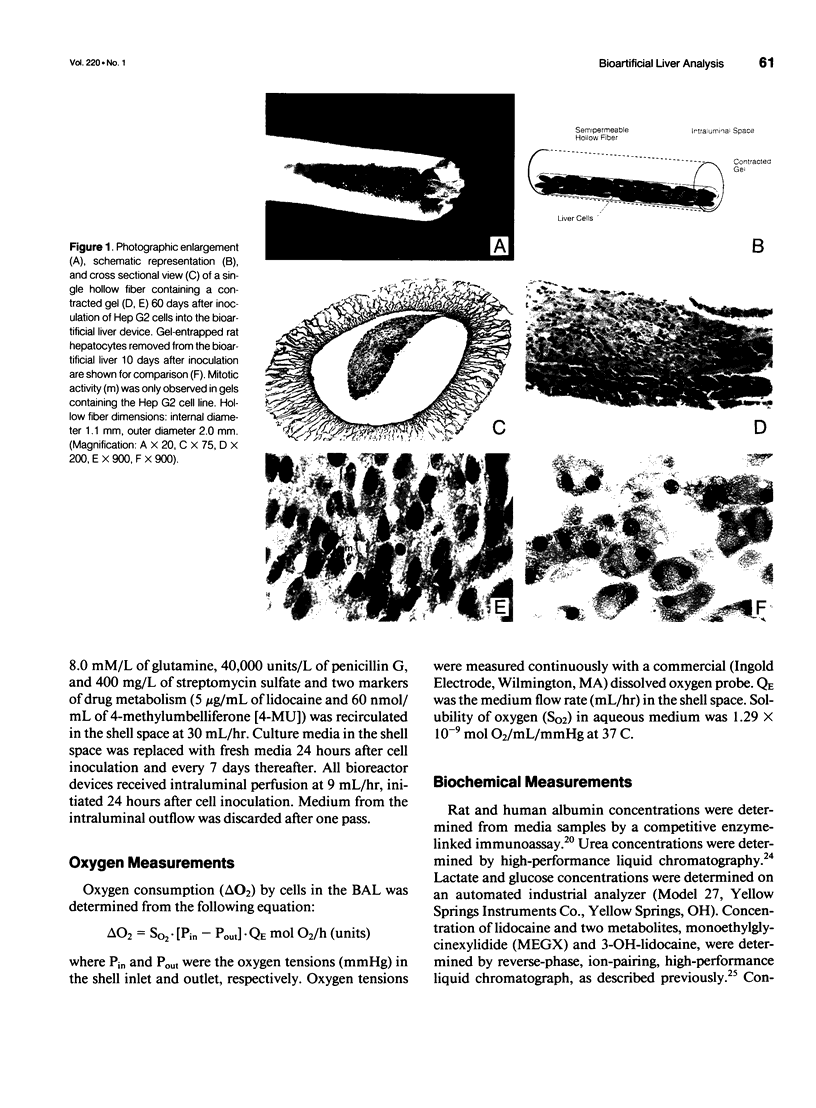
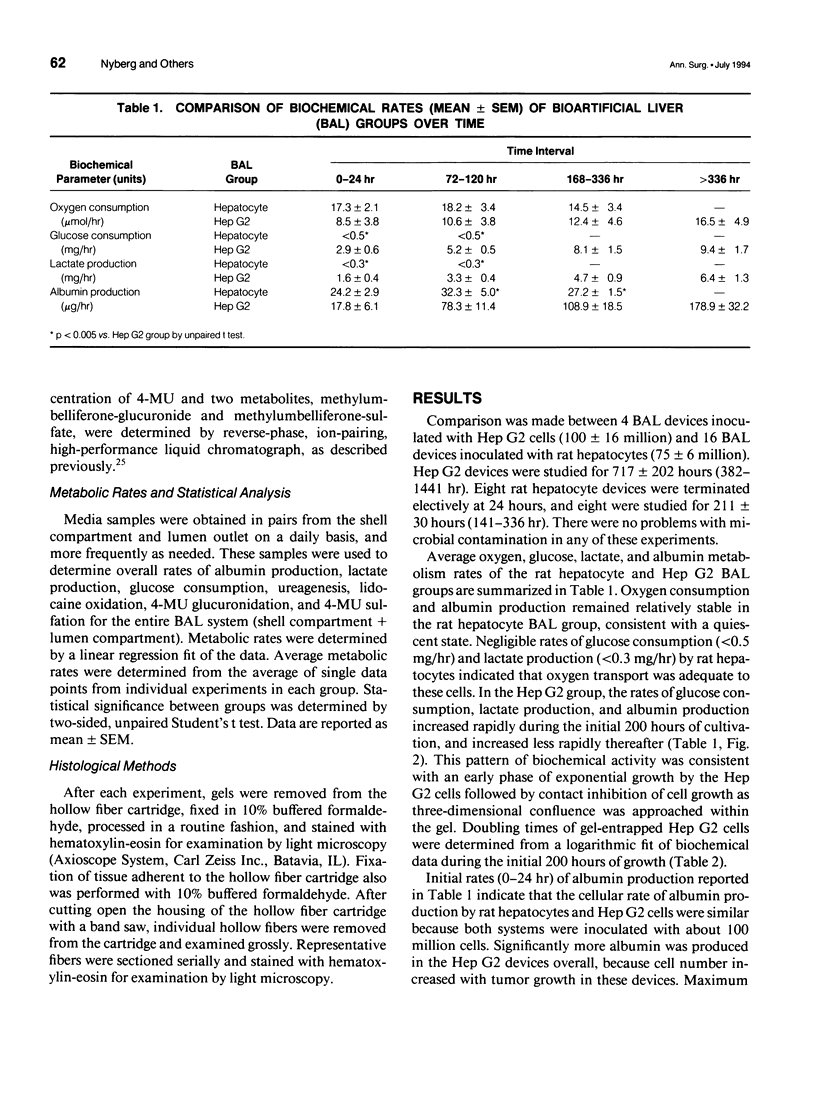
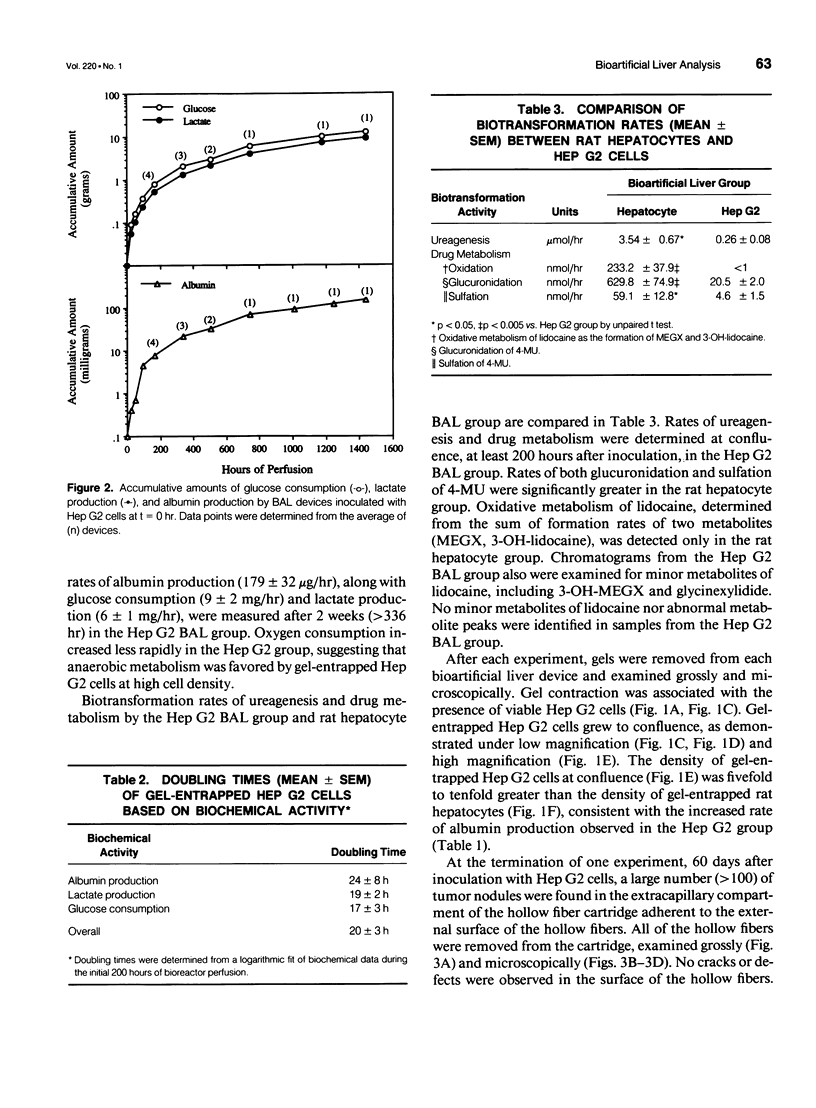
Abstract
OBJECTIVE: Metabolic activity of transformed human liver (Hep G2) cells and primary rat hepatocytes were compared during in vitro application of a gel entrapment bioartificial liver. BACKGROUND: Clinical trials of bioartificial liver devices containing either transformed liver cells or primary hepatocytes have been initiated. A study comparing transformed liver cells and primary hepatocytes in a bioartificial liver under similar conditions has not been reported previously. METHODS: Gel entrapment bioartificial liver devices were inoculated with 100 million cells, Hep G2 cell line (n = 4), or rat hepatocytes (n = 16), and studied for up to 60 days of in vitro cultivation. RESULTS: Hep G2 cells grew to confluence within the gel entrapment configuration with a doubling time of 20 +/- 3 hours. Rat hepatocytes significantly outperformed Hep G2 cells at confluence in all categories of biotransformation, including ureagenesis (3.5 +/- 0.7 vs. 0.3 +/- 0.1 mumol/hr, p < 0.05), glucuronidation (630 +/- 75 vs. 21 +/- 2 nmol/hr, p < 0.005), sulfation (59 +/- 13 vs. 5 +/- 2 nmol/hr, p < 0.05), and oxidation (233 +/- 38 vs. < 1 nmol/hr, p < 0.005). At the conclusion of one experiment, Hep G2 cells were found in the extracapillary compartment of the bioartificial liver, analogous to the patient's compartment during clinical application. CONCLUSIONS: Primary rat hepatocytes were superior to the Hep G2 cell line as the source of hepatic function in a bioartificial liver and avoided the potential risk of tumor transmigration from the bioartificial liver into the patient's circulation.
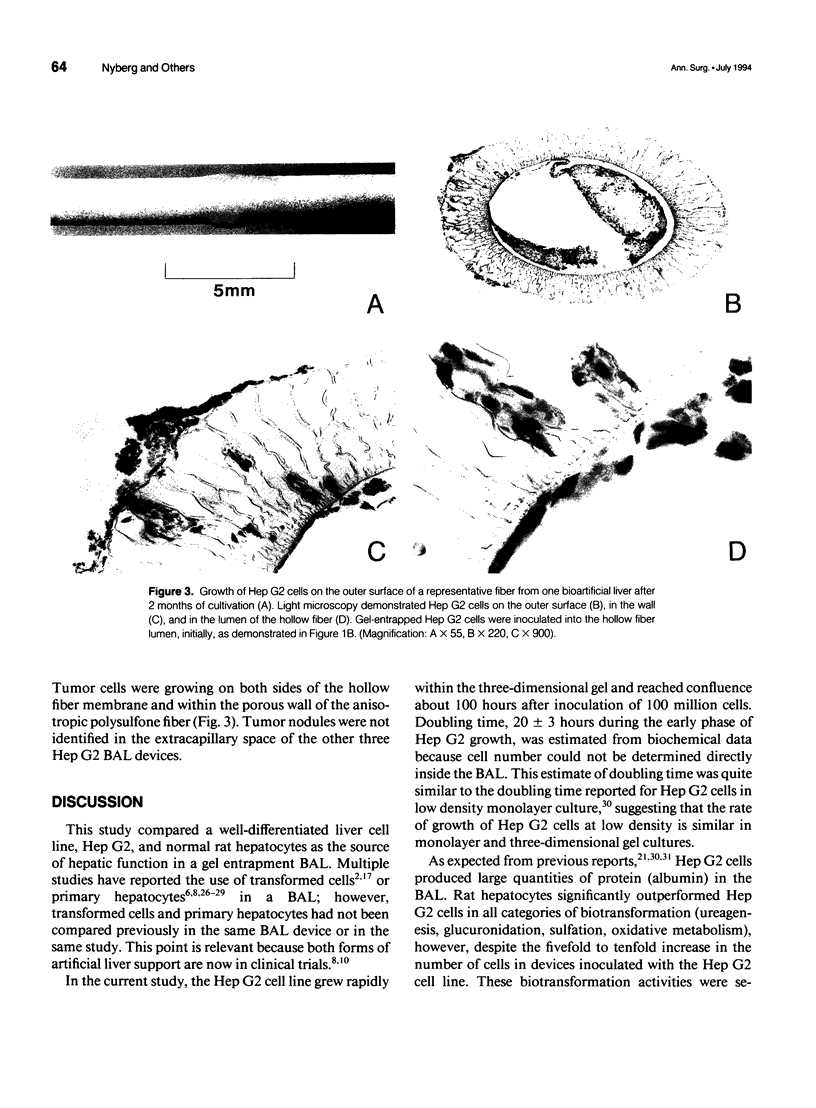
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Armbruster L., Cavard C., Briand P., Bertolotti R. Selection of variant hepatoma cells in liver-specific growth media: regulation at the mRNA level. Differentiation. 1992 May;50(1):25–33. doi: 10.1111/j.1432-0436.1992.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Bargetzi M. J., Aoyama T., Gonzalez F. J., Meyer U. A. Lidocaine metabolism in human liver microsomes by cytochrome P450IIIA4. Clin Pharmacol Ther. 1989 Nov;46(5):521–527. doi: 10.1038/clpt.1989.180. [DOI] [PubMed] [Google Scholar]
- Basile A. S., Jones E. A., Skolnick P. The pathogenesis and treatment of hepatic encephalopathy: evidence for the involvement of benzodiazepine receptor ligands. Pharmacol Rev. 1991 Mar;43(1):27–71. [PubMed] [Google Scholar]
- Bock K. W., Bock-Hennig B. S. Differential induction of human liver UDP-glucuronosyltransferase activities by phenobarbital-type inducers. Biochem Pharmacol. 1987 Dec 1;36(23):4137–4143. doi: 10.1016/0006-2952(87)90572-7. [DOI] [PubMed] [Google Scholar]
- Burchell B., Coughtrie M. W. UDP-glucuronosyltransferases. Pharmacol Ther. 1989;43(2):261–289. doi: 10.1016/0163-7258(89)90122-8. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Kelly J. H., Buffone G. J. Growth and hepatospecific gene expression of human hepatoma cells in a defined medium. In Vitro Cell Dev Biol. 1987 May;23(5):349–354. doi: 10.1007/BF02620991. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Adams D. J., Wolf C. R. Induction of drug metabolizing enzymes in human liver cell line Hep G2. FEBS Lett. 1985 Apr 22;183(2):219–222. doi: 10.1016/0014-5793(85)80780-8. [DOI] [PubMed] [Google Scholar]
- Doostdar H., Demoz A., Burke M. D., Melvin W. T., Grant M. H. Variation in drug-metabolizing enzyme activities during the growth of human Hep G2 hepatoma cells. Xenobiotica. 1990 Apr;20(4):435–441. doi: 10.3109/00498259009046859. [DOI] [PubMed] [Google Scholar]
- Doostdar H., Duthie S. J., Burke M. D., Melvin W. T., Grant M. H. The influence of culture medium composition on drug metabolising enzyme activities of the human liver derived Hep G2 cell line. FEBS Lett. 1988 Dec 5;241(1-2):15–18. doi: 10.1016/0014-5793(88)81021-4. [DOI] [PubMed] [Google Scholar]
- Everson G. T., Polokoff M. A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986 Feb 15;261(5):2197–2201. [PubMed] [Google Scholar]
- Fair D. S., Bahnak B. R. Human hepatoma cells secrete single chain factor X, prothrombin, and antithrombin III. Blood. 1984 Jul;64(1):194–204. [PubMed] [Google Scholar]
- Fukuda Y., Ishida N., Noguchi T., Kappas A., Sassa S. Interleukin-6 down regulates the expression of transcripts encoding cytochrome P450 IA1, IA2 and IIIA3 in human hepatoma cells. Biochem Biophys Res Commun. 1992 Apr 30;184(2):960–965. doi: 10.1016/0006-291x(92)90684-d. [DOI] [PubMed] [Google Scholar]
- Garfield S., Huber B. E., Nagy P., Cordingley M. G., Thorgeirsson S. S. Neoplastic transformation and lineage switching of rat liver epithelial cells by retrovirus-associated oncogenes. Mol Carcinog. 1988;1(3):189–195. doi: 10.1002/mc.2940010307. [DOI] [PubMed] [Google Scholar]
- Gerlach J., Klöppel K., Schauwecker H. H., Tauber R., Müller C., Bücherl E. S. Use of hepatocytes in adhesion and suspension cultures for liver support bioreactors. Int J Artif Organs. 1989 Dec;12(12):788–792. [PubMed] [Google Scholar]
- Grant M. H., Duthie S. J., Gray A. G., Burke M. D. Mixed function oxidase and UDP-glucuronyltransferase activities in the human Hep G2 hepatoma cell line. Biochem Pharmacol. 1988 Nov 1;37(21):4111–4116. doi: 10.1016/0006-2952(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Tokiwa T., Bennett W., Metcalf R. A., Welsh J. A., Sun T., Harris C. C. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis. 1993 May;14(5):987–992. doi: 10.1093/carcin/14.5.987. [DOI] [PubMed] [Google Scholar]
- Jauregui H. O., Gann K. L. Mammalian hepatocytes as a foundation for treatment in human liver failure. J Cell Biochem. 1991 Apr;45(4):359–365. doi: 10.1002/jcb.240450409. [DOI] [PubMed] [Google Scholar]
- Jauregui H. O., Muller T. E. Long-term cultures of adult mammalian hepatocytes in hollow fibers as the cellular component of extracorporeal (hybrid) liver assist devices. Artif Organs. 1992 Apr;16(2):209–212. doi: 10.1111/j.1525-1594.1992.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Javitt N. B. Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. FASEB J. 1990 Feb 1;4(2):161–168. doi: 10.1096/fasebj.4.2.2153592. [DOI] [PubMed] [Google Scholar]
- Kelly J. H., Darlington G. J. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989 Feb;25(2):217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Konstantinides F. N., Garr L., Li J. C., Cerra F. B. Overcoming retention time shifts as a source of error in HPLC analysis. J Chromatogr Sci. 1987 Apr;25(4):158–163. doi: 10.1093/chromsci/25.4.158. [DOI] [PubMed] [Google Scholar]
- Li A. P., Barker G., Beck D., Colburn S., Monsell R., Pellegrin C. Culturing of primary hepatocytes as entrapped aggregates in a packed bed bioreactor: a potential bioartificial liver. In Vitro Cell Dev Biol. 1993 Mar;29A(3 Pt 1):249–254. doi: 10.1007/BF02634192. [DOI] [PubMed] [Google Scholar]
- Neuzil D. F., Rozga J., Moscioni A. D., Ro M. S., Hakim R., Arnaout W. S., Demetriou A. A. Use of a novel bioartificial liver in a patient with acute liver insufficiency. Surgery. 1993 Mar;113(3):340–343. [PubMed] [Google Scholar]
- Nyberg S. L., Payne W. D., Amiot B., Shirabe K., Remmel R. P., Hu W. S., Cerra F. B. Demonstration of biochemical function by extracorporeal xenohepatocytes in an anhepatic animal model. Transplant Proc. 1993 Apr;25(2):1944–1945. [PubMed] [Google Scholar]
- Nyberg S. L., Platt J. L., Shirabe K., Payne W. D., Hu W. S., Cerra F. B. Immunoprotection of xenocytes in a hollow fiber bioartificial liver. ASAIO J. 1992 Jul-Sep;38(3):M463–M467. doi: 10.1097/00002480-199207000-00077. [DOI] [PubMed] [Google Scholar]
- Nyberg S. L., Shatford R. A., Hu W. S., Payne W. D., Cerra F. B. Hepatocyte culture systems for artificial liver support: implications for critical care medicine (bioartificial liver support). Crit Care Med. 1992 Aug;20(8):1157–1168. doi: 10.1097/00003246-199208000-00016. [DOI] [PubMed] [Google Scholar]
- Nyberg S. L., Shirabe K., Peshwa M. V., Sielaff T. D., Crotty P. L., Mann H. J., Remmel R. P., Payne W. D., Hu W. S., Cerra F. B. Extracorporeal application of a gel-entrapment, bioartificial liver: demonstration of drug metabolism and other biochemical functions. Cell Transplant. 1993 Nov-Dec;2(6):441–452. doi: 10.1177/096368979300200602. [DOI] [PubMed] [Google Scholar]
- Oda Y., Imaoka S., Nakahira Y., Asada A., Fujimori M., Fujita S., Funae Y. Metabolism of lidocaine by purified rat liver microsomal cytochrome P-450 isozymes. Biochem Pharmacol. 1989 Dec 15;38(24):4439–4444. doi: 10.1016/0006-2952(89)90654-0. [DOI] [PubMed] [Google Scholar]
- Richards C. A., Short S. A., Thorgeirsson S. S., Huber B. E. Characterization of a transforming N-ras gene in the human hepatoma cell line Hep G2: additional evidence for the importance of c-myc and ras cooperation in hepatocarcinogenesis. Cancer Res. 1990 Mar 1;50(5):1521–1527. [PubMed] [Google Scholar]
- Roe A. L., Snawder J. E., Benson R. W., Roberts D. W., Casciano D. A. HepG2 cells: an in vitro model for P450-dependent metabolism of acetaminophen. Biochem Biophys Res Commun. 1993 Jan 15;190(1):15–19. doi: 10.1006/bbrc.1993.1003. [DOI] [PubMed] [Google Scholar]
- Rozga J., Holzman M. D., Ro M. S., Griffin D. W., Neuzil D. F., Giorgio T., Moscioni A. D., Demetriou A. A. Development of a hybrid bioartificial liver. Ann Surg. 1993 May;217(5):502–511. doi: 10.1097/00000658-199305010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Sugita O., Galbraith R. A., Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987 Feb 27;143(1):52–57. doi: 10.1016/0006-291x(87)90628-0. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Schuetz J. D., Strom S. C., Thompson M. T., Fisher R. A., Molowa D. T., Li D., Guzelian P. S. Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology. 1993 Nov;18(5):1254–1262. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shatford R. A., Nyberg S. L., Meier S. J., White J. G., Payne W. D., Hu W. S., Cerra F. B. Hepatocyte function in a hollow fiber bioreactor: a potential bioartificial liver. J Surg Res. 1992 Dec;53(6):549–557. doi: 10.1016/0022-4804(92)90253-v. [DOI] [PubMed] [Google Scholar]
- Shnyra A., Bocharov A., Bochkova N., Spirov V. Bioartificial liver using hepatocytes on biosilon microcarriers: treatment of chemically induced acute hepatic failure in rats. Artif Organs. 1991 Jun;15(3):189–197. doi: 10.1111/j.1525-1594.1991.tb03039.x. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Chong M. G., Koussayer T., He D. E., Shang T. A., Whisennand H. H., Kelly J. H. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology. 1992 Jul;16(1):60–65. doi: 10.1002/hep.1840160112. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Kelly J. H. Artificial liver: a forthcoming attraction. Hepatology. 1993 Jun;17(6):1163–1164. doi: 10.1002/hep.1840170632. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Kelly J. H. Improved liver function following treatment with an extracorporeal liver assist device. Artif Organs. 1993 Jan;17(1):27–30. doi: 10.1111/j.1525-1594.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Kelly J. H. Liver assist devices (LADs) will not be used to treat fulminant hepatic failure (FHF), but its consequences, namely hepatic encephalopathy (HE) Artif Organs. 1993 Jan;17(1):43–45. [PubMed] [Google Scholar]
- Takahashi M., Matsue H., Matsushita M., Sato K., Nishikawa M., Koike M., Noto H., Nakajima Y., Uchino J., Komai T. Does a porcine hepatocyte hybrid artificial liver prolong the survival time of anhepatic rabbits? ASAIO J. 1992 Jul-Sep;38(3):M468–M472. doi: 10.1097/00002480-199207000-00078. [DOI] [PubMed] [Google Scholar]
- Uchino J., Matsue H., Takahashi M., Nakajima Y., Matsushita M., Hamada T., Hashimura E. A hybrid artificial liver system. Function of cultured monolayer pig hepatocytes in plasma from hepatic failure patients. ASAIO Trans. 1991 Jul-Sep;37(3):M337–M338. [PubMed] [Google Scholar]
- Vickers A. E., Fischer V., Connors S., Fisher R. L., Baldeck J. P., Maurer G., Brendel K. Cyclosporin A metabolism in human liver, kidney, and intestine slices. Comparison to rat and dog slices and human cell lines. Drug Metab Dispos. 1992 Nov-Dec;20(6):802–809. [PubMed] [Google Scholar]
- Wolf C. F., Munkelt B. E. Bilirubin conjugation by an artificial liver composed of cultured cells and synthetic capillaries. Trans Am Soc Artif Intern Organs. 1975;21:16–27. [PubMed] [Google Scholar]
- Wortelboer H. M., de Kruif C. A., de Boer W. I., van Iersel A. A., Falke H. E., Blaauboer B. J. Induction and activity of several isoenzymes of cytochrome P-450 in primary cultures of rat hepatocytes, in comparison with in vivo data. Mol Toxicol. 1987;1(4):373–381. [PubMed] [Google Scholar]
- Yarmush M. L., Dunn J. C., Tompkins R. G. Assessment of artificial liver support technology. Cell Transplant. 1992;1(5):323–341. doi: 10.1177/096368979200100501. [DOI] [PubMed] [Google Scholar]
- Zeindl-Eberhart E., Rabes H. M. Variant protein patterns in hepatomas and transformed liver cell lines as determined by high resolution two-dimensional gel electrophoresis (2DE). Carcinogenesis. 1992 Jul;13(7):1177–1183. doi: 10.1093/carcin/13.7.1177. [DOI] [PubMed] [Google Scholar]