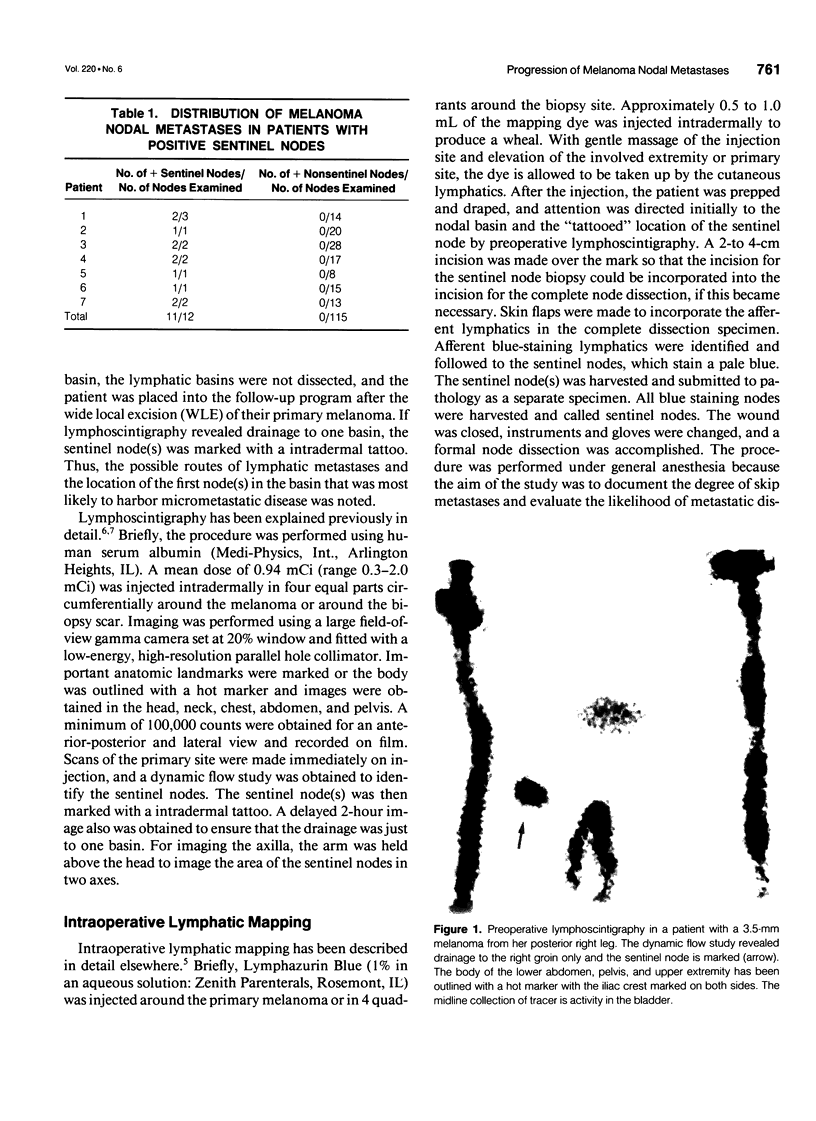
Abstract
OBJECTIVE. The aim of this study was to determine the order of melanoma nodal metastases. SUMMARY BACKGROUND DATA. Most solid tumors are thought to demonstrate a random nodal metastatic pattern. The incidence of skip nodal metastases precluded the use of sampling procedures of first station nodal basins to achieve adequate pathological staging. Malignant melanoma may be different from other malignancies in that the cutaneous lymphatic flow is better defined and can be mapped accurately. The concept of an orderly progression of nodal metastases is radically different than what is thought to occur in the natural history of metastases from most other solid malignancies. METHODS. The investigators performed preoperative and intraoperative mapping of the cutaneous lymphatics from the primary melanoma in an attempt to identify the "sentinel" lymph node in the regional basin. All patients had primary melanomas with tumor thicknesses > 0.76 mm and were considered candidates for elective lymph node dissection. The sentinel lymph node was harvested and submitted separately to pathology, followed by a complete node dissection. The null hypothesis tested was whether nodal metastases from malignant melanoma occurred in equal proportions among sentinel and nonsentinel nodes. RESULTS. Forty-two patients met the criteria of the protocol based on prognostic factors of their primary melanoma. Thirty-four patients had histologically negative sentinel nodes, with the rest of the nodes in the basin also being negative. Thus, there were no skip metastases documented. Eight patients had positive sentinel nodes, with seven of the eight having the sentinel node as the only site of disease. In these seven patients, the frequency of sentinel nodal metastases was 92%, whereas none of the higher nodes had documented metastatic disease. Nodal involvement was compared between the sentinel and nonsentinel nodal groups, based on the binomial distribution. Under the null hypothesis of equality in distribution of nodal metastases, the probability that all seven unpaired observations would demonstrate that involvement of the sentinel node is 0.008. CONCLUSIONS. The data presented demonstrate that nodal metastases from cutaneous melanoma are not random events. The sentinel lymph nodes in the lymphatic basins can be mapped and identified individually, and they have been shown to contain the first evidence of melanoma metastases. This information can be used to revolutionize melanoma care so that only those patients with evidence of nodal metastatic disease are subjected to the morbidity and expense of a complete node dissection. Because sentinel node histology accurately reflects the histology of the remainder of the lymphatic basin, information gained from the sentinel node biopsy can be used as a prognostic factor for melanoma. These findings demonstrate effective pathologic staging, no decrease in standards of care, and a reduction of morbidity with a less aggressive, rational surgical approach.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman C. G., Norman J., Cruse C. W., Reintgen D. S., Clark R. A. Lymphoscintigraphy in malignant melanoma. Ann Plast Surg. 1992 Jan;28(1):29–32. doi: 10.1097/00000637-199201000-00010. [DOI] [PubMed] [Google Scholar]
- Halsted W. S. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg. 1907 Jul;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., McCarthy W. H., Pearson L., Balch C. M., Soong S. J. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: results of surgical treatment in 1319 patients. Br J Surg. 1982 Feb;69(2):108–111. doi: 10.1002/bjs.1800690217. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Wen D. R., Wong J. H., Economou J. S., Cagle L. A., Storm F. K., Foshag L. J., Cochran A. J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- Msuya C. A., Hartveit F. Skip lesions in the axilla in breast cancer, and their association with micrometastases. Breast Cancer Res Treat. 1991 Nov;19(3):277–281. doi: 10.1007/BF01961164. [DOI] [PubMed] [Google Scholar]
- Norman J., Cruse C. W., Espinosa C., Cox C., Berman C., Clark R., Saba H., Wells K., Reintgen D. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991 Nov;162(5):432–437. doi: 10.1016/0002-9610(91)90255-c. [DOI] [PubMed] [Google Scholar]
- Norman J., Wells K., Kearney R., Cruse C. W., Berman C., Reintgen D. Identification of lymphatic drainage basins in patients with cutaneous melanoma. Semin Surg Oncol. 1993 May-Jun;9(3):224–227. [PubMed] [Google Scholar]
- Reintgen D. S., Cox C., Slingluff C. L., Jr, Seigler H. F. Recurrent malignant melanoma: the identification of prognostic factors to predict survival. Ann Plast Surg. 1992 Jan;28(1):45–49. doi: 10.1097/00000637-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Reintgen D. S., Cox E. B., McCarty K. S., Jr, Vollmer R. T., Seigler H. F. Efficacy of elective lymph node dissection in patients with intermediate thickness primary melanoma. Ann Surg. 1983 Sep;198(3):379–385. doi: 10.1097/00000658-198309000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P. P., Lesser M. L., Kinne D. W., Beattie E. J. Discontinuous or "skip" metastases in breast carcinoma. Analysis of 1228 axillary dissections. Ann Surg. 1983 Mar;197(3):276–283. doi: 10.1097/00000658-198303000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. I., Reintgen D., Balch C. M. Selective lymphadenectomy: emerging role for lymphatic mapping and sentinel node biopsy in the management of early stage melanoma. Semin Surg Oncol. 1993 May-Jun;9(3):219–223. [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Ivins J. C., Pritchard D. J., Soule E. H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978 Mar;41(3):948–956. doi: 10.1002/1097-0142(197803)41:3<948::aid-cncr2820410324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stankard C., Cruse C. W., Cox C., Wells K. E., King J., Reintgen D. S. The concept of lymph node dissections in patients with malignant melanoma. Ann Plast Surg. 1992 Jan;28(1):33–38. doi: 10.1097/00000637-199201000-00011. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Rilke F., Luini A., Sacchini V., Galimberti V., Campa T., Dei Bei E., Greco M., Magni A., Merson M. Distribution of axillary node metastases by level of invasion. An analysis of 539 cases. Cancer. 1987 Feb 15;59(4):682–687. doi: 10.1002/1097-0142(19870215)59:4<682::aid-cncr2820590403>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wong J. H., Cagle L. A., Morton D. L. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991 Nov;214(5):637–641. doi: 10.1097/00000658-199111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman T. J., Nicolson G. L. Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin Surg Oncol. 1993 May-Jun;9(3):256–263. [PubMed] [Google Scholar]