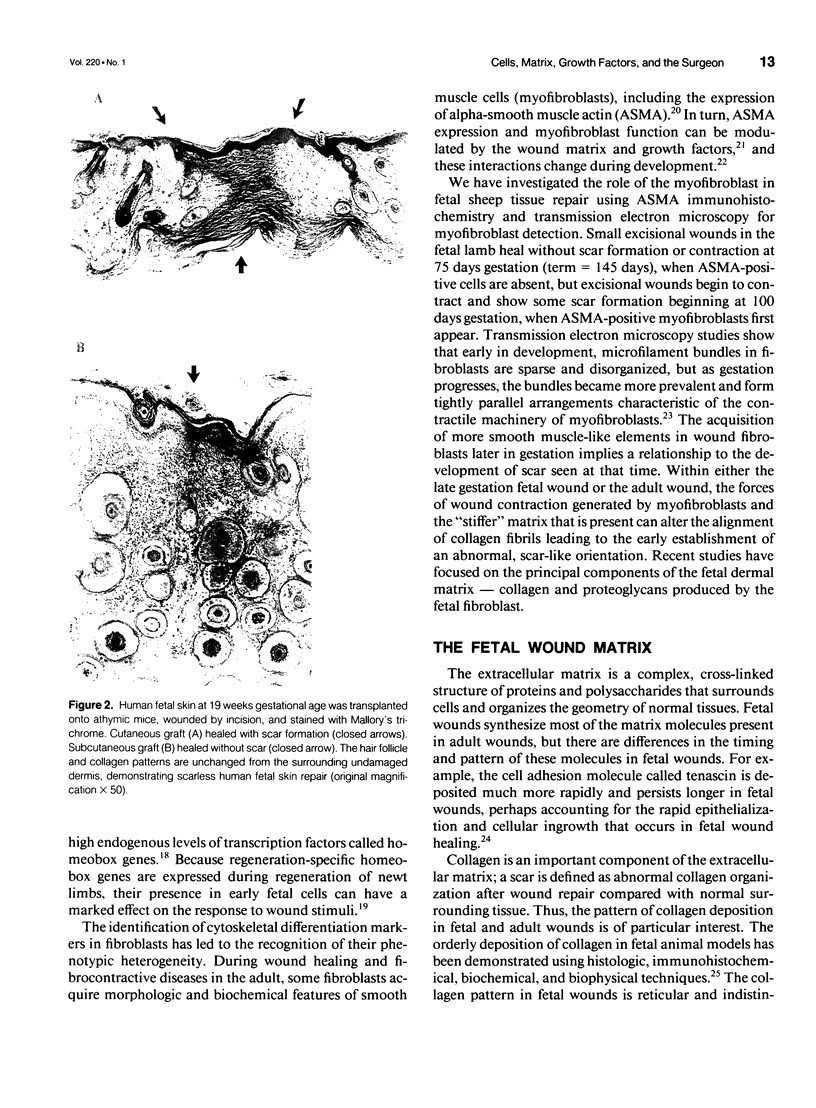
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- DePalma R. L., Krummel T. M., Durham L. A., 3rd, Michna B. A., Thomas B. L., Nelson J. M., Diegelmann R. F. Characterization and quantitation of wound matrix in the fetal rabbit. Matrix. 1989 Jun;9(3):224–231. doi: 10.1016/s0934-8832(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. M., Adzick N. S., Harrison M. R., Longaker M. T., Stern R. Hyaluronate metabolism undergoes an ontogenic transition during fetal development: implications for scar-free wound healing. J Pediatr Surg. 1993 Oct;28(10):1227–1231. doi: 10.1016/s0022-3468(05)80303-3. [DOI] [PubMed] [Google Scholar]
- Flake A. W., Harrison M. R., Adzick N. S., Zanjani E. D. Transplantation of fetal hematopoietic stem cells in utero: the creation of hematopoietic chimeras. Science. 1986 Aug 15;233(4765):776–778. doi: 10.1126/science.2874611. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Ihara S., Motobayashi Y. Wound closure in foetal rat skin. Development. 1992 Mar;114(3):573–582. doi: 10.1242/dev.114.3.573. [DOI] [PubMed] [Google Scholar]
- Jennings R. W., Adzick N. S., Longaker M. T., Duncan B. W., Scheuenstuhl H., Hunt T. K. Ontogeny of fetal sheep polymorphonuclear leukocyte phagocytosis. J Pediatr Surg. 1991 Jul;26(7):853–855. doi: 10.1016/0022-3468(91)90155-m. [DOI] [PubMed] [Google Scholar]
- Jonsson K., Jensen J. A., Goodson W. H., 3rd, Scheuenstuhl H., West J., Hopf H. W., Hunt T. K. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991 Nov;214(5):605–613. doi: 10.1097/00000658-199111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel T. M., Ehrlich H. P., Nelson J. M., Michna B. A., Thomas B. L., Haynes J. H., Cohen I. K., Diegelmann R. F. In vitro and in vivo analysis of the inability of fetal rabbit wounds to contract. Wound Repair Regen. 1993 Jan;1(1):15–21. doi: 10.1046/j.1524-475X.1993.10106.x. [DOI] [PubMed] [Google Scholar]
- Krummel T. M., Michna B. A., Thomas B. L., Sporn M. B., Nelson J. M., Salzberg A. M., Cohen I. K., Diegelmann R. F. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988 Jul;23(7):647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Logan A., Frautschy S. A., Gonzalez A. M., Sporn M. B., Baird A. Enhanced expression of transforming growth factor beta 1 in the rat brain after a localized cerebral injury. Brain Res. 1992 Aug 7;587(2):216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker M. T., Adzick N. S., Hall J. L., Stair S. E., Crombleholme T. M., Duncan B. W., Bradley S. M., Harrison M. R., Stern R. Studies in fetal wound healing, VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg. 1990 Apr;25(4):430–433. doi: 10.1016/0022-3468(90)90387-o. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Burd D. A., Gown A. M., Yen T. S., Jennings R. W., Duncan B. W., Harrison M. R., Adzick N. S. Midgestational excisional fetal lamb wounds contract in utero. J Pediatr Surg. 1991 Aug;26(8):942–948. doi: 10.1016/0022-3468(91)90841-g. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Chiu E. S., Harrison M. R., Crombleholme T. M., Langer J. C., Duncan B. W., Adzick N. S., Verrier E. D., Stern R. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann Surg. 1989 Nov;210(5):667–672. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Adzick N. S., Crombleholme T. M., Langer J. C., Duncan B. W., Bradley S. M., Stern R., Ferguson M. W., Harrison M. R. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990 Jan;25(1):63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
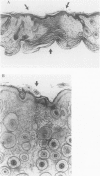
- Longaker M. T., Whitby D. J., Ferguson M. W., Lorenz H. P., Harrison M. R., Adzick N. S. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994 Jan;219(1):65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Jennings R. W., Duncan B. W., Ferguson M. W., Harrison M. R., Adzick N. S. Fetal diaphragmatic wounds heal with scar formation. J Surg Res. 1991 Apr;50(4):375–385. doi: 10.1016/0022-4804(91)90206-2. [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Longaker M. T., Perkocha L. A., Jennings R. W., Harrison M. R., Adzick N. S. Scarless wound repair: a human fetal skin model. Development. 1992 Jan;114(1):253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Whitby D. J., Longaker M. T., Adzick N. S. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Ann Surg. 1993 Apr;217(4):391–396. doi: 10.1097/00000658-199304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A., Leeming G. L., Jowett A. K., Ferguson M. W., Sharpe P. T. The homeobox gene Hox 7.1 has specific regional and temporal expression patterns during early murine craniofacial embryogenesis, especially tooth development in vivo and in vitro. Development. 1991 Feb;111(2):269–285. doi: 10.1242/dev.111.2.269. [DOI] [PubMed] [Google Scholar]
- Martin P., Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992 Nov 12;360(6400):179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- Merkel J. R., DiPaolo B. R., Hallock G. G., Rice D. C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988 Apr;187(4):493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Kähäri L., Jaakkola S., Kähäri V. M., Varga J., Uitto J., Jimenez S. A. Evaluation of transforming growth factor beta and type I procollagen gene expression in fibrotic skin diseases by in situ hybridization. J Invest Dermatol. 1990 Mar;94(3):365–371. doi: 10.1111/1523-1747.ep12874491. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Dietrich P. Y., Skalli O., Widgren S., Gabbiani G. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch A Pathol Anat Histopathol. 1989;415(6):551–557. doi: 10.1007/BF00718649. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992 Jan 25;339(8787):213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990 Oct;2(5):839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991 Sep;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991 Jun;112(2):651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Longaker M. T., Harrison M. R., Adzick N. S., Ferguson M. W. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci. 1991 Jul;99(Pt 3):583–586. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Rossi A. M., Chegini N., Schultz G. Effect of transforming growth factor beta on postoperative adhesion formation and intact peritoneum. J Surg Res. 1992 Jan;52(1):65–70. doi: 10.1016/0022-4804(92)90280-d. [DOI] [PubMed] [Google Scholar]
- Yeo T. K., Brown L., Dvorak H. F. Alterations in proteoglycan synthesis common to healing wounds and tumors. Am J Pathol. 1991 Jun;138(6):1437–1450. [PMC free article] [PubMed] [Google Scholar]