Abstract
Phosducin proteins are known to inhibit G protein-mediated signaling by sequestering Gβγ subunits. However, Dictyostelium discoideum cells lacking the phosducin-like protein PhLP1 display defective rather than enhanced G protein signaling. Here we show that green fluorescent protein (GFP)-tagged Gβ (GFP-Gβ) and GFP-Gγ subunits exhibit drastically reduced steady-state levels and are absent from the plasma membrane in phlp1− cells. Triton X-114 partitioning suggests that lipid attachment to GFP-Gγ occurs in wild-type cells but not in phlp1− and gβ− cells. Moreover, Gβγ dimers could not be detected in vitro in coimmunoprecipitation assays with phlp1− cell lysates. Accordingly, in vivo diffusion measurements using fluorescence correlation spectroscopy showed that while GFP-Gγ proteins are present in a complex in wild-type cells, they are free in phlp1− and gβ− cells. Collectively, our data strongly suggest the absence of Gβγ dimer formation in Dictyostelium cells lacking PhLP1. We propose that PhLP1 serves as a cochaperone assisting the assembly of Gβ and Gγ into a functional Gβγ complex. Thus, phosducin family proteins may fulfill hitherto unsuspected biosynthetic functions.
Phosducin family proteins have classically been linked to G protein regulation. Both phosducin (Phd) and the related phosducin-like protein (PhLP) have been shown to bind Gβγ subunits (18, 32, 53, 57, 64, 69). In so doing, they are thought to function as a cellular “sink” which sequesters free Gβγ subunits following their dissociation from receptor-activated G proteins (2, 19, 33, 41, 58). As G protein-coupled receptors only couple to Gαβγ trimers, the sequestration of Gβγ attenuates transmembrane signaling. Thus, phosducin family proteins may adapt the cell's sensitivity to extracellular signals.
Being interested in factors underlying the adaptation of G protein-mediated chemotactic signaling in Dictyostelium discoideum, we recently identified three Dictyostelium Phd-like protein genes (3). To test whether PhLP1, the Dictyostelium protein that is most similar to mammalian Phd and PhLP, is involved in modulating G protein signaling, we analyzed phlp1 knockout cells. Surprisingly, G protein signaling is completely defective rather than enhanced in phlp1− cells, which exhibit a phenotype that is remarkably similar to that of gβ knockout cells. Fluorescence confocal microscopy experiments with cells expressing green fluorescent protein (GFP)-tagged Gβ (GFP-Gβ) or GFP-Gγ fusion proteins indicated that Gβγ complexes are absent from the plasma membrane of phlp1− cells, providing a possible explanation for the abrogation of signal transduction (3). These findings suggested that Gβ and Gγ fail to be assembled into a Gβγ complex in phlp1− cells or that the complex is not properly routed to the plasma membrane in these cells.
In this paper, we have further investigated the Gβγ defect in phlp1− cells. We show that steady-state levels of (GFP-tagged) Gβ and Gγ subunits are dramatically reduced and that these proteins are detected in the cytosol when the PhLP1 protein is not present. Triton X-114 partitioning experiments suggest that Gγ is not lipid modified in phlp1 knockout cells. Prenylation is normally the first step in a sequence of posttranslational modification events following Gβγ dimerization (22, 70) and is essential for membrane association (45, 60). Moreover, a complementary combination of in vitro biochemical and in vivo spectroscopic experiments strongly suggests that Gβ and Gγ do not form a complex in the absence of PhLP1: tagged Gβ and Gγ subunits are not detectably coimmunoprecipitated, and cytosolic diffusion measurements using fluorescence correlation spectroscopy (FCS) reveal that the diffusion of GFP-Gγ expressed in phlp1− cells is similar to that of free GFP-Gγ monomers in cells lacking Gβ.
Collectively, our data suggest that the phosducin-like protein PhLP1 serves a role in Gβγ dimer formation. We propose that PhLP1 may function as a cytosolic cochaperone for Gβγ assembly.
MATERIALS AND METHODS
Cell culture.
All cell lines were grown in 9-cm dishes containing HG5 medium (14.3 g/liter of peptone, 7.15 g/liter of yeast extract, 10 g/liter of glucose, 0.49 g/liter of KH2PO4, and 1.36 g/liter of Na2HPO4 · 2H2O). The Dictyostelium discoideum AX3 strain was used as a wild-type control in all experiments. Cells were grown in HG5 medium that was supplemented with 30 μg/ml G418 (GibcoBRL) for the transfectants expressing tagged Gβ or Gγ subunits. The gβ− (LW6) and phlp1− knockout cell lines, as well as cells expressing either GFP-Gβ or GFP-Gγ, have been described previously (3, 36, 68).
Plasmid construction and transfection.
An expression plasmid encoding both GFP-tagged Gβ and hemagglutinin (HA)-tagged Gγ (HA-Gγ) was constructed as follows. DNA encoding Dictyostelium Gγ was isolated as a BglII-SpeI fragment from a pGEM-T Gγ-Easy construct (3) and subcloned in pMB74 downstream of the actin15 promoter. The Dictyostelium expression vector pMB74 is derived from pMB12Neo (37) by replacement of the 2H3 terminator with an actin8 terminator. A double-stranded oligonucleotide containing a Dictyostelium translational initiation site (A5ATG) and a sequence encoding an N-terminal HA epitope (MISYPYDVPDYA) was inserted in frame with the Gγ coding sequence by ligating it as a BamHI-BglII fragment into the BglII site of Gγ/pMB74. The resulting expression cassette, with HA-Gγ codons flanked by an actin15 promoter and an actin8 terminator, was isolated by XbaI digestion, Klenow treatment, and ClaI digestion and subcloned in the SmaI and ClaI sites of a pLB5Neo-based expression plasmid encoding GFP-Gβ (3). The resultant plasmid, pGβγ Express, contains tandem expression cassettes for GFP-Gβ and HA-Gγ.
To express a GFP-PhLP1 fusion construct, DNA encoding PhLP1 was amplified by PCR using the forward primer 5′-TCTCAGATCTAAAGAATGGAACAAAACATTTTAAATAG-3′ and the reverse primer 5′-GGACTAGTATCGTCATTATCATCATCGGAC-3′, with the DNA encoding the open reading frame of phlp1 as the template (3). The DNA fragment was subcloned in pGEM-T Easy (Promega) and sequenced. Subsequently, the phlp1 insert was released and ligated into the BglII and SpeI sites of MB74GFP. The MB74GFP plasmid is similar to MB74 but contains the S65T GFP gene behind the SpeI site. The final fusion protein consists of the open reading frame of PhLP1, two serines, and the complete S65T GFP protein. The plasmids were introduced into Dictyostelium cells by electroporation following standard procedures, and transfectants were selected with G418.
Western blot analysis.
Cells were lysed either directly by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer or by filter lysis and detergent solubilization (see below). Equal amounts of lysates were loaded; lysates containing GFP-Gβ or GFP-Gγ were applied to standard 10 or 12% SDS-PAGE gels. Lysates containing the small HA-Gγ protein were applied to 15% Tricine-SDS-PAGE gels (54). Following electrophoresis, proteins were electroblotted onto polyvinylidene difluoride membranes (Millipore). For confirming equal loading, blots were stained with Ponceau S. For Western blot analysis, the membranes were blocked for 2 h in 5% low-fat milk in Tris-buffered saline plus Tween 20 (TBST) (20 mM Tris-HCl [pH 7.4], 137 mM NaCl, 0.05% Tween 20). Subsequently, membranes were incubated overnight at 4°C with polyclonal anti-GFP antibody ab6556 (Abcam) diluted 1:5,000 in blocking buffer, washed several times with TBST, incubated for 1 h at room temperature with a peroxidase-coupled sheep anti-rabbit antibody (Roche), washed several times with TBST and once with TBS without Tween 20, and developed with an ECL kit (Roche).
Crude cell fractionation.
Cells were harvested from confluent 9-cm dishes and washed twice with phosphate buffer (pH 6.5). Cell pellets were taken up in 150 to 300 μl ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 5 mM EGTA, 150 mM NaCl, 1 mM dithiothreitol, containing Complete protease inhibitor cocktail [Roche]) and lysed through a 3-μm Nuclepore filter (Whatman). Lysates were centrifuged for 5 min in an Eppendorf centrifuge at 4°C, and a sample of the supernatant was carefully removed while taking care not to take along any particulate matter. The pellets were washed and then resuspended in an original volume of lysis buffer. Equal samples of supernatant (cytosolic) and pellet (particulate) fractions were used for Western blot analysis.
Triton X-114 partitioning assay.
Cells were prepared and lysed as described above. Subsequently, 100 μl lysate was diluted with 100 μl lysis buffer containing 2% Triton X-114, which had been precondensed thrice (4), and rotated for 40 min at 4°C. Insoluble material was removed by spinning for 15 min at 4°C in an Eppendorf centrifuge, and supernatants were subjected to phase partitioning by incubation at 30°C for 3 min and spinning for 5 min at room temperature. The upper, water phases were reextracted with an equal volume of 2% Triton X-114, and the lower, detergent phases were reextracted with 0.2% Triton X-114. Following partitioning at 30°C, detergent phases were combined, and the volume was adjusted with lysis buffer to match the volume of the water phase samples. Equal samples of water and detergent phases were used for Western blot analysis.
Immunoprecipitation.
Cells were prepared and lysed as described above. All subsequent procedures were performed at 4°C. Following lysis, 100 μl lysate was diluted with 400 μl lysis buffer containing 1.25% Triton X-100 and rotated for 45 min. Insoluble material was removed by centrifugation for 15 min in an Eppendorf centrifuge, and the lysate was precleared twice by rotating with 50 μl 50% (vol/vol) protein A-Sepharose beads for 1 h and removal of the beads by centrifugation for 10 min. Precleared supernatants were then rotated for 1 h with 2 μl monoclonal anti-GFP antibody ab1218 (Abcam), and immunocomplexes were collected by rotation with 25 μl 50% (vol/vol) protein A-Sepharose beads for 1 h and centrifugation for 1 min. The beads were washed four times with lysis buffer containing 1% Triton X-100 and once with lysis buffer without Triton X-100 while transferring the beads to a fresh tube. Beads were taken up in 2× concentrated SDS-PAGE sample buffer, heated for 3 min at 95°C, and spun down for 5 min in an Eppendorf centrifuge. Supernatants were stored at −20°C prior to Western blot analysis.
Fluorescence correlation spectroscopy.
The diffusion measurements of GFP-tagged proteins in cells were performed on a ConforCor 2 (Carl Zeiss). The details of the setup have been described previously (23). In our experiments, GFP was excited with the 488-nm line from an argon-ion laser and focused into the sample with a water immersion C-Apochromat 40× lens objective (Zeiss). The excitation intensity was ∼11 μW. The excitation and emission light was separated by a dichroic beam splitter (HFT 488/633). The fluorescence was detected by an avalanche photodiode after being filtered through a band-pass filter of 505 to 550 nm. The pinhole was set at 70 μm.
Cells from a confluent dish were transferred to a 96-chamber glass-bottomed microplate (Whatman, Inc.) and washed twice with potassium phosphate buffer (17 mM, pH 6.5). Measurements were performed for cells incubated in buffer at room temperature. Around 100 autocorrelation traces were obtained for each cell line from five measurements made at a randomly chosen spot in the cytoplasm of around 20 different cells. The expression levels in cells were too high for FCS measurements, and so the GFP was photobleached to acceptable fluorescence intensity levels by exposing the cells to a high-intensity laser beam (∼1 mW) for 1 s. The measurement duration for all cell lines was 10 s.
The data were analyzed using the software FCS Data Processor (61). The autocorrelation traces were fitted with a model describing Brownian motion of a single species in three dimensions (equation 1), with an additional offset term to account for artifacts caused by drifts in average fluorescence on time scales of >1 s arising from cellular and intracellular movement (5).
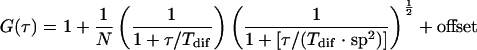 |
(1) |
In equation 1, G(τ) is the autocorrelation function, N is the average number of molecules in the detection volume, Tdif is the average diffusion time of the molecules, and sp is the structural parameter. A global-analysis approach was used to fit up to five traces simultaneously with the Tdif linked and a fixed value of sp. The value of sp, determined from calibration measurements with a rhodamine 110 solution, was around 5. The translational diffusion coefficient D was calculated from the diffusion time Tdif by using equation 2,
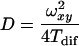 |
(2) |
where ωxy is the equatorial radius of the confocal volume element and is obtained from calibration measurements.
RESULTS
Expression of GFP-PhLP1 rescues phlp1− cells.
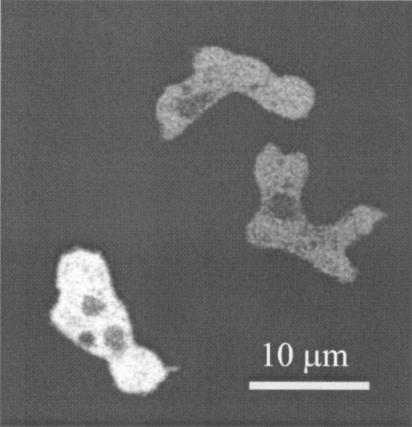
Northern blots revealed that PhLP1 is expressed throughout development (3; unpublished observations). To investigate the cellular localization of PhLP1 and the potential interaction of PhLP1 with membrane-associated Gβγ complexes, PhLP1 was fused to GFP and expressed in phlp1− cells. This resulted in a cell line with normal development and a complete rescue of the aggregation-negative phenotype (unpublished observations). Confocal microscopy revealed that PhLP1-GFP is localized predominantly in the cytosol, without indications for any membrane enrichment (Fig. 1). Stimulation of these cells with 1 μM cyclic AMP in a perfusion chamber does not lead to a significant change in the fluorescence intensity of PhLP1-GFP in the cytosol (detection limit of 4% change in fluorescence intensity [50]) or any detectable translocation of PhLP1-GFP from the cytosol to other cellular compartments (unpublished observations). These observations suggest that PhLP may function in the cytoplasm and that we have no evidence for an interaction of PhLP1 with Gβγ complexes at the plasma membrane.
FIG. 1.
PhLP1-GFP is localized in the cytosol. An extrachromosomal plasmid expressing a C-terminal fusion of GFP to PhLP1 was expressed in phlp1− cells, which completely restored the aggregation-negative phenotype. PhLP1-GFP appears to be localized in the cytosol in both weakly and strongly expressing cells, without indications for association to membranes or membrane-associated proteins.
GFP-Gβ and GFP-Gγ are expressed at reduced levels and do not associate with the plasma membrane in phlp1− cells.
To be able to monitor the fate of Gβ and Gγ in intact cells, we expressed GFP-Gβ or GFP-Gγ fusion proteins in wild-type AX3 cells and in phlp1− cells (3). Complexes of Gβ and Gγ tagged with GFP variants have been demonstrated to be fully functional in Dictyostelium amoebae (26, 27). As a reference, we also employed gβ− cells, which do not express Gβ and therefore lack normal Gβγ complexes (36, 68).
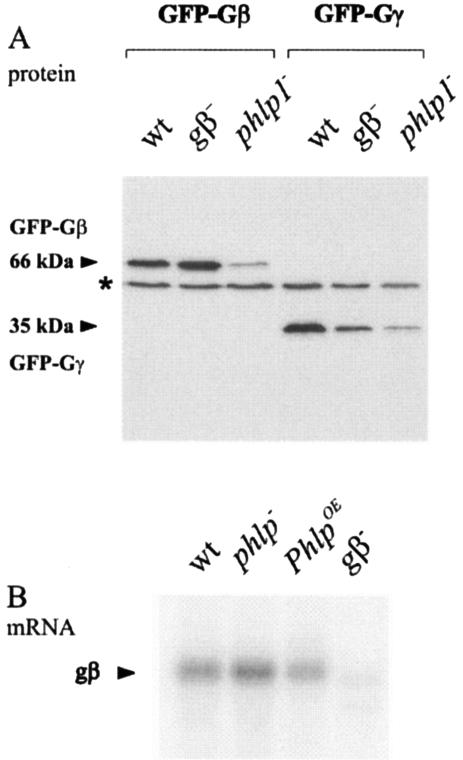
Western blot analysis revealed that correctly sized proteins are expressed in all cell lines (Fig. 2A). However, the steady-state levels of GFP-Gβ and GFP-Gγ proteins are dramatically reduced (at least 20-fold) in phlp1− cells compared to the levels in wild-type cells. Such reduced protein levels were also observed for endogenously expressed Gβ by use of an anti-Gβ antibody (unpublished observations). Interestingly, the mRNA levels for Gβ are not significantly different between control and phlp1− cells (Fig. 2B), suggesting that the reduced levels of Gβ, GFP-Gβ, and GFP-Gγ proteins are due to increased instability of the proteins in phlp1− cells. The cellular level of GFP-Gγ in gβ− cells is also significantly lower than in control cells (Fig. 2A). This indicates that Gγ subunits are less stable in the absence of Gβ. Such a mutual dependency of binding partners for stable expression has been previously documented for various protein complexes, including Gβγ dimers (24, 51, 55, 60, 67).
FIG. 2.
Expression of Gβ and Gγ in phlp1− cells. (A) Steady-state protein levels of GFP-Gβ and GFP-Gγ are reduced in phlp1− cells. Wild-type AX3 cells (wt) and gβ− and phlp1− knockout cells were transfected with plasmids encoding GFP-Gβ or GFP-Gγ. Cell lysates were prepared and analyzed through Western blot analysis with a GFP antibody. The molecular masses of GFP-Gβ and GFP-Gγ are indicated. The asterisk denotes a cross-reacting AX3 band. (B) Northern blots show normal expression of Gβ in phlp1− cells. Poly(A) mRNA was isolated from wild-type AX3 cells (wt), phlp1− knockout cells, phlp1− cells overexpressing PhLP1 (PhlpOE), and gβ− knockout cells. RNA was size fractionated, transferred, and probed with gβ.
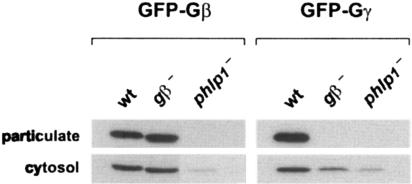
Crude cell fractionation of AX3 transfectants revealed that GFP-Gβ and GFP-Gγ can be detected both in a cytosolic fraction and in a particulate fraction containing plasma membranes (Fig. 3). Jin and coworkers similarly reported a considerable amount of GFP-Gβ (about 30%) to be present in cytosolic fractions of transfected Dictyostelium amoebae and claimed that the same holds true for endogenous Gβ (27). In striking contrast to the situation for wild-type cells, GFP-Gβ and GFP-Gγ cannot be detected in particulate fractions of phlp1− transfectants, although they are detected in cytosolic fractions. Again, in gβ− cells, GFP-Gβ can combine with endogenous Gγ and exhibits behavior similar to that in wild-type cells, whereas GFP-Gγ does not reach the plasma membrane in default of a Gβ partner. These results are fully in line with our previous fluorescence confocal microscopy studies (3) and explain why G protein signaling is defective in cells lacking the PhLP1 protein. Either a Gβγ complex is formed but not transported to its final destination, or the complex is not formed at all, with individual Gβ and Gγ subunits not being able to associate with the plasma membrane. In either case, this largely results in the demise of the nonfunctional protein.
FIG. 3.
GFP-Gβ and GFP-Gγ are absent from plasma membranes in phlp1− cells. Cells expressing GFP-Gβ or GFP-Gγ were subjected to crude fractionation by filter lysis and collection of particulate and cytosolic fractions after centrifugation in a microcentrifuge. Equal samples were used for Western blot analysis with a GFP antibody, as described in the legend for Fig. 2A. wt, wild type.
Triton X-114 partitioning suggests that GFP-Gγ is not prenylated in phlp1− cells.
To further define the stage at which the formation of a functional Gβγ complex goes awry in phlp1− cells, we assessed whether Gγ proteins are still posttranslationally modified. During normal Gβγ synthesis, a CAAX motif at the C terminus of immature Gγ subunits is prenylated, followed by proteolytic removal of the last three residues (AAX) and carboxyl methylation of the now C-terminal prenyl-cysteine moiety (22, 70). The attachment of a prenyl group is mandatory for stable membrane association of Gβγ subunits (45, 60).
Lipid modification of Gγ can be demonstrated by metabolic labeling with radioactive precursors but can also be investigated in a nonradioactive fashion by using the Triton X-114 partitioning assay originally devised for integral membrane proteins (4). In this assay, cell lysates containing Triton X-114 are prepared and then induced to undergo phase separation by transient warming to 30°C. Proteins carrying a significantly hydrophobic moiety, such as a prenyl group, are drawn from the water phase into the detergent phase. The partitioning assay has been used not only for small monomeric G proteins but also for heterotrimeric G proteins (28).
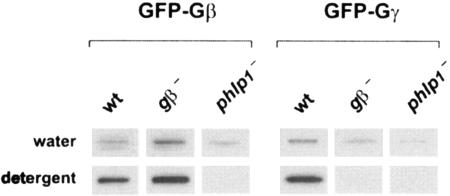
As can be seen in Fig. 4, substantial amounts of both GFP-Gβ and GFP-Gγ expressed in AX3 cells are drawn into the detergent phase after partitioning. This indicates that GFP-Gγ is lipid modified in wild-type cells and that GFP-Gβ must form a tight complex with endogenous Gγ since it lacks hydrophobic modifications. When expressed in gβ− cells, GFP-Gβ also forms a complex with endogenous Gγ proteins and therefore partitions into the detergent phase. However, GFP-Gγ expressed in gβ− cells can only be detected in the water phase. This indicates that Gγ is not (efficiently) modified unless it is present in Gβγ heterodimers. Such a finding corroborates suggestions in the literature that the Gβγ complex is the substrate for the prenylation machinery (22).
FIG. 4.
Triton X-114 partitioning suggests absence of lipid modification on GFP-Gγ in phlp1− cells. Triton X-114-containing extracts were prepared from cells expressing GFP-Gβ or GFP-Gγ and subjected to phase partitioning at 30°C. Water phases and detergent phases were separated and reextracted, and then equal samples were used for Western blot analysis with a GFP antibody, as described in the legend for Fig. 2A. wt, wild type.
Significantly, GFP-Gβ and GFP-Gγ expressed in phlp1− cells could only be detected in the water phase after extraction and partitioning. Thus, Gγ remains unmodified in phlp1− cells and fails to draw Gβ into the detergent phase. A lack of lipid attachment in phlp1− cells might be explained by defective modification of an otherwise properly formed Gβγ complex or by the failure of Gβ and Gγ to form a complex in the first place.
Coimmunoprecipitation studies suggest that Gβ and Gγ do not form a complex in phlp1− cells.
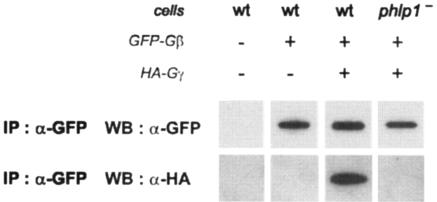
To assess whether there is indeed a lack of Gβγ complex formation in phlp1− cells, we performed coimmunoprecipitation experiments. In order to be able to detect both Gβ and Gγ, we transfected AX3 and phlp1− cells with a plasmid which directs the coexpression of GFP-tagged Gβ (GFP-Gβ) and hemagglutinin epitope-tagged Gγ (HA-Gγ). Cell lysates were subjected to immunoprecipitation with an anti-GFP antibody, and subsequently aliquots of the precipitate were analyzed by Western blot experiments with antibodies recognizing GFP (for immunoprecipitated GFP-Gβ) or HA (for coimmunoprecipitated HA-Gγ).
As can be seen in Fig. 5, GFP-Gβ can be immunoprecipitated from both wild-type AX3 and phlp1− knockout cell lysates. HA-Gγ was clearly coprecipitated with GFP-Gβ when these proteins were coexpressed in wild-type cells. In contrast, a similar band could not be detected in the precipitate of phlp1− cells coexpressing GFP-Gβ and HA-Gγ, even though significant amounts of GFP-Gβ had been precipitated. Furthermore, we have mixed small amounts of a lysate prepared from wild-type cells expressing GFP-Gβ and HA-Gγ with large amounts of lysate from phlp1− cells in a 1:20 ratio and could easily observe the presence of the Gβγ dimer (unpublished observations). These results suggest that a normal Gβγ complex is not present at significant levels in phlp1− cells, and we propose that the levels of Gβ and Gγ in phlp1− cells are low because they are not in dimers and are hence destabilized, rather than the opposite, that dimers are rare because Gβ and Gγ levels are too low in the absence of PhLP1. Unfortunately we have not yet been able to induce Gβγ dimer formation in vitro by using a mixture of lysates from GFP-Gβ/HA-Gγ-expressing phlp1− cells (providing unassembled GFP-Gβ and HA-Gγ) with wild-type cells (providing phosducin).
FIG. 5.
GFP-Gβ and HA-Gγ do not coimmunoprecipitate in extracts of phlp1− cells. Cell lysates were prepared from nontransfected cells and from cells expressing either GFP-Gβ alone or both GFP-Gβ and HA-Gγ and immunoprecipitated with a monoclonal GFP antibody. Half of the immunoprecipitates were used for Western blot analysis with a polyclonal GFP antibody (α-GFP), and the other half were used for Western blot analysis with an antibody recognizing hemagglutinin tags (α-HA). wt, wild type.
Similar diffusion of GFP-Gγ in phlp1− and gβ− cells suggests absence of Gβγ complexes in phlp1− cells.
The coimmunoprecipitation experiments described above indicate that Gβ and Gγ subunits do not form a complex in cells lacking PhLP1. Further experiments were complicated by the reduced steady-state levels of GFP-Gβ and GFP-Gγ in phlp1− cells. To overcome this problem, and to substantiate our in vitro findings in an in vivo environment, we used FCS, which is a sensitive technique that can be used to monitor the diffusion of proteins expressed at low concentrations in living cells (20, 30, 43). The diffusion characteristics of a protein provide valuable clues about its aggregation state or intracellular interactions. Thus, the cytoplasmic diffusion coefficients obtained from FCS measurements can be used to discriminate free subunits from Gβγ complexes in different cell lines.
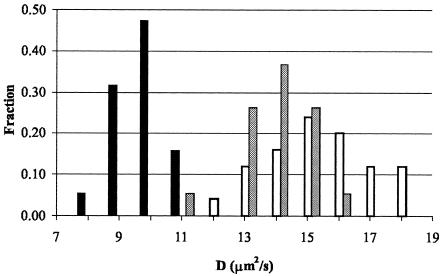
The 7-kDa Gγ is much smaller than the 40-kDa Gβ and, thus, diffusion characteristics of GFP-Gγ would be more strongly affected than those of GFP-Gβ when Gβγ no longer forms a complex. Therefore, we chose to analyze the diffusion of GFP-Gγ in the cytoplasm of wild-type and mutant cell lines (Fig. 6 and Table 1).
FIG. 6.
GFP-Gγ exhibits monomer-type diffusion characteristics in phlp1− cells. Comparison of the diffusion coefficient distribution of GFP-Gγ in wild-type AX3 (black bars), phlp1− (open bars), and gβ− (gray bars) cells. The diffusion coefficient distribution of GFP-Gγ proteins was determined in the cytosol of different cell lines by using FCS. Bars indicate the fraction of the total number of observations in which a specific diffusion coefficient (D) was found.
TABLE 1.
Diffusion of GFP-Gγ in wild-type and mutant cell lines
| Cell line | D (μm2/s)a |
|---|---|
| AX3 | 10 ± 1 |
| phlp1− | 15 ± 2 |
| gβ− | 14 ± 1 |
Average diffusion coefficients (D) and standard deviation values were determined for GFP-Gγ in the cytoplasm of wild-type and mutant Dictyostelium cells incubated in 17 mM phosphate buffer (pH 6.5) (n = 20).
The experimental data fit well to a model describing the diffusion of a single population of fluorescing molecules in three dimensions. Figure 6 shows that the diffusion coefficient distribution for GFP-Gγ in phlp1− cells is shifted to higher values than that in wild-type AX3 cells. The slow diffusion of GFP-Gγ in wild-type cells indicates that it is predominantly present as a complex in wild-type cells but not in phlp1− cells. To determine if the diffusion of GFP-Gγ in phlp1− cells was indeed the diffusion of free GFP-Gγ monomers, we measured the diffusion of GFP-Gγ in gβ− cells. As can be seen in Fig. 6, the diffusion coefficient distribution for GFP-Gγ expressed in gβ− cells strongly overlaps with that obtained for phlp1− cells, clearly indicating that GFP-Gγ in phlp1− cells is free. The average diffusion coefficient of GFP-Gγ molecules in gβ− cells is very similar to the value obtained for GFP-Gγ in phlp1− cells (14 and 15 μm2/s, respectively). These values are slightly smaller than the value of 20 μm2/s obtained for freely diffusing GFP monomers in Dictyostelium cells (52). Since GFP-Gγ and GFP are similar in size (35 and 27 kDa, respectively), their diffusion coefficients are also expected to be similar. However, GFP is approximately globular (49) whereas Gγ in a Gβγ dimer has a rod-like structure (63), and though it is not known how the structure changes when Gγ is not associated with Gβ, it is likely that it is (partially) unfolded. The difference in the shapes of GFP and GFP-Gγ can thus be the reason for the discrepancy seen between their diffusion coefficients. Taken together, the FCS data strongly suggest that, in cells that are devoid of PhLP1, GFP-Gγ molecules are present as free monomers. We therefore conclude that Gβγ complexes, if present at all, form a very small minority in living phlp1− cells.
DISCUSSION
Absence of Gβγ dimers in cells lacking phosducin-like protein PhLP1.
Dictyostelium amoebae lacking the phosducin-like protein PhLP1 exhibit a phenotype which is strikingly similar to the phenotype observed upon gβ gene disruption (3). Our present findings suggest that the gβ−-like phenotype of phlp1− cells is due to defective Gβγ dimer formation. We observed a dramatic reduction in Gβ and Gγ steady-state protein levels in phlp1 knockout cells, while mRNA levels were unaltered. This might be explained with the notion that Gβγ complex formation is required for stable expression of either partner (24, 51, 55, 60, 67). Our inability to detect posttranslational lipid attachment to GFP-Gγ in phlp1 knockout cells would also be in keeping with a lack of Gβγ dimer formation. It has been suggested that Gβγ assembly precedes cytosolic prenylation of the C-terminal CAAX motif of Gγ (22). Indeed, when we overexpress GFP-Gγ in gβ− cells which cannot form Gβγ complexes, the protein is unstable and does not seem to be lipid modified in Triton X-114 partitioning assays.
The absence of Gβγ dimer formation is also strongly suggested by our inability to coimmunoprecipitate GFP-tagged Gβ and HA-tagged Gγ in vitro after coexpression in phlp1− cells. Furthermore, in vivo FCS experiments revealed that the diffusion of GFP-Gγ in phlp1− cells is similar to that in gβ− cells, whereas it is significantly slower in wild-type AX3 cells. The average diffusion coefficient values can be explained assuming the presence of free GFP-Gγ monomers in phlp1− and gβ− cells and of GFP-Gαβγ complexes in AX3 cells. Therefore, the FCS results indicate that Gβ and Gγ are indeed apart in cells which lack PhLP1.
Taken together, the above data strongly suggest that without the PhLP1 protein, Gβγ dimer formation is defunct in Dictyostelium cells. We emphasize that it cannot be ruled out that small amounts of Gβγ dimers are still being formed in phlp1− cells. Maybe such traces of Gβγ can still provide some modulation of (a subset of) effectors, but in the assays which we reported previously, we failed to detect any Gβγ function (3).
PhLP1: a cochaperone for Gβγ dimer formation?
Whereas the Gγ subunit is a relatively small and flexible peptide lacking tertiary structure (7, 55), the Gβ subunit exhibits complex folding. Gβ is a member of the WD repeat protein family (47, 62), which in turn forms part of a larger family of β-propeller proteins (46, 48). Gβ proteins contain seven WD repeats which form seven β sheets making up the blades of a propeller-like structure. The last blade is made up of one β strand donated by the first WD repeat and three β strands donated by the last, C-terminal repeat, forming a noncovalent “Velcro snap” (6) which closes the ring structure of the propeller. Such a repetitive and highly integrated structure may not allow proper Gβ folding and/or assembly to occur spontaneously.
The structural difference between Gβ and Gγ is reflected in a difference of ease of production. Gγ can be produced in any expression system, but Gβ is more demanding. Assembly-competent Gβ cannot be produced in Escherichia coli or wheat germ extracts (21, 44) and only inefficiently in rabbit reticulocyte lysates (11, 12, 44, 55). This indicates the need for an accessory factor during Gβ synthesis which may have diverged between plant and mammalian cells and is in limiting supply in reticulocyte lysates. The need for one or more cellular factors is also indicated by the finding that assembly-competent Gβ can only be collected from recombinant baculovirus-infected Sf9 cells during early infection stages, when host cell protein expression is not shut down yet (14, 21). Thus, Gβ proteins may require molecular chaperones (10, 17) for their incorporation into functional Gβγ complexes.
A likely candidate as one of these factors is the group II chaperonin CCT (chaperonin containing TCP-1), which is also known as TRiC (34, 66). The barrel-shaped cylinder of CCT, a folding cage made up of two rings of eight subunits each, may provide a favorable environment for, and participate in, the folding and assembly of various proteins. Intriguingly, an unexpected proportion of yeast proteins interacting with CCT subunits harbor seven WD repeats (66). Indeed, this set of proteins includes STE4, the yeast Gβ subunit. These findings have recently been verified for both STE4 and five other, unrelated WD repeat proteins in yeast (59).
Apart from protein folding, CCT has also been implicated in the assembly of multimeric proteins. The WD repeat domain of the VHL tumor suppressor protein has been shown to bind to CCT prior to VHL assembly with the elongin BC complex (8, 16). Moreover, with the help of five additional cofactors, α- and β-tubulin are dimerized following their folding by CCT (15, 66). Thus, CCT, aided by cochaperones, may assist both folding and assembly of multimeric proteins, including WD repeat proteins such as Gβ.
The picture that emerges from our studies on Dictyostelium phlp1− cells is that Gβγ complex formation is abolished in the absence of PhLP1. It is unlikely that PhLP1 deficiency leads to a loss of function of the CCT machinery, as the CCT machinery is absolutely required for actin and tubulin folding and its absence is lethal in yeast (35, 65). Rather, it is tempting to speculate that PhLP1 serves a role as a cofactor cooperating with CCT in the formation of native Gβγ heterodimers. Interestingly, mammalian PhLP, but not Phd, has been shown to coimmunoprecipitate CCT subunits (42).
While the current work was under review, three reports were published providing substantial support for the hypothesis that PhLP is a molecular chaperone for Gβγ assembly (25, 39, 40). The structure of the complex between CCT and PhLP reveals PhLP binding at the apical top of CCT, above the folding activity. This leads to the hypothesis that PhLP delivers its binding partner (possibly Gβγ) to the CCT complex for folding (40). Consistent with this hypothesis is the observation that RNA interference inhibition of the CCT subunit TCP-1α leads to a strong reduction of the level of Gβγ subunits (25). Very recently, Lukov et al. (39) have shown that reduced expression of PhLP by RNA interference results in inhibition of Gβγ expression and G protein signaling, similar to PhLP-null cells in Dictyostelium (3) and Cryphonectria parasitica (29). Furthermore, Lukov et al. demonstrated that the inhibition of G protein signaling is due to an inability of nascent Gβγ to form dimers, as also demonstrated in the present work. They provide a model in which PhLP binds to Gβ and stabilizes the nascent Gβ polypeptide until Gγ will associate and PhLP will dissociate to catalyze another round of assembly (39). Phosphorylation of PhLP at Ser18 to Ser20 enhances binding to CCT and is essential for Gβγ folding (39). However, the role of CCT remains unclear, since a PhLP mutant with reduced CCT binding (PhLP133-135A) is fully capable in Gβγ folding (39).
The structure of Phd-Gβγ complexes may provide clues as to what the cochaperoning role of phosducin-type proteins could possibly be. Phd interacts with blades 6 and 7 of native Gβγ and can induce conformational changes in that region while transporting Gβγ away from the membrane (13, 38). Intriguingly, during Gβ biosynthesis, blade 7 must be formed by bringing together remote β strands from the N-terminal and C-terminal WD repeats of Gβ (6). Also, assembly-competent Gβ has an open structure with a Stokes radius larger than that of native Gβγ complexes (21, 55, 56). Thus, one might hypothesize that molecules such as PhLP facilitate ring closure of the Gβ propeller while Gβ and Gγ assemble. The fact that phlp1− cells grow significantly more slowly than both wild-type and gβ− cells (3) could indicate that the cellular chaperone machinery is to some extent obstructed in the absence of PhLP1 or that PhLP1 may assist in the folding of other proteins that are required for optimal cell growth.
Three families of PhLP proteins have been recognized, with one member of each family in Dictyostelium (3). The PhLP1 subfamily incorporates the mammalian PhLP involved in Gβγ folding (39) and the PhLP proteins from Cryphonectria parasitica and Dictyostelium that are essential for Gβγ functioning and folding (3, 29). Members of the PhLP2 and PhLP3 families bind Gβγ poorly and have no Gβγ-malfunctioning phenotypes. Deletion of PhLP2 is lethal in both Dictyostelium and yeast (3, 9). Interestingly, in yeast, PhLP2 forms a complex with CCT and VID27, a protein with a WD40 propeller structure similar to that of Gβ (1). Deletion of PhLP3 in yeast and Dictyostelium is not lethal (3, 9), but genetic evidence suggests that the yeast PhLP3 family member (named Plp1p) transfers the nascent β-tubulin polypeptides to the CCT folding apparatus (31). An interesting possibility that the PhLP families act as chaperones for the assembly of several proteins arises.
In conclusion, we have found that cells lacking the phosducin-like protein PhLP1 are not able to form functional Gβγ dimers, and we propose that this defect is related to an essential cochaperone function of PhLP1 during Gβγ assembly.
Acknowledgments
We thank Peter Devreotes for providing us with the gβ− cell line LW6 and Mark Hink for useful discussions on the FCS data.
The work by R.E. was financially supported by the Research Council for Earth and Life Sciences of the Netherlands Organization for Scientific Research.
REFERENCES
- 1.Aloy, P., B. Bottcher, H. Ceulemans, C. Leutwein, C. Mellwig, S. Fischer, A. C. Gavin, P. Bork, G. Superti-Furga, L. Serrano, and R. B. Russell. 2004. Structure-based assembly of protein complexes in yeast. Science 303:2026-2029. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, P. H., S. Muller, M. Puzicha, S. Pippig, B. Obermaier, E. J. Helmreich, and M. J. Lohse. 1992. Phosducin is a protein kinase A-regulated G-protein regulator. Nature 358:73-76. [DOI] [PubMed] [Google Scholar]
- 3.Blaauw, M., J. C. Knol, A. Kortholt, J. Roelofs, R. Engel, M. Postma, A. J. Visser, and P. J. van Haastert. 2003. Phosducin-like proteins in Dictyostelium discoideum: implications for the phosducin family of proteins. EMBO J. 22:5047-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 5.Brock, R., G. Vamosi, G. Vereb, and T. M. Jovin. 1999. Rapid characterization of green fluorescent protein fusion proteins on the molecular and cellular level by fluorescence correlation microscopy. Proc. Natl. Acad. Sci. USA 96:10123-10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham, D. E., and E. J. Neer. 1997. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 37:167-203. [DOI] [PubMed] [Google Scholar]
- 7.Dues, G., S. Muller, and N. Johnsson. 2001. Detection of a conformational change in G gamma upon binding G beta in living cells. FEBS Lett. 505:75-80. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, D. E., V. Thulasiraman, R. G. Ferreyra, and J. Frydman. 1999. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell 4:1051-1061. [DOI] [PubMed] [Google Scholar]
- 9.Flanary, P. L., P. R. DiBello, P. Estrada, and H. G. Dohlman. 2000. Functional analysis of Plp1 and Plp2, two homologues of phosducin in yeast. J. Biol. Chem. 275:18462-18469. [DOI] [PubMed] [Google Scholar]
- 10.Frydman, J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70:603-647. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Higuera, I., J. Fenoglio, Y. Li, C. Lewis, M. P. Panchenko, O. Reiner, T. F. Smith, and E. J. Neer. 1996. Folding of proteins with WD-repeats: comparison of six members of the WD-repeat superfamily to the G protein beta subunit. Biochemistry 35:13985-13994. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Higuera, I., C. Gaitatzes, T. F. Smith, and E. J. Neer. 1998. Folding a WD repeat propeller. Role of highly conserved aspartic acid residues in the G protein beta subunit and Sec13. J. Biol. Chem. 273:9041-9049. [DOI] [PubMed] [Google Scholar]
- 13.Gaudet, R., A. Bohm, and P. B. Sigler. 1996. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell 87:577-588. [DOI] [PubMed] [Google Scholar]
- 14.Graber, S. G., R. A. Figler, V. K. Kalman-Maltese, J. D. Robishaw, and J. C. Garrison. 1992. Expression of functional G protein beta gamma dimers of defined subunit composition using a baculovirus expression system. J. Biol. Chem. 267:13123-13126. [PubMed] [Google Scholar]
- 15.Gutsche, I., L. O. Essen, and W. Baumeister. 1999. Group II chaperonins: new TRiC(k)s and turns of a protein folding machine. J. Mol. Biol. 293:295-312. [DOI] [PubMed] [Google Scholar]
- 16.Hansen, W. J., M. Ohh, J. Moslehi, K. Kondo, W. G. Kaelin, and W. J. Welch. 2002. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol. Cell. Biol. 22:1947-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 18.Hawes, B. E., K. Touhara, H. Kurose, R. J. Lefkowitz, and J. Inglese. 1994. Determination of the G beta gamma-binding domain of phosducin. A regulatable modulator of G beta gamma signaling. J. Biol. Chem. 269:29825-29830. [PubMed] [Google Scholar]
- 19.Hekman, M., P. H. Bauer, P. Sohlemann, and M. J. Lohse. 1994. Phosducin inhibits receptor phosphorylation by the beta-adrenergic receptor kinase in a PKA-regulated manner. FEBS Lett. 343:120-124. [DOI] [PubMed] [Google Scholar]
- 20.Hess, S. T., S. Huang, A. A. Heikal, and W. W. Webb. 2002. Biological and chemical applications of fluorescence correlation spectroscopy: a review. Biochemistry 41:697-705. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, J. B., and P. J. Casey. 1994. In vitro processing of recombinant G protein gamma subunits. Requirements for assembly of an active beta gamma complex. J. Biol. Chem. 269:9067-9073. [PubMed] [Google Scholar]
- 22.Higgins, J. B., and P. J. Casey. 1996. The role of prenylation in G-protein assembly and function. Cell. Signal. 8:433-437. [DOI] [PubMed] [Google Scholar]
- 23.Hink, M. A., J. W. Borst, and A. J. Visser. 2003. Fluorescence correlation spectroscopy of GFP fusion proteins in living plant cells. Methods Enzymol. 361:93-112. [DOI] [PubMed] [Google Scholar]
- 24.Hirschman, J. E., G. S. De Zutter, W. F. Simonds, and D. D. Jenness. 1997. The G beta gamma complex of the yeast pheromone response pathway. Subcellular fractionation and protein-protein interactions. J. Biol. Chem. 272:240-248. [DOI] [PubMed] [Google Scholar]
- 25.Humrich, J., C. Bermel, M. Bunemann, L. Harmark, R. Frost, U. Quitterer, and M. J. Lohse. 2005. Phosducin-like protein regulates G-protein βγ folding by interaction with tailless complex polypeptide-1α: dephosphorylation or splicing of PhLP turns the switch toward regulation of Gβγ folding. J. Biol. Chem. 280:20042-20050. [DOI] [PubMed] [Google Scholar]
- 26.Janetopoulos, C., T. Jin, and P. Devreotes. 2001. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291:2408-2411. [DOI] [PubMed] [Google Scholar]
- 27.Jin, T., N. Zhang, Y. Long, C. A. Parent, and P. N. Devreotes. 2000. Localization of the G protein betagamma complex in living cells during chemotaxis. Science 287:1034-1036. [DOI] [PubMed] [Google Scholar]
- 28.Justice, J. M., J. J. Murtagh, Jr., J. Moss, and M. Vaughan. 1995. Hydrophobicity and subunit interactions of rod outer segment proteins investigated using Triton X-114 phase partitioning. J. Biol. Chem. 270:17970-17976. [DOI] [PubMed] [Google Scholar]
- 29.Kasahara, S., P. Wang, and D. L. Nuss. 2000. Identification of bdm-1, a gene involved in G protein beta-subunit function and alpha-subunit accumulation. Proc. Natl. Acad. Sci. USA 97:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, S. A., and P. Schwille. 2003. Intracellular applications of fluorescence correlation spectroscopy: prospects for neuroscience. Curr. Opin. Neurobiol. 13:583-590. [DOI] [PubMed] [Google Scholar]
- 31.Lacefield, S., and F. Solomon. 2003. A novel step in beta-tubulin folding is important for heterodimer formation in Saccharomyces cerevisiae. Genetics 165:531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, R. H., B. S. Lieberman, and R. N. Lolley. 1987. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry 26:3983-3990. [DOI] [PubMed] [Google Scholar]
- 33.Lee, R. H., T. D. Ting, B. S. Lieberman, D. E. Tobias, R. N. Lolley, and Y. K. Ho. 1992. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J. Biol. Chem. 267:25104-25112. [PubMed] [Google Scholar]
- 34.Leroux, M. R., and F. U. Hartl. 2000. Protein folding: versatility of the cytosolic chaperonin TRiC/CCT. Curr. Biol. 10:R260-R264. [DOI] [PubMed] [Google Scholar]
- 35.Li, W. Z., P. Lin, J. Frydman, T. R. Boal, T. S. Cardillo, L. M. Richard, D. Toth, M. A. Lichtman, F. U. Hartl, F. Sherman, et al. 1994. Tcp20, a subunit of the eukaryotic TRiC chaperonin from humans and yeast. J. Biol. Chem. 269:18616-18622. [PubMed] [Google Scholar]
- 36.Lilly, P., L. Wu, D. L. Welker, and P. N. Devreotes. 1993. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 7:986-995. [DOI] [PubMed] [Google Scholar]
- 37.Linskens, M. H., P. D. Grootenhuis, M. Blaauw, B. Huisman-de Winkel, A. Van Ravestein, P. J. Van Haastert, and J. C. Heikoop. 1999. Random mutagenesis and screening of complex glycoproteins: expression of human gonadotropins in Dictyostelium discoideum. FASEB J. 13:639-645. [DOI] [PubMed] [Google Scholar]
- 38.Loew, A., Y. K. Ho, T. Blundell, and B. Bax. 1998. Phosducin induces a structural change in transducin beta gamma. Structure 6:1007-1019. [DOI] [PubMed] [Google Scholar]
- 39.Lukov, G. L., T. Hu, J. N. McLaughlin, H. E. Hamm, and B. M. Willardson. 2005. Phosducin-like protein acts as a molecular chaperone for G protein betagamma dimer assembly. EMBO J. 24:1965-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Benito, J., S. Bertrand, T. Hu, P. J. Ludtke, J. N. McLaughlin, B. M. Willardson, J. L. Carrascosa, and J. M. Valpuesta. 2004. Structure of the complex between the cytosolic chaperonin CCT and phosducin-like protein. Proc. Natl. Acad. Sci. USA 101:17410-17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin, J. N., C. D. Thulin, S. M. Bray, M. M. Martin, T. S. Elton, and B. M. Willardson. 2002. Regulation of angiotensin II-induced G protein signaling by phosducin-like protein. J. Biol. Chem. 277:34885-34895. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin, J. N., C. D. Thulin, S. J. Hart, K. A. Resing, N. G. Ahn, and B. M. Willardson. 2002. Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc. Natl. Acad. Sci. USA 99:7962-7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medina, M. A., and P. Schwille. 2002. Fluorescence correlation spectroscopy for the detection and study of single molecules in biology. Bioessays 24:758-764. [DOI] [PubMed] [Google Scholar]
- 44.Mende, U., C. J. Schmidt, F. Yi, D. J. Spring, and E. J. Neer. 1995. The G protein gamma subunit. Requirements for dimerization with beta subunits. J. Biol. Chem. 270:15892-15898. [DOI] [PubMed] [Google Scholar]
- 45.Muntz, K. H., P. C. Sternweis, A. G. Gilman, and S. M. Mumby. 1992. Influence of gamma subunit prenylation on association of guanine nucleotide-binding regulatory proteins with membranes. Mol. Biol. Cell 3:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murzin, A. G. 1992. Structural principles for the propeller assembly of beta-sheets: the preference for seven-fold symmetry. Proteins 14:191-201. [DOI] [PubMed] [Google Scholar]
- 47.Neer, E. J., C. J. Schmidt, R. Nambudripad, and T. F. Smith. 1994. The ancient regulatory-protein family of WD-repeat proteins. Nature 371:297-300. [DOI] [PubMed] [Google Scholar]
- 48.Neer, E. J., and T. F. Smith. 2000. A groovy new structure. Proc. Natl. Acad. Sci. USA 97:960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ormo, M., A. B. Cubitt, K. Kallio, L. A. Gross, R. Y. Tsien, and S. J. Remington. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392-1395. [DOI] [PubMed] [Google Scholar]
- 50.Postma, M., J. Roelofs, J. Goedhart, T. W. J. Gadella, A. J. W. G. Visser, and P. J. M. Van Haastert. 2003. Uniform cAMP stimulation of Dictyostelium cells induces localized patches of signal transduction and pseudopodia. Mol. Biol. Cell 14:5019-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pronin, A. N., and N. Gautam. 1993. Proper processing of a G protein gamma subunit depends on complex formation with a beta subunit. FEBS Lett. 328:89-93. [DOI] [PubMed] [Google Scholar]
- 52.Ruchira, M. A. Hink, L. Bosgraaf, P. J. van Haastert, and A. J. Visser. 2004. Pleckstrin homology domain diffusion in Dictyostelium cytoplasm studied using fluorescence correlation spectroscopy. J. Biol. Chem. 279:10013-10019. [DOI] [PubMed] [Google Scholar]
- 53.Savage, J. R., J. N. McLaughlin, N. P. Skiba, H. E. Hamm, and B. M. Willardson. 2000. Functional roles of the two domains of phosducin and phosducin-like protein. J. Biol. Chem. 275:30399-30407. [DOI] [PubMed] [Google Scholar]
- 54.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt, C. J., and E. J. Neer. 1991. In vitro synthesis of G protein beta gamma dimers. J. Biol. Chem. 266:4538-4544. [PubMed] [Google Scholar]
- 56.Schmidt, C. J., T. C. Thomas, M. A. Levine, and E. J. Neer. 1992. Specificity of G protein beta and gamma subunit interactions. J. Biol. Chem. 267:13807-13810. [PubMed] [Google Scholar]
- 57.Schroder, S., K. Bluml, C. Dees, and M. J. Lohse. 1997. Identification of a C-terminal binding site for G-protein betagamma-subunits in phosducin-like protein. FEBS Lett. 401:243-246. [DOI] [PubMed] [Google Scholar]
- 58.Schroder, S., and M. J. Lohse. 1996. Inhibition of G-protein betagamma-subunit functions by phosducin-like protein. Proc. Natl. Acad. Sci. USA 93:2100-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegers, K., B. Bolter, J. P. Schwarz, U. M. Bottcher, S. Guha, and F. U. Hartl. 2003. TRiC/CCT cooperates with different upstream chaperones in the folding distinct protein classes. EMBO J. 22:5230-5240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Simonds, W. F., J. E. Butrynski, N. Gautam, C. G. Unson, and A. M. Spiegel. 1991. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J. Biol. Chem. 266:5363-5366. [PubMed] [Google Scholar]
- 61.Skakun, V. V., M. A. Hink, A. V. Digris, E. R., E. G. Novikov, V. V. Apanasovic, and A. J. W. G. Visser. 2005. Global analysis of fluorescence fluctuation data. Eur. Biophys. J. 34:323-334. [DOI] [PubMed] [Google Scholar]
- 62.Smith, T. F., C. Gaitatzes, K. Saxena, and E. J. Neer. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181-185. [DOI] [PubMed] [Google Scholar]
- 63.Sondek, J., A. Bohm, D. G. Lambright, H. E. Hamm, and P. B. Sigler. 1996. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature 379:369-374. [DOI] [PubMed] [Google Scholar]
- 64.Thibault, C., M. W. Sganga, and M. F. Miles. 1997. Interaction of phosducin-like protein with G protein betagamma subunits. J. Biol. Chem. 272:12253-12256. [DOI] [PubMed] [Google Scholar]
- 65.Ursic, D., and M. R. Culbertson. 1991. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol. Cell. Biol. 11:2629-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valpuesta, J. M., J. Martin-Benito, P. Gomez-Puertas, J. L. Carrascosa, and K. R. Willison. 2002. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 529:11-16. [DOI] [PubMed] [Google Scholar]
- 67.Wang, Q., B. K. Mullah, and J. D. Robishaw. 1999. Ribozyme approach identifies a functional association between the G protein beta1gamma7 subunits in the beta-adrenergic receptor signaling pathway. J. Biol. Chem. 274:17365-17371. [DOI] [PubMed] [Google Scholar]
- 68.Wu, L., R. Valkema, P. J. Van Haastert, and P. N. Devreotes. 1995. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J. Cell Biol. 129:1667-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu, J., D. Wu, V. Z. Slepak, and M. I. Simon. 1995. The N terminus of phosducin is involved in binding of beta gamma subunits of G protein. Proc. Natl. Acad. Sci. USA 92:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, F. L., and P. J. Casey. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241-269. [DOI] [PubMed] [Google Scholar]