Abstract
IQGAP1 modulates many cellular functions such as cell-cell adhesion, transcription, cytoskeletal architecture, and selected signaling pathways. We previously documented that IQGAP1 binds extracellular signal-regulated kinase (ERK) 2 and regulates growth factor-stimulated ERK activity. Here we show that MEK, the molecule immediately upstream of ERK in the Ras/mitogen-activated protein (MAP) kinase signaling cascade, also interacts directly with IQGAP1. Both MEK1 and MEK2 bound IQGAP1 in vitro and coimmunoprecipitated with IQGAP1. The addition of ERK2 enhanced by fourfold the in vitro interaction of MEK2 with IQGAP1 without altering binding of MEK1. Similarly, ERK1 promoted MEK binding to IQGAP1, but either MEK protein altered the association between IQGAP1 and ERK. Epidermal growth factor (EGF) differentially regulated binding, enhancing MEK1 interaction while reducing MEK2 binding to IQGAP1. In addition, both knockdown and overexpression of IQGAP1 reduced EGF-stimulated activation of MEK and ERK. Analyses with selective IQGAP1 mutant constructs indicated that MEK binding is crucial for IQGAP1 to modulate EGF activation of ERK. Our data strongly suggest that IQGAP1 functions as a molecular scaffold in the Ras/MAP kinase pathway.
The mitogen-activated protein (MAP) kinase cascades, which are activated in response to a variety of extracellular stimuli, mediate signal transduction from the cell surface to the nucleus (9, 36). The most widely studied MAP kinase pathway is the extracellular signal-regulated kinase (ERK) cascade. ERK has an important role in the transduction of several proliferative and differentiating signals in normal cellular development and oncogenesis. The components of the ERK module form a linear cascade consisting of Raf (MAP kinase kinase kinase [MAP3K]), MEK (MAP or ERK kinase-MAP2K), and ERK (MAPK) kinases.
Diverse MAP kinase pathways respond to different external cues and exert distinct biological functions. However, there seems to be potential for extensive overlap with regard not only to the activating stimuli but also to downstream targets. The mechanism by which the specificity and fidelity of distinct biological responses are maintained is largely unknown. Recent reports indicate that specificity in MAP kinase signaling in Saccharomyces cerevisiae is attained predominantly by scaffolding proteins (38). Similarly, a number of scaffolding proteins that modulate signaling from Raf to MEK and ERK have been identified in mammalian cells (for reviews, see references 23 and 36). These include kinase suppressor of Ras (KSR) (37), connector enhancer of KSR (19), Sur8 (28), MEK partner 1 (MP1) (42), MAP kinase organizer 1 (48), Sef (45), and Raf kinase inhibitor protein (51).
IQGAPs are multidomain molecules that contain several protein-interacting motifs (for reviews, see references 4 and 5). The name is derived from the presence of an IQ domain (with four tandem IQ motifs) and a region with significant sequence similarity to the catalytic domain of Ras-GTPase-activating proteins (GAPs). Other motifs include a calponin homology domain (CHD), a coiled-coil region and a WW domain. The multiple conserved modules in IQGAP1 suggest that it participates in protein-protein interactions and may be a component of several signaling pathways. This hypothesis has been validated by both our group and others who documented that IQGAP1 binds activated Cdc42 and Rac1 (but not RhoA or Ras) (11, 16, 20), actin (10, 17, 32), calmodulin (17, 20, 32), E-cadherin (25, 29), β-catenin (3, 25), S100B (33), nectin (21), CLIP-170 (12), ERK2 (40), and adenomatous polyposis coli (49). The diversity of IQGAP1 targets has led to the hypothesis that IQGAP1 functions as a scaffolding protein that can assemble multiprotein complexes within various subcellular domains (4, 17). In support of this concept, published evidence reveals that IQGAP1 is capable of binding several proteins simultaneously. For example, complexes of IQGAP1 with actin and Cdc42 have been isolated in vitro (10). Moreover, complexes of IQGAP1 with Cdc42 and calmodulin (17), Rac1 and calmodulin (41), or Rac1/Cdc42 and CLIP-170 (12) have been reported. These findings strongly suggest that IQGAP1 functions as a scaffold within cells.
We previously demonstrated that IQGAP1 binds directly to ERK2 and regulates its activity (40). Moreover, altering intracellular IQGAP1 levels inhibited epidermal growth factor (EGF)-stimulated activation of ERK. These findings led to the question of whether IQGAP1 may modulate MEK, the molecule immediately upstream of ERK in the Ras-Raf-MEK-ERK pathway. Here, we present evidence that IQGAP1 and MEK associate both in vitro and in intact cells. Analogous to our observations with ERK, the ability of EGF to stimulate phosphorylation of MEK was abrogated in cells in which endogenous IQGAP1 was specifically knocked down by small interfering RNA (siRNA). Both overexpression and specific knockdown of endogenous IQGAP1 significantly impaired EGF-stimulated MEK and ERK activities. These data suggest that IQGAP1 participates as a scaffolding protein in MEK-ERK signaling.
MATERIALS AND METHODS
Materials.
Tissue culture reagents were obtained from Life Technologies, Inc. Fetal bovine serum was purchased from Biowhittaker. Anti-phospho-ERK antibody was obtained from Promega. Anti-ERK, anti-MEK, and anti-phospho-MEK antibodies were obtained from Cell Signaling Technology. Antihemagglutinin (anti-HA) antibody was purchased from Santa Cruz Biotechnology. Anti-myc monoclonal antibody was manufactured by Maine Biotechnology. The anti-IQGAP1 polyclonal antibody has been previously characterized (17). The anti-IQGAP1 monoclonal antibody was a generous gift from Andre Bernards (MGH Center for Cancer Research, Charlestown, MA). Secondary antibodies for enhanced chemiluminescence (ECL) detection were from Amersham Pharmacia Biotech. All other reagents were of standard analytical grade.
Plasmid construction and expression.
Myc-tagged human IQGAP1 in pcDNA3 vector (17) and plasmids pMCL-MKK1 and pMCL-MKK2, for expression of HA-tagged MEK1 and MEK2, respectively, were used for expression in mammalian cells. For in vitro studies, the NpT7-5 His6-ERK1 (39), NpT7-5 His6-ERK2 (39), pRSET His6-MEK1 (30), and pRSET His6-MEK2 (30) constructs were expressed in Escherichia coli and purified by nickel affinity chromatography using Ni2+-nitrilotriacetic acid (NTA) agarose (QIAGEN). Glutathione S-transferase (GST) fusion proteins of IQGAP1 were also expressed in E. coli and isolated with glutathione-Sepharose as previously described (17). All proteins were >90% pure by Coomassie blue staining. All plasmids were purified using QIAGEN DNA purification kits (QIAGEN), according to the manufacturer's instructions.
Cell lines.
MCF-7 human breast epithelial cells which stably overexpress either pcDNA3 (termed MCF/V cells) or pcDNA3-myc-IQGAP1 (termed MCF/I cells) have been described previously (3, 44). MCF/I cells have threefold more IQGAP1 than MCF/V cells (44). Stable expression of siRNA for IQGAP1 was described earlier (31). The IQGAP1 protein level in these cells (termed MCF-siIQ8 cells) is reduced by 80%.
Cell culture and transfection.
MCF-7 cells and HEK-293H (Gibco-BRL) were maintained in Dulbecco's modified Eagle medium supplemented with 10% (vol/vol) fetal bovine serum. MCF-7 cells were transfected using FuGENE 6 (Roche Molecular Biochemicals) as previously described (29). HEK-293H cells were transfected using Lipofectamine 2000 (Gibco-BRL) according to the manufacturer's instructions. Nucleofection of MCF-7 cells with HA-tagged MEK1 and MEK2 was performed using Nucleofector solution V (Amaxa) and the program P-20 according to the manufacturer's instructions.
Transient knockdown of IQGAP1.
Transient knockdown of IQGAP1 by siRNA was carried out as previously described (31). Briefly, siRNA directed against IQGAP1, cloned into the mU6pro vector (mU6siRNA 5 or 8), and mU6pro vector alone were transfected into MCF-7 cells with FuGENE 6. After 24 h, cells were processed as described below for measurement of MAP kinase activity.
Measurement of MAP kinase activity.
Equal numbers of MCF-7 cells were incubated in Dulbecco's modified Eagle medium containing 0.5% serum at 37°C. Sixteen hours later, 100 ng/ml EGF or an equal volume of vehicle was added for 10 min at 25°C. The reactions were terminated by removing the medium, washing the cells twice with phosphate-buffered saline (PBS) at 4°C, and lysing in buffer A (20 mM Tris, pH 8.0, 137 mM NaCl, 10% glycerol, and 0.2% Triton X-100) containing phosphatase inhibitor cocktail II (Sigma). ERK activity was assayed by Western blotting cell lysates using an antibody specific for phosphorylated ERK to detect phospho (active) ERK (40). MEK is activated by phosphorylation on two serine residues. In order to monitor MEK activity, Western blots of lysates were probed with anti-phospho-MEK antibody that detects active (phosphorylated) MEK. In all cases, cell lysates were equalized for protein concentration prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Where indicated, blots were stripped by incubating in 62.5 mM Tris, pH 6.8, 2% (wt/vol) SDS, and 0.7% (vol/vol) β-mercaptoethanol for 30 min at 50°C and reprobed with anti-ERK or anti-MEK antibodies to measure total ERK and MEK, respectively. Blots were also probed with anti-myc and anti-IQGAP1 antibodies as specified.
Cross-linking of proteins.
MCF-7 cells, starved of serum, were incubated with EGF or vehicle for 10 min. Where indicated, 1 mM dithiobis succinimidyl propionate (DSP) (Pierce) in PBS was added, followed 10 min later with Tris (pH 7.4) to a final concentration of 50 mM. DSP reacts with primary amine groups and cross-links bound proteins. The cells were washed with PBS 15 min later and lysed in buffer A. Immunoprecipitation was performed as described below.
Immunoprecipitation.
MCF-7 cells were transiently transfected with HA-MEK1 or HA-MEK2. After 24 h, cells were starved of serum, followed by incubation with EGF where indicated (see below). Immunoprecipitation was performed essentially as previously described (29). Equal amounts of protein lysate were incubated for 3 h at 4°C with anti-IQGAP1 polyclonal antibody immobilized on protein A-Sepharose (Amersham) beads. Samples were washed five times with buffer A, and Western blotting was performed. Immunoprecipitation with nonimmune rabbit serum (NIRS) was performed in parallel. Blots were probed with anti-IQGAP1 monoclonal antibody and anti-MEK antibodies, and antigen-antibody complexes were identified with horseradish peroxidase-conjugated sheep anti-mouse and donkey anti-rabbit antibodies, respectively. ECL was used for detection.
TNT product production and binding analysis.
[35S]methionine-labeled transcription and translation (TNT) products were produced with the TNT Quick Coupled Transcription/Translation system (Promega) according to the manufacturer's instructions. The construction of IQGAP1 and IQGAP1-C (C-terminal region, amino acids 864 to 1657), IQGAP1-N1 (comprising amino acids 2 to 431), IQGAP1-N2 (amino acids 432 to 864), IQGAP1ΔWW (amino acids 643 to 744 deleted), IQGAP1ΔIQ (amino acids 699 to 905 deleted), and IQGAP1ΔCHD, (amino acids 35 to 365 deleted) was described previously (17, 40, 43). Briefly, 0.5 μg of the IQGAP1 plasmids was incubated with 40 μl of TNT Quick Master Mix (Promega) and 20 μCi of [35S]methionine (New England Nuclear) at 30°C for 1 h. Products were confirmed by SDS-PAGE and autoradiography.
For in vitro binding experiments with [35S]methionine-labeled products, the IQGAP1 constructs described in the previous paragraph were incubated with His6-MEK1 or His6-MEK2 in 500 μl of buffer A for 3 h at 4°C. Samples were washed six times in buffer A, and complexes were isolated with Ni2+-NTA agarose and resolved by SDS-PAGE. Bands were analyzed after autoradiography of the dried gel.
In vitro binding.
GST (1 μg) or GST-IQGAP1 (∼0.7 μg) bound to glutathione-Sepharose was incubated with equimolar quantities of His6-MEK1 or His6-MEK2 in 500 μl of buffer A for 3 h at 4°C. Where indicated, equivalent amounts of His6-ERK1 or His6-ERK2 was added. After centrifugation, samples were washed and resolved by SDS-PAGE, and the gel was cut in half; the top portion (containing IQGAP1) was stained with Coomassie blue, and the bottom half was transferred to polyvinylidene difluoride (PVDF) membrane; blots were probed with anti-MEK antibody. Blots were stripped thereafter by incubating in 62.5 mM Tris, pH 6.8, 2% (wt/vol) SDS, and 0.7% (vol/vol) β-mercaptoethanol for 30 min at 50°C and reprobed with anti-ERK antibody.
Miscellaneous.
Densitometry of ECL signals was analyzed with UN-SCAN-IT software (Silk Scientific Corporation). Statistical analysis was assessed by a Student's t test with InStat software (GraphPad Software, Inc.). Protein concentrations were determined with a DC Protein Assay kit (Bio-Rad).
RESULTS
IQGAP1 binds MEK1 and MEK2 in vitro.
Analysis with pure proteins was used to examine a possible interaction between IQGAP1 and MEK1 or MEK2. A GST fusion protein of full-length IQGAP1 expressed in E. coli was incubated with bacterially produced, recombinant purified His6-MEK1 or His6-MEK2, and complexes were isolated with glutathione-Sepharose. Analysis by Western blotting revealed that MEK1 and MEK2 each bound to IQGAP1 (Fig. 1). Binding was specific as neither MEK protein was detected in the samples incubated with GST alone. Coomassie staining of the top half of the gel revealed that approximately equivalent amounts of GST-IQGAP1 were present (Fig. 1, upper panel).
FIG. 1.
MEK1 and MEK2 bind to IQGAP1. GST alone or GST-IQGAP1 (IQGAP1) bound to glutathione-Sepharose was incubated with purified His6-MEK1 (A) or His6-MEK2 (B). Complexes were isolated and washed as described in Materials and Methods. A total of 100 ng of purified His-tagged protein (MEK1 in panel A or MEK2 in panel B) was loaded as input. After samples were resolved by SDS-PAGE, the gel was cut into two pieces; the top portion (containing IQGAP1) was stained with Coomassie blue, whereas the bottom half was transferred to PVDF membrane and probed with anti-MEK antibody. The positions of migration of MEK1 and MEK2 are depicted. The data are representative of six independent experiments.
IQGAP1 binds MEK in cells.
To ascertain whether endogenous IQGAP1 interacts with MEK in a normal cell milieu, immunoprecipitation was performed. MCF-7 cells were transiently transfected with HA-MEK1 or HA-MEK2 using Nucleofector (Amaxa) as described in Materials and Methods. Immunoprecipitating lysates with anti-IQGAP1 antibody revealed that endogenous IQGAP1 bound both MEK1 and MEK2 (Fig. 2A). In unstimulated cells, the amount of MEK2 that coimmunoprecipitated with IQGAP1 was slightly more than the amount of MEK1. No MEK1 or MEK2 was detected in samples immunoprecipitated with NIRS, confirming that IQGAP1 and MEK specifically interact in vivo.
FIG. 2.
MEK1, MEK2, and ERK1 coimmunoprecipitate with IQGAP1. (A) MCF-7 cells were transiently transfected with pMCL-MKK1 (MEK1) or pMCL-MKK2 (MEK2). Twenty-four hours later, cells were starved of serum overnight and then incubated with vehicle (−) or 100 ng/ml EGF (+) for 10 min. After lysis, equal amounts of protein were incubated with NIRS or immunoprecipitated (IP) with anti-IQGAP1 polyclonal antibody. Both immune complexes (IP) and unfractionated lysates (Lysate) were resolved by SDS-PAGE. After transfer to PVDF membranes, the blots were probed with monoclonal anti-IQGAP1 and anti-MEK antibodies. Data are representative of two independent experiments for MEK1 and four independent experiments for MEK2. (B) Untransfected MCF-7 cells were starved of serum for 16 h and then incubated with vehicle (−) or 100 ng/ml EGF (+) for 10 min. Cells were exposed to a cross-linker, DSP, before lysis. Equal amounts of protein lysate were immunoprecipitated (IP) as described for panel A. Western blots were probed with anti-IQGAP1 and anti-ERK1 antibodies. Data are representative of three independent experiments.
EGF activates the MAPK cascade, enhancing both MEK and ERK activity (18, 27). We previously observed that incubation of cells with EGF did not change the amount of ERK2 that coimmunoprecipitated with IQGAP1 (40). To determine whether activating MEK modulates its interaction with IQGAP1, MCF-7 cells were stimulated with EGF for 10 min prior to lysis and immunoprecipitation. EGF enhanced the binding of MEK1 to IQGAP1 by sixfold (Fig. 2A). In contrast, under identical assay conditions, EGF reduced by 3.5-fold the amount of MEK2 that coimmunoprecipitated with IQGAP1. Our results reveal that IQGAP1 and MEK associate in human breast epithelial cells and that EGF differentially modulates the interaction of MEK1 and MEK2 with IQGAP1.
ERK1 coimmunoprecipitated with IQGAP1.
Immunoprecipitation was performed to determine whether endogenous ERK1 binds endogenous IQGAP1 in cells. Immunoprecipitation with anti-IQGAP1 antibody revealed that ERK1 bound to endogenous IQGAP1 (Fig. 2B). Binding was specific as no ERK1 was detected in samples immunoprecipitated with nonimmune rabbit serum. Incubation with EGF did not significantly change the amount of ERK1 that bound to IQGAP1 (Fig. 2B). These results indicate that, analogous to ERK2 (40), ERK1 associates with IQGAP1 in human breast epithelial cells and that this interaction is not altered by EGF.
IQGAP1 modulates EGF-stimulated activation of MEK.
We have previously documented that IQGAP1 binds ERK and regulates its activity (40). Because IQGAP1 functions as a scaffolding protein in several signaling pathways (4, 5, 17), we hypothesized that IQGAP1 might serve as a scaffold in the MAP kinase signaling cascade. If this hypothesis is correct, one would anticipate that IQGAP1 would modulate activation of MEK, the molecule immediately upstream of ERK in the ERK/MAP kinase cascade. This concept was evaluated initially by examining EGF-stimulated phosphorylation of MEK in cells expressing different levels of IQGAP1. MCF-7 cells that stably overexpress IQGAP1 (MCF/I cells) or empty vector (MCF/V cells) (3, 44) were used. MCF/I cells express threefold more IQGAP1 than MCF/V cells (3) (Fig. 3A). To stably downregulate IQGAP1 in MCF-7 cells, we used a retroviral vector to integrate a specific siRNA for human IQGAP1 into the genome. The IQGAP1 protein level in these cells (MCF-siIQ8 cells) was reduced by 80% (31) (Fig. 3A). The relative amounts of (active) phospho-MEK were determined by probing blots with an antibody that specifically recognizes MEK phosphorylated at Ser217/219. EGF stimulated MEK phosphorylation by 3.47-fold ± 0.6-fold (mean ± standard error [SE]; n = 3) in MCF/V cells (Fig. 3). Modulation of intracellular IQGAP1 levels impaired the ability of EGF to induce MEK phosphorylation. Following EGF treatment, levels of phospho-MEK in MCF/I cells were significantly lower (P < 0.001) than those in MCF/V cells (Fig. 3). The ability of EGF to induce MEK phosphorylation was also markedly attenuated by knockdown of IQGAP1 in MCF-siIQ8 cells. These results are analogous to our prior observations with ERK, where both overexpression and knockdown of IQGAP1 significantly reduced EGF stimulation of ERK activity (40).
FIG. 3.
IQGAP1 modulates EGF-stimulated activation of MEK. (A) MCF/V, MCF/I, and MCF-siIQ8 (siIQ8) cells were starved of serum overnight and treated with vehicle or 100 ng/ml EGF for 10 min. Equal amounts of protein from the cell lysates were resolved by SDS-PAGE, and Western blots were probed with anti-IQGAP1 and anti-phospho-MEK antibodies. The membranes were stripped and reprobed with anti-MEK antibody. (B) The amount of phospho-MEK was quantified by densitometry and corrected for the amount of MEK in the corresponding lysate. Data, expressed relative to the amount of phospho-MEK in vehicle-treated MCF/V cells, represent the means ± SE (n = 3). *, significantly different from vehicle-treated MCF/V cells (P < 0.001).
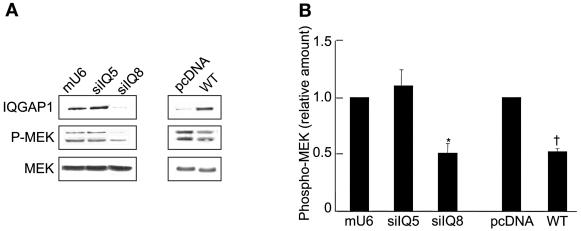
The effect of IQGAP1 on EGF-stimulated MEK activity was confirmed in transiently transfected cells. Transient transfection of siRNA 8 in MCF-7 cells reduced IQGAP1 protein expression by 70 to 80% (Fig. 4A). MEK phosphorylation in these cells was 50% lower than in vector-transfected cells. By contrast, siRNA 5—which is directed against a different region of IQGAP1 and does not reduce IQGAP1 protein expression (Fig. 4A)—had no effect on MEK phosphorylation (Fig. 4). Analogous to the results with MCF/I cells, transient overexpression of IQGAP1 in MCF-7 cells significantly reduced activation of MEK by EGF (Fig. 4).
FIG. 4.
Altering intracellular IQGAP1 concentrations reduces EGF-stimulated MEK activity. (A) MCF-7 cells were transiently transfected with vectors mU6pro (mU6) or pcDNA3 (pcDNA), IQGAP1 (WT), or siRNAs for IQGAP1 (siIQ5 and siIQ8). Cells were processed as described in the legend of Fig. 3A. (B) The amount of phospho-MEK was quantified by densitometry and corrected for the amount of MEK in the corresponding lysate. Data, expressed relative to the amount of phospho-MEK in cells transfected with the appropriate vector represent the means ± SE (n = 3). *, significantly different from mU6-transfected cells (P < 0.001); †, significantly different from pcDNA-transfected cells (P < 0.01).
Identification of the MEK1 and MEK2 binding domain on IQGAP1.
The MEK binding domain on IQGAP1 was investigated using deletion mutants and fragments of IQGAP1. Selected constructs of IQGAP1 (Fig. 5) were labeled with [35S]methionine in a reticulocyte lysate and incubated with His6-MEK1 or His6-MEK2. His-tagged MEK proteins were isolated by Ni2+ affinity chromatography, and the fragments of IQGAP1 that bound were resolved by SDS-PAGE and identified by autoradiography. [35S]methionine-labeled full-length IQGAP1 bound to His6-MEK1 and to His6-MEK2 (Fig. 6A and B). The lower-molecular-weight bands are degradation fragments of IQGAP1. Specificity of binding was verified by the absence of IQGAP1 from samples with Ni2+ beads alone (Fig. 6A and B). Examination of the two halves of IQGAP1 revealed that only the N-terminal half (amino acid residues 1 to 863) bound to MEK1 and MEK2; no interaction between the C-terminal half of IQGAP1 and MEK was observed (Fig. 6A and B). Inspection of the input (Fig. 6C) indicates that approximately equal amounts of the N- and C-terminal halves were incubated with MEK. Similarly, the amounts of His6-MEK1 and His6-MEK2 in each sample were essentially identical (data not shown).
FIG. 5.
Schematic representation of IQGAP1 constructs depicting full-length IQGAP1, truncated IQGAP1 fragments, and deletion mutants. The identified protein interaction motifs and the specific amino acid residues absent from each mutant are indicated. WW, polyproline binding domain; IQ, four tandem calmodulin-binding motifs; IQGAP1-N, N-terminal half of IQGAP1; IQGAP1-C, C-terminal half of IQGAP1; IQGAP1-N1, comprising amino acids 2 to 431, and IQGAP1-N2, harboring amino acids 432 to 863.
FIG. 6.
Identification of amino acid residues of IQGAP1 necessary for binding MEK. (A and B) [35S]methionine-labeled pcDNA vector (V), wild-type IQGAP1 (WT), IQGAP1-C (C), IQGAP1-N (N), IQGAP1-N1 (N1), IQGAP2-N2 (N2), IQGAP1ΔCHD (ΔCHD), IQGAP1ΔIQ (ΔIQ), and IQGAP1ΔWW (ΔWW), produced with the TNT Quick Coupled Transcription/Translation system, were incubated with Ni2+-NTA beads (last lane in panels A and B) or equal amounts of His6-MEK1 (panel A) or His6-MEK2 (panel B). Complexes were isolated with Ni2+-NTA agarose beads and resolved by SDS-PAGE. Gels were dried and processed by autoradiography. Data are representative of three independent experiments. (C) An aliquot of [35S]methionine-labeled TNT product (equivalent to 10% of the amount that was subjected to pull-down) for the His6-MEK pull-down assays was resolved by SDS-PAGE, dried, and processed by autoradiography (Input). Data are representative of three independent experiments.
In order to narrow the binding site, the N-terminal portion of IQGAP1 was divided into two equal halves, termed N1 and N2 (Fig. 5). Both His6-MEK1 and His6-MEK2 bound N2 exclusively (Fig. 6A and B), revealing that the binding domain is between residues 432 and 863 of IQGAP1. In addition, the identified protein interaction domains in the N-terminal half of IQGAP1 were deleted (Fig. 5). Analysis of binding showed that removal of neither the CHD (amino acids 37 to 265 deleted) nor the WW region (amino acids 643 to 744 deleted) from IQGAP1 attenuated its binding to MEK1 or MEK2 (Fig. 6A and B). In contrast, absence of the region that includes the IQ domain (deletion of amino acids 699 to 905) completely disrupted the interaction of IQGAP1 with MEK. These data demonstrate that the IQ region of IQGAP1 is necessary for MEK binding. The input for each IQGAP1 construct is depicted in Fig. 6C.
Effects of IQGAP1ΔIQ and IQGAP1ΔWW on MEK and ERK activities.
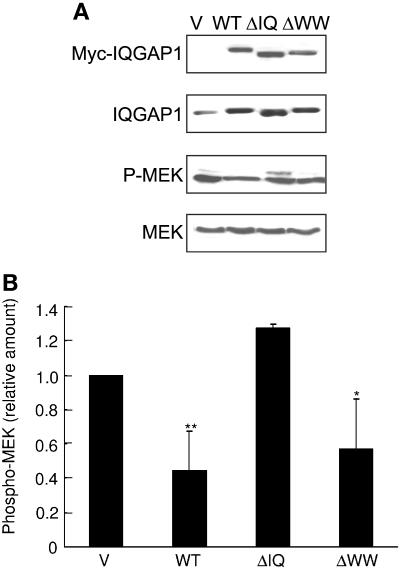
We previously documented that transient overexpression of full-length IQGAP1 in MCF-7 and HEK 293H cells significantly reduced activation of ERK1 and ERK2 by EGF (40). Therefore, we determined the effect of wild-type and mutant IQGAP1 constructs on MEK activity. Transient transfection of HEK-293H cells with wild-type IQGAP1 reduced EGF-stimulated MEK activity by 55% (Fig. 7A and B). Similarly, transfection of IQGAP1ΔWW, which binds MEK with an affinity similar to that of wild-type IQGAP1 (Fig. 6A and B), significantly reduced EGF stimulation of MEK. In contrast, transfection of an equivalent amount of IQGAP1ΔIQ, which lacks the MEK binding domain, did not impair the ability of EGF to activate MEK (Fig. 7A and B). These data reveal that interaction of MEK with IQGAP1 is necessary for IQGAP1 to modulate EGF-stimulated MEK activity.
FIG. 7.
Binding to MEK is necessary for IQGAP1 to alter EGF-stimulated MEK-ERK signaling. (A) HEK-293H cells were transiently transfected with pcDNA3 vector (V), IQGAP1 (WT), IQGAP1ΔIQ (ΔIQ), or IQGAP1ΔWW (ΔWW). Cells were starved of serum for 16 h, followed by incubation with 100 ng/ml EGF for 10 min. Equal amounts of protein from the cell lysates were resolved by SDS-PAGE, transferred to PVDF membrane, and probed with anti-myc antibody (Myc-IQGAP1) (the transfected IQGAP1 constructs are myc-tagged) and an antibody specific for phosphorylated MEK isoforms (P-MEK). The membrane was stripped and reprobed with anti-IQGAP1 (which recognizes both endogenous and transfected IQGAP1) and anti-MEK antibodies. Data are representative of four independent experiments. (B) The amount of phosphorylated MEK isoforms (phospho-MEK) was quantified by densitometry and corrected for the amount of total MEK in the corresponding lysate. Data, expressed relative to the amount of phospho-MEK in cells transfected with pcDNA3 vector, represent the means ± SE (n = 4). *, significantly different from vector-transfected cells (P < 0.01); **, significantly different from vector-transfected cells (P < 0.001).
In an earlier report, we showed that IQGAP1ΔWW—which does not bind ERK—had no effect on EGF-stimulated ERK activation (40). (Note that when IQGAP1ΔWW is expressed at very high levels [exceeding approximately 10 times endogenous IQGAP1], ERK activity is progressively reduced [data not shown]. This effect is probably due to sequestration of MEK by IQGAP1ΔWW, preventing the normal coupling of MEK to ERK that is mediated by endogenous IQGAP1.) To extend our prior observations, we examined whether ERK activity was affected by deleting the MEK binding domain from IQGAP1. Although IQGAP1ΔIQ binds normally to ERK (40), transfection of IQGAP1ΔIQ to the same level as wild-type IQGAP1 did not change the level of phosphorylated ERK upon EGF stimulation (Fig. 8A and B). These data indicate that binding to IQGAP1 regulates the ability of MEK to activate ERK.
FIG. 8.
Deletion of residues 699 to 905 of IQGAP1 has no effect on IQGAP1-mediated attenuation of EGF-stimulated ERK activity. (A) HEK-293H cells were transiently transfected and processed as described in Fig. 7A. Western blots were probed with anti-myc antibody (Myc-IQGAP1) and an antibody specific for phosphorylated ERK isoforms (P-ERK). The membrane was stripped and reprobed with anti-IQGAP1 and anti-ERK antibodies. Data are representative of three independent experiments. (B) The amount of phosphorylated ERK isoforms (phospho-ERK) was quantified by densitometry and corrected for the amount of total ERK in the corresponding lysate. Data, expressed relative to the amount of phospho-ERK in cells transfected with pcDNA3 vector, represent the means ± SE (n = 4 for V, WT, and ΔWW; n = 3 for ΔIQ). *, significantly different from vector-transfected cells (P < 0.05).
Effect of ERK on the binding of MEK to IQGAP1.
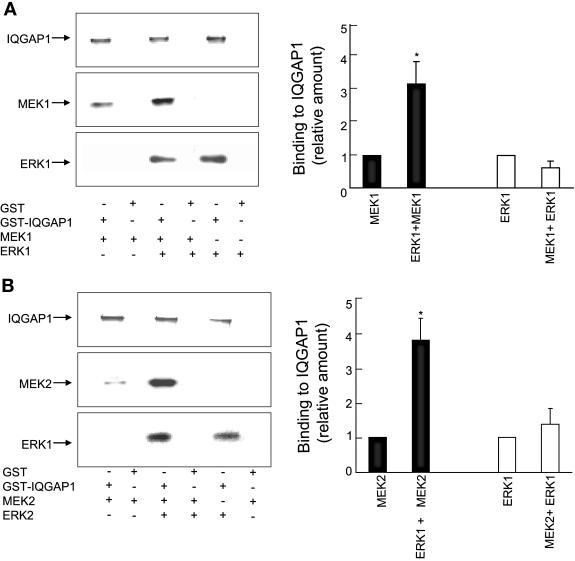
In vitro analysis with pure proteins was conducted to examine whether ERK1 or ERK2 has any effect on the binding of IQGAP1 to MEK1 or MEK2 and vice versa. A GST fusion protein of full-length IQGAP1 was incubated with purified His6-MEK1 or His6-ERK1 separately or with His6-MEK1 and His6-ERK1 together. Complexes were isolated with glutathione-Sepharose, and the relative amounts of binding were compared by Western blotting. ERK1 increased the binding of MEK1 to IQGAP1 by threefold (Fig. 9A). In contrast, the presence of MEK1 did not significantly alter the interaction of ERK1 with IQGAP1. Staining the upper portion of the gel with Coomassie blue indicated that equal amounts of GST-IQGAP1 were present (Fig. 9). Specificity of the interactions was revealed by the absence of bands from samples incubated with GST alone. Both ERK1 (Fig. 9B) and ERK2 (Fig. 10A) enhanced the interaction of MEK2 with IQGAP1 by fourfold. However, ERK2 had no effect on the binding of MEK1 to IQGAP1 (Fig. 10B). Note that neither MEK1 nor MEK2 modulated the interaction of ERK1 or ERK2 with IQGAP1 (Fig. 9 and 10). These data reveal that ERK1 promoted the binding of MEK1 and MEK2 to IQGAP1, while ERK2 specifically enhanced the interaction of MEK2 with IQGAP1.
FIG. 9.
ERK1 facilitates MEK binding to IQGAP1. (A) GST-IQGAP1 or GST alone bound to glutathione-Sepharose was incubated with purified His6-MEK1, His6-ERK1, or both MEK1 and ERK1. Complexes were isolated and washed as described in Materials and Methods. After samples were resolved by SDS-PAGE, the gel was cut into two pieces; the top portion (containing IQGAP1) was stained with Coomassie blue, whereas the bottom half was transferred to PVDF membrane and probed with anti-MEK and anti-ERK antibodies. The positions of migration of MEK1 and ERK1 are depicted. The data are representative of three independent experiments. The amounts of MEK1 and ERK1 bound to IQGAP1 were quantified by densitometry. Data, expressed relative to the amount of MEK1 in samples without ERK1 (filled bars) and ERK1 without MEK1 (open bars), represent the means ± SE (n = 6). *, significantly different from MEK1 alone (P < 0.05). (B) In vitro binding was performed as described in panel A using MEK2 and ERK1. *, significantly different from MEK2 alone (P < 0.001).
FIG. 10.
ERK2 facilitates MEK2 binding to IQGAP1. In vitro binding was performed as described in the legend of Fig. 9 using MEK2 and ERK2 (panel A) and MEK1 and ERK2 (panel B). Data, expressed relative to the amount of MEK in samples without ERK2 (filled bars) and ERK2 without MEK (open bars), represent the means ± SE (n = 3). *, significantly different from MEK2 alone (P < 0.0001).
IQGAP1 modulates EGF-stimulated activation of ERK and MEK in a concentration-dependent manner.
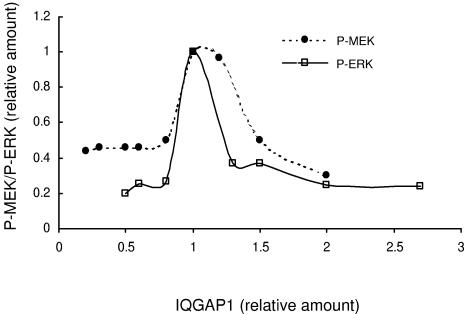
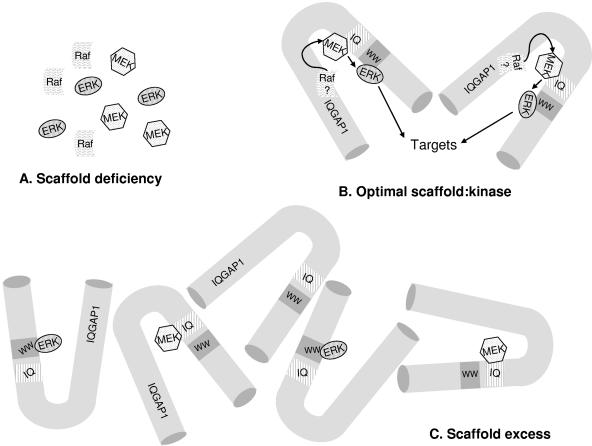
Because IQGAP1 participates, at least in part, in MEK and ERK signaling, we assessed whether IQGAP1 modulated MEK and ERK activities in a dose-dependent manner. To obtain different expression levels, IQGAP1 was transiently overexpressed or knocked down (by siRNA). Serum-starved cells were stimulated with EGF, and lysates were analyzed by Western blotting. We observed that EGF was able to activate ERK only within a very narrow range of IQGAP1 concentrations close to endogenous levels (Fig. 11). Interestingly, increasing and decreasing intracellular IQGAP1 levels reduced activation of ERK by virtually identical amounts. Both overexpression and knockdown of endogenous IQGAP1 by ≥30% impaired by >65% the ability of EGF to stimulate ERK activity (Fig. 11). Similarly, maximal stimulation of MEK phosphorylation by EGF occurred only when intracellular IQGAP1 concentration was close to endogenous levels (Fig. 11). Increasing or reducing IQGAP1 markedly attenuated the ability of EGF to activate MEK. These data lead us to speculate that, analogous to other scaffolding proteins of the MAP kinase cascade (36, 45, 48), a functional and productive signaling unit can be assembled only when the relative stoichiometries of the kinases and IQGAP1 are optimal.
FIG. 11.
IQGAP1 modulates MEK and ERK activation in a concentration-dependent manner. Data from EGF-treated MCF-7 cells were analyzed to compare the extent of phosphorylation of MEK and ERK in cells expressing different concentrations of IQGAP1. The y axis represents relative phosphorylation of MEK (•) (P-MEK) and ERK (□) (P-ERK), corrected for total MEK and ERK, respectively. The x axis represents the relative amounts of IQGAP1. Vector-transfected cells are set at 1.0.
DISCUSSION
IQGAP1 binds a diverse array of proteins including calmodulin, Cdc42, Rac1, actin, β-catenin, E-cadherin, and CLIP-170 (4). Interaction with IQGAP1 regulates the function of its target proteins (reviewed in references 4 and 5). For example, IQGAP1 inhibits cell-cell adhesion mediated by the E-cadherin-β-catenin complex (25, 29), increases β-catenin-mediated transcriptional activation (3), and captures growing microtubules via CLIP-170 at the leading edge of migrating fibroblasts (12). Prior work from our laboratory documented that IQGAP1 binds ERK2 and modulates its activity (40). In this study, we investigated the interaction with IQGAP1 of MEK1 and MEK2, the kinases immediately upstream of ERK, and explored the functional sequelae in the ERK/MAP kinase cascade.
We observed direct binding between IQGAP1 and MEK1, as well as between IQGAP1 and MEK2. Association in cells was revealed by coimmunoprecipitation of MEK1 and MEK2 with endogenous IQGAP1 from epithelial cell lysates. Analogous to the effects on its other targets (4), IQGAP1 modulated MEK function. Changing intracellular concentrations of wild-type IQGAP1 impaired the ability of EGF to activate MEK, but a mutant IQGAP1 construct that is unable to bind MEK had no effect. These findings confirm that the binding of IQGAP1 to MEK regulates its activation by EGF.
MAP kinases are evolutionarily conserved enzymes that connect cell-surface receptors to critical regulatory targets within cells (8). Mammals express at least four distinctly regulated groups of MAP kinases. Specificity in MAP kinase activation is secured by several mechanisms. The kinase components of a particular MAP kinase module may interact stepwise through a series of sequential binary interactions creating a signaling cascade. These kinases may be structurally organized into signaling complexes for proper triggering of the cascade. Alternatively, a scaffold protein may intervene and tether the different components of the cascade together to create a functional module. Scaffolding proteins were originally identified in yeast (14), but a large body of evidence reveals that scaffolds also contribute to the regulation of MAP kinase pathways in mammalian cells (8). For example, KSR, MP1, β-arrestins, and MEK kinase 1 are important scaffolding molecules that are involved in regulating ERK activity (36). KSR1 and MP1 contribute to the normal regulation of ERK activity, implying that scaffolding proteins are used in a nonredundant manner. In addition, different scaffolds may permit the MAP kinase signaling complexes to target specific substrates (36). Thus, selected scaffolding proteins mediate diverse MEK/ERK functions. Based on the experimental evidence suggesting that IQGAP1 functions as a scaffolding protein that integrates diverse signaling pathways (4, 5), we hypothesized that IQGAP1 may be an unrecognized scaffold for the Ras/MAP kinase signaling pathway. The evidence presented here supports this hypothesis.
IQGAP1 binds directly to at least two components of the MAP kinase pathway, namely MEK and its target ERK. These interactions modulate the ability of EGF to activate both MEK and ERK. Although necessary, by themselves these factors are not sufficient to establish that IQGAP1 functions as a scaffold in MEK/ERK signaling. Validation of the scaffold concept is provided by the data obtained with selected mutant IQGAP1 constructs. Analysis was performed with IQGAP1ΔIQ, which binds ERK but not MEK, and IQGAP1ΔWW, which binds MEK but not ERK. Transfection of IQGAP1ΔIQ had no effect on activation of MEK or ERK by EGF. In contrast, overexpression of IQGAP1ΔWW attenuated EGF-stimulated MEK activity to the same extent as that produced by wild-type IQGAP1. However, IQGAP1ΔWW did not alter the ability of EGF to activate ERK (Fig. 8B) (40). Thus, binding of both MEK and ERK to IQGAP1 is required for IQGAP1 to modulate EGF-stimulated ERK activity. These data support the postulate that MEK phosphorylates and thereby activates ERK in a processive manner whereby both the molecules are bound simultaneously to the adaptor molecule (26).
Increasing and decreasing intracellular IQGAP1 concentrations produced the same outcome, namely marked impairment of stimulation of MEK and ERK by EGF. Maximal activation of MEK and ERK by EGF was observed only when cellular IQGAP1 concentrations were close to normal levels. Deviation by ≥50% from these values reduced active MEK and ERK by >50%. Although these findings initially seem counterintuitive, they actually support the notion that IQGAP1 is a scaffold that links MEK to ERK. This concept is illustrated schematically in Fig. 12. IQGAP1 acting as a scaffold can assemble its client proteins (MEK and ERK) only when all components are present in an appropriate stoichiometric ratio. The concentrations of IQGAP1, MEK2, and ERK2 in MCF-7 cells were determined to be 0.8 μM, 6.9 μM, and 15.5 μM, respectively. These values correspond to relative stoichiometries of IQGAP1:MEK2:ERK2 of 1:9:20. Thus, relatively small changes in the amount of IQGAP1 will result in large changes in the relative stoichiometries of IQGAP1 to MEK and ERK. When IQGAP1 is in excess, nonfunctional binary complexes of IQGAP1 with only one of the components of the kinase cascade are formed (Fig. 12). This model is consistent with published data which reveal that overexpression of the scaffold KSR inhibited ERK-dependent biological effects (6) and high concentrations of MP1 decreased MEK1-ERK1 binding (42). Our data derived from overexpression of IQGAP1 are analogous to these findings. Computer modeling of the MAP kinase cascade further supports these observations and suggests that increasing or reducing the scaffold concentration decreases maximal activation of the MAP kinase cascade (26). In support of the latter concept, activation of ERKs by EGF was reduced by 50% in KSR-deficient mouse fibroblasts (37). These results are consistent with our data on knockdown of IQGAP1. In vivo scaffold levels can be regulated by modulation of gene expression or protein stability or via sequestration in specific subcellular compartments (22). Although we manipulated intracellular IQGAP1 concentrations in this study, IQGAP1 is not uniformly distributed in the cell. Increased levels of IQGAP1 are found in discrete subcellular locations (29, 34). Moreover, several stimuli, including manipulation of intracellular Ca2+ concentrations (32, 33), alteration of calmodulin function (29), and activation of E-cadherin (29), induce the movement of IQGAP1 between different subcellular regions. It is plausible, therefore, that changes in IQGAP1 concentrations in microdomains of the cell can influence MAP kinase in distinct signaling compartments.
FIG. 12.
Model of IQGAP1 functioning as a scaffold in the MAP kinase pathway. (A) When the concentration of the scaffolding protein, IQGAP1, is too low, functional IQGAP1 complexes containing MEK and ERK are unable to form. (B) At the optimal scaffold concentration, the kinases are in an appropriate stoichiometric ratio with IQGAP1. Functional MAP kinase signaling complexes are formed. (C) When the concentration of IQGAP1 is excessive, many different IQGAP1 molecules harboring only one of the components of the cascade form complexes, which cannot function optimally. The binding site for ERK2 on IQGAP1 is the region containing the polyproline binding domain (WW) and that of MEK is the region spanning the calmodulin-binding motif (IQ). Raf binding to IQGAP1 has not been documented.
The biological functions of ERK1 and ERK2 are usually considered to be equivalent. Similarly, potential functional differences between MEK1 and MEK2 are rarely explored. A possible explanation for the lack of discrimination in the past between the role of MEK1 versus MEK2 and ERK1 versus ERK2 is the inability of pharmacologic inhibitors to distinguish between these kinases. This limitation is exemplified by the recent demonstration using specific siRNAs that MEK2 and ERK2, but not MEK1 and ERK1, mediate programmed cell death (7). Moreover, older evidence also suggests that MEK1 and MEK2 may not have fully overlapping functions. For example, only MEK1 is activated by serum in some cell lines (50). MEK1 knockout leads to embryonic lethality from defects in placental vascularization (13), while mouse growth and development are independent of MEK2 (1). A very recent report disclosed that the two MEK subtypes have different effects on cell cycle progression (46). Analogous observations have been made for ERK. In rat fibroblasts v-Raf selectively induced ERK2 activity (24), and ERK1 and ERK2 are regulated by distinct machinery during differentiation of HL-60 cells by 12-O-tetradecanoyl-phorbol 13-acetate (35).
Notwithstanding this evidence, very little is known about the molecular mechanism underlying the selectivity. It seems reasonable to postulate that, analogous to their ability to separate MEK/ERK signaling from the p38 MAP kinase cascade (36), distinct scaffolding proteins might mediate signaling specificity. This notion has been described for MP1, which binds specifically to MEK1 and ERK1 and facilitates their activation (42). On the basis of data presented here, we propose that IQGAP1 may serve such a function. In quiescent MCF-7 cells, IQGAP1 bound slightly more MEK2 than MEK1, and differences were markedly amplified on EGF stimulation. The binding of MEK1 to IQGAP1 increased, while that of MEK2 was reduced dramatically by EGF. These data reveal that EGF differentially regulates the binding of MEK1 and MEK2 to IQGAP1. By this mechanism, EGF could promote signaling from MEK1 to ERK while simultaneously attenuating MEK2/ERK signaling. In this context, it is noteworthy that EGF did not alter the binding of either ERK1 (Fig. 2B) or ERK2 (40) to IQGAP1.
The hypothesis that IQGAP1 functions as a scaffold that enables differential regulation of MEK1 and MEK2 is further bolstered by our in vitro binding studies. Analysis with purified proteins revealed that ERK2 specifically enhanced binding of MEK2 to IQGAP1, without altering the IQGAP1-MEK1 interaction. In contrast, ERK1 increased the interaction of MEK1 and MEK2 with IQGAP1. Because neither MEK protein modulated ERK binding to IQGAP1, the increased binding to IQGAP1 is probably not due to ERK-MEK interactions. The most likely mechanism is that ERK1 and ERK2 change the tertiary conformation of IQGAP1, selectively increasing the accessibility of MEK to its binding site. An alteration in the conformation of IQGAP1 on binding calmodulin has been postulated to explain how calmodulin reduces binding of Cdc42 and E-cadherin to IQGAP1 (17, 29). Our in vitro observation raises the possibility of a positive feedback loop in cells where association of ERK1 with IQGAP1 enhances its proximity to, and therefore interaction with, MEK1, leading to increased phosphorylation and activation of ERK1. EGF also increases MEK1 binding to IQGAP1, further augmenting MEK1/ERK1 signaling. For MEK2, the pathway would be different. EGF reduced the binding of MEK2 to IQGAP1, while both ERK1 and ERK2 increased the IQGAP1-MEK2 interaction.
The results of these studies raise an intriguing question as to the mechanism by which IQGAP1 mediates the coupling between the EGF receptor and its downstream signaling partners MEK and ERK. One possible model is that IQGAP1 binds directly to components of the MAP kinase cascade upstream of MEK, such as Raf or Ras or even the EGF receptor itself. In this context, the observation that IQGAP1 is recruited to a complex of activated Grb2-EGF receptors (2) is particularly relevant. Prior reports failed to identify an interaction between H-Ras and IQGAP1 (16, 34). (A recent publication detected IQGAP1 as an interaction partner of M-Ras [47], but M-Ras is not believed to be an important participant in EGF signaling.) We are not aware of studies that have examined a possible association between IQGAP1 and Raf. Another possibility, which may complement the first model, concerns the spatiotemporal regulation of the MAP kinase cascade. It is becoming increasingly apparent that spatiotemporal factors are important in Ras/MAP kinase signaling (15, 36, 45). Ras and MAP kinases can be targeted to different compartments of the cell. It is conceivable that IQGAP1 may be recruited to a particular subcellular domain by the EGF receptor. Consistent with this hypothesis is the documentation that IQGAP1 can move between different subcellular regions. For example, activation of E-cadherin (25, 29) and antagonism of calmodulin (29) induce IQGAP1 accumulation at the plasma membrane, while increasing intracellular Ca2+ concentrations removed IQGAP1 from the cell cortex (32). Further work is necessary to separate out the intricate molecular mechanisms by which IQGAP1 modulates the MAP kinase cascade.
In the current study, we gain further insight into the participation of IQGAP1 in the MAP kinase signaling cascade. A novel association between IQGAP1 and MEK that modulated MEK activation by EGF was identified. Evidence obtained using diverse experimental approaches strongly supports the concept that IQGAP1 is a scaffold molecule involved in some aspects of MEK-ERK signaling. Finally, a combination of direct in vitro binding and coimmunoprecipitation analyses imply that IQGAP1 may enable cells to differentially regulate individual isoforms of MEK/ERK, suggesting that it may contribute to differential signaling in the MAP kinase cascade.
Acknowledgments
We are grateful to Natalie Ahn (University of Colorado) for generously donating the HA-tagged and His6-tagged MEK1 and MEK2 constructs, to Melanie Cobb (University of Texas) for the gift of His6-tagged ERK1 and ERK2, and to David Turner (University of Michigan) for the mU6pro vector. We thank Chris French for the use of Nucleofector (Amaxa) and Angela Lam for technical assistance. We appreciate Rob Krikorian's expert help in the preparation of the manuscript.
This study was supported in part by grants from the National Institutes of Health (to D.B.S.) and a United States Army Fellowship grant DAMD17-02-1-0304 (to M.R.).
REFERENCES
- 1.Belanger, L. F., S. Roy, M. Tremblay, B. Brott, A. M. Steff, W. Mourad, P. Hugo, R. Erikson, and J. Charron. 2003. Mek2 is dispensable for mouse growth and development. Mol. Cell. Biol. 23:4778-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blagoev, B., I. Kratchmarova, S. E. Ong, M. Nielsen, L. J. Foster, and M. Mann. 2003. A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat. Biotechnol. 21:315-318. [DOI] [PubMed] [Google Scholar]
- 3.Briggs, M. W., Z. Li, and D. B. Sacks. 2002. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J. Biol. Chem. 277:7453-7465. [DOI] [PubMed] [Google Scholar]
- 4.Briggs, M. W., and D. B. Sacks. 2003. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 542:7-11. [DOI] [PubMed] [Google Scholar]
- 5.Briggs, M. W., and D. B. Sacks. 2003. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 4:571-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacace, A. M., N. R. Michaud, M. Therrien, K. Mathes, T. Copeland, G. M. Rubin, and D. K. Morrison. 1999. Identification of constitutive and Ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol. Cell. Biol. 19:229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Obregon, S., R. V. Rao, G. del Rio, S. F. Chen, K. S. Poksay, S. Rabizadeh, S. Vesce, X. K. Zhang, R. A. Swanson, and D. E. Bredesen. 2004. Alternative, nonapoptotic programmed cell death: mediation by arrestin 2, ERK2, and Nur77. J. Biol. Chem. 279:17543-17553. [DOI] [PubMed] [Google Scholar]
- 8.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z., T. B. Gibson, F. Robinson, L. Silvestro, G. Pearson, B. Xu, A. Wright, C. Vanderbilt, and M. H. Cobb. 2001. MAP kinases. Chem. Rev. 101:2449-2476. [DOI] [PubMed] [Google Scholar]
- 10.Erickson, J. W., R. A. Cerione, and M. J. Hart. 1997. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J. Biol. Chem. 272:24443-24447. [DOI] [PubMed] [Google Scholar]
- 11.Fukata, M., S. Kuroda, K. Fujii, T. Nakamura, I. Shoji, Y. Matsuura, K. Okawa, A. Iwamatsu, A. Kikuchi, and K. Kaibuchi. 1997. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J. Biol. Chem. 272:29579-29583. [DOI] [PubMed] [Google Scholar]
- 12.Fukata, M., T. Watanabe, J. Noritake, M. Nakagawa, M. Yamaga, S. Kuroda, Y. Matsuura, A. Iwamatsu, F. Perez, and K. Kaibuchi. 2002. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109:873-885. [DOI] [PubMed] [Google Scholar]
- 13.Giroux, S., M. Tremblay, D. Bernard, J. F. Cardin-Girard, S. Aubry, L. Larouche, S. Rousseau, J. Huot, J. Landry, L. Jeannotte, and J. Charron. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369-372. [DOI] [PubMed] [Google Scholar]
- 14.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock, J. F. 2003. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell. Biol. 4:373-384. [DOI] [PubMed] [Google Scholar]
- 16.Hart, M. J., M. G. Callow, B. Souza, and P. Polakis. 1996. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 15:2997-3005. [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, Y. D., J. L. Joyal, Z. Li, and D. B. Sacks. 1999. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J. Biol. Chem. 274:464-470. [DOI] [PubMed] [Google Scholar]
- 18.Hunter, T. 2000. Signaling-2000 and beyond. Cell 100:113-127. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe, A. B., P. Aspenstrom, and A. Hall. 2004. Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways. Mol. Cell. Biol. 24:1736-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyal, J. L., R. S. Annan, Y. D. Ho, M. E. Huddleston, S. A. Carr, M. J. Hart, and D. B. Sacks. 1997. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J. Biol. Chem. 272:15419-15425. [DOI] [PubMed] [Google Scholar]
- 21.Katata, T., K. Irie, A. Fukuhara, T. Kawakatsu, A. Yamada, K. Shimizu, and Y. Takai. 2003. Involvement of nectin in the localization of IQGAP1 at the cell-cell adhesion sites through the actin cytoskeleton in Madin-Darby canine kidney cells. Oncogene 22:2097-2109. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. H., S. K. Lee, and K. Y. Choi. 1998. Saccharomyces cerevisiae STE11 may contribute to the stabilities of a scaffold protein, STE5, in the pheromone signaling pathway. Mol. Cell 8:130-137. [PubMed] [Google Scholar]
- 23.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 24.Kortenjann, M., and P. E. Shaw. 1995. Raf-1 kinase and ERK2 uncoupled from mitogenic signals in rat fibroblasts. Oncogene 11:2105-2112. [PubMed] [Google Scholar]
- 25.Kuroda, S., M. Fukata, M. Nakagawa, K. Fujii, T. Nakamura, T. Ookubo, I. Izawa, T. Nagase, N. Nomura, H. Tani, I. Shoji, Y. Matsuura, S. Yonehara, and K. Kaibuchi. 1998. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science 281:832-835. [DOI] [PubMed] [Google Scholar]
- 26.Levchenko, A., J. Bruck, and P. W. Sternberg. 2000. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl. Acad. Sci. USA 97:5818-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 28.Li, W., M. Han, and K. L. Guan. 2000. The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf. Genes Dev. 14:895-900. [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Z., S. H. Kim, J. M. Higgins, M. B. Brenner, and D. B. Sacks. 1999. IQGAP1 and calmodulin modulate E-cadherin function. J. Biol. Chem. 274:37885-37892. [DOI] [PubMed] [Google Scholar]
- 30.Mansour, S. J., W. T. Matten, A. S. Hermann, J. M. Candia, S. Rong, K. Fukasawa, G. F. Vande Woude, and N. G. Ahn. 1994. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265:966-970. [DOI] [PubMed] [Google Scholar]
- 31.Mataraza, J. M., M. W. Briggs, Z. Li, A. Entwistle, A. J. Ridley, and D. B. Sacks. 2003. IQGAP1 promotes cell motility and invasion. J. Biol. Chem. 278:41237-41245. [DOI] [PubMed] [Google Scholar]
- 32.Mateer, S. C., A. E. McDaniel, V. Nicolas, G. M. Habermacher, M. J. Lin, D. A. Cromer, M. E. King, and G. S. Bloom. 2002. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J. Biol. Chem. 277:12324-12333. [DOI] [PubMed] [Google Scholar]
- 33.Mbele, G. O., J. C. Deloulme, B. J. Gentil, C. Delphin, M. Ferro, J. Garin, M. Takahashi, and J. Baudier. 2002. The zinc- and calcium-binding S100B interacts and co-localizes with IQGAP1 during dynamic rearrangement of cell membranes. J. Biol. Chem. 277:49998-50007. [DOI] [PubMed] [Google Scholar]
- 34.McCallum, S. J., W. J. Wu, and R. A. Cerione. 1996. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J. Biol. Chem. 271:21732-21737. [DOI] [PubMed] [Google Scholar]
- 35.Meighan-Mantha, R. L., A. Wellstein, and A. T. Riegel. 1997. Differential regulation of extracellular signal-regulated kinase 1 and 2 activity during 12-O-tetradecanoylphorbol 13-acetate-induced differentiation of HL-60 cells. Exp. Cell Res. 234:321-328. [DOI] [PubMed] [Google Scholar]
- 36.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91-118. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen, A., W. R. Burack, J. L. Stock, R. Kortum, O. V. Chaika, M. Afkarian, W. J. Muller, K. M. Murphy, D. K. Morrison, R. E. Lewis, J. McNeish, and A. S. Shaw. 2002. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, S. H., A. Zarrinpar, and W. A. Lim. 2003. Rewiring MAP kinase pathways using alternative scaffold assembly mechanisms. Science 299:1061-1064. [DOI] [PubMed] [Google Scholar]
- 39.Robbins, D. J., E. Zhen, H. Owaki, C. A. Vanderbilt, D. Ebert, T. D. Geppert, and M. H. Cobb. 1993. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 268:5097-5106. [PubMed] [Google Scholar]
- 40.Roy, M., Z. Li, and D. B. Sacks. 2004. IQGAP1 binds ERK2 and modulates its activity. J. Biol. Chem. 279:17329-17337. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Velasco, R., C. C. Lanning, and C. L. Williams. 2002. The activation of Rac1 by M3 muscarinic acetylcholine receptors involves the translocation of Rac1 and IQGAP1 to cell junctions and changes in the composition of protein complexes containing Rac1, IQGAP1, and actin. J. Biol. Chem. 277:33081-33091. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 43.Sokol, S. Y., Z. Li, and D. B. Sacks. 2001. The effect of IQGAP1 on Xenopus embryonic ectoderm requires Cdc42. J. Biol. Chem. 276:48425-48430. [DOI] [PubMed] [Google Scholar]
- 44.Swart-Mataraza, J. M., Z. Li, and D. B. Sacks. 2002. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 277:24753-24763. [DOI] [PubMed] [Google Scholar]
- 45.Torii, S., M. Kusakabe, T. Yamamoto, M. Maekawa, and E. Nishida. 2004. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev. Cell 7:33-44. [DOI] [PubMed] [Google Scholar]
- 46.Ussar, S., and T. Voss. 2004. MEK1 and MEK2, different regulators of the G1/S transition. J. Biol. Chem. 279:43861-43869. [DOI] [PubMed] [Google Scholar]
- 47.Vasilescu, J., X. Guo, and J. Kast. 2004. Identification of protein-protein interactions using in vivo cross-linking and mass spectrometry. Proteomics 4:3845-3854. [DOI] [PubMed] [Google Scholar]
- 48.Vomastek, T., H. J. Schaeffer, A. Tarcsafalvi, M. E. Smolkin, E. A. Bissonette, and M. J. Weber. 2004. Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc. Natl. Acad. Sci. USA 101:6981-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe, T., S. Wang, J. Noritake, K. Sato, M. Fukata, M. Takefuji, M. Nakagawa, N. Izumi, T. Akiyama, and K. Kaibuchi. 2004. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell 7:871-883. [DOI] [PubMed] [Google Scholar]
- 50.Xu, B., J. L. Wilsbacher, T. Collisson, and M. H. Cobb. 1999. The N-terminal ERK-binding site of MEK1 is required for efficient feedback phosphorylation by ERK2 in vitro and ERK activation in vivo. J. Biol. Chem. 274:34029-34035. [DOI] [PubMed] [Google Scholar]
- 51.Yeung, K., T. Seitz, S. Li, P. Janosch, B. McFerran, C. Kaiser, F. Fee, K. D. Katsanakis, D. W. Rose, H. Mischak, J. M. Sedivy, and W. Kolch. 1999. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401:173-177. [DOI] [PubMed] [Google Scholar]