Abstract
OBJECTIVE. The authors investigated the role of mucin and secretory immunoglobulin A (slgA) in a model of nutritionally induced bacterial translocation. BACKGROUND. Parenteral and certain elemental diets have been shown to impair intestinal barrier function, whereas fiber has been shown to protect against nutritionally induced bacterial translocation. However, the factors responsible for these phenomenon have not been fully determined. METHODS. Intestinal mucin levels, mucosal protein content, slgA, intestinal morphology, and permeability to horseradish peroxidase, bacterial translocation, and intestinal bacterial population levels were measured in rats 7 days after receiving total parenteral nutrition (TPN) solution (28% glucose, 4.25% amino acids; 307 kcal/kg/day) enterally (ORAL-TPN) or parenterally (IV-TPN) with or without enteral bulk fiber supplementation. Chow-fed rats served as control subjects. RESULTS. The incidence of bacterial translocation in the ORAL-TPN and IV-TPN groups was reduced significantly by the provision of fiber (p < 0.05). Mucosal protein, slgA, and insoluble mucin levels were decreased in the jejunum of the ORAL-TPN and IV-TPN groups, with mucosal protein levels being decreased to a greater extent than slgA or mucin. Although similar decreases in these parameters were observed in the fiber-fed groups, fiber appeared to improve intestinal barrier function as measured by horseradish peroxidase permeability. CONCLUSIONS. The provision of bulk-forming fiber improves intestinal barrier function as measured by peroxidase permeability and bacterial translocation, but does not restore mucosal protein content, intestinal mucin, or slgA levels to normal.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Cunliffe W. J., Pearson J. P., Sellers L. A., Ward R. Studies on gastrointestinal mucus. Scand J Gastroenterol Suppl. 1984;93:101–113. [PubMed] [Google Scholar]
- Alverdy J. C., Aoys E., Moss G. S. Effect of commercially available chemically defined liquid diets on the intestinal microflora and bacterial translocation from the gut. JPEN J Parenter Enteral Nutr. 1990 Jan-Feb;14(1):1–6. doi: 10.1177/014860719001400101. [DOI] [PubMed] [Google Scholar]
- Alverdy J. C., Aoys E., Moss G. S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988 Aug;104(2):185–190. [PubMed] [Google Scholar]
- Barber A. E., Jones W. G., 2nd, Minei J. P., Fahey T. J., 3rd, Moldawer L. L., Rayburn J. L., Fischer E., Keogh C. V., Shires G. T., Lowry S. F. Harry M. Vars award. Glutamine or fiber supplementation of a defined formula diet: impact on bacterial translocation, tissue composition, and response to endotoxin. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4):335–343. doi: 10.1177/0148607190014004335. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown R. C., Kelleher J., Losowsky M. S. The effect of pectin on the structure and function of the rat small intestine. Br J Nutr. 1979 Nov;42(3):357–365. doi: 10.1079/bjn19790125. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Alverdy J. C., Aoys E., Moss G. S. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989 Dec;124(12):1396–1399. doi: 10.1001/archsurg.1989.01410120042009. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Pavlat W. A., Urban E. Adaptive responses to total intravenous feeding. J Surg Res. 1974 Jul;17(1):45–52. doi: 10.1016/0022-4804(74)90167-x. [DOI] [PubMed] [Google Scholar]
- Cassidy M. M., Lightfoot F. G., Grau L. E., Story J. A., Kritchevsky D., Vahouny G. V. Effect of chronic intake of dietary fibers on the ultrastructural topography of rat jejunum and colon: a scanning electron microscopy study. Am J Clin Nutr. 1981 Feb;34(2):218–228. doi: 10.1093/ajcn/34.2.218. [DOI] [PubMed] [Google Scholar]
- Clamp J. R. The relationship between secretory immunoglobulin A and mucus [proceedings]. Biochem Soc Trans. 1977;5(5):1579–1581. doi: 10.1042/bst0051579. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Maejima K., Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985 May;25(5):385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L. Small bowel morphology and epithelial proliferation in intravenously alimented rabbits. Surgery. 1977 Nov;82(5):613–620. [PubMed] [Google Scholar]
- Ecknauer R., Sircar B., Johnson L. R. Effect of dietary bulk on small intestinal morphology and cell renewal in the rat. Gastroenterology. 1981 Oct;81(4):781–786. [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Townsend C. M., Jr, Uchida T., Thompson J. C. Differential effects of gut hormones on pancreatic and intestinal growth during administration of an elemental diet. Ann Surg. 1990 May;211(5):630–638. [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Models for studying the role of bacterial attachment in virulence and pathogenesis. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S647–S658. doi: 10.1093/clinids/5.supplement_4.s647. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Effects of addition of kaolin or cellulose to an elemental diet on intestinal cell proliferation in the mouse. Br J Nutr. 1983 Jul;50(1):91–98. doi: 10.1079/bjn19830075. [DOI] [PubMed] [Google Scholar]
- Haskel Y., Xu D., Lu Q., Deitch E. Elemental diet-induced bacterial translocation can be hormonally modulated. Ann Surg. 1993 Jun;217(6):634–643. doi: 10.1097/00000658-199306000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda N., Nishi M., Nakagawa M., Hiramatsu Y., Hioki K., Yamamoto M. Structural and functional alterations in the gut of parenterally or enterally fed rats. J Surg Res. 1989 Aug;47(2):129–133. doi: 10.1016/0022-4804(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Hosoda N., Nishi M., Nakagawa M., Hiramatsu Y., Hioki K., Yamamoto M. Structural and functional alterations in the gut of parenterally or enterally fed rats. J Surg Res. 1989 Aug;47(2):129–133. doi: 10.1016/0022-4804(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Hughes C. A., Dowling R. H. Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clin Sci (Lond) 1980 Nov;59(5):317–327. doi: 10.1042/cs0590317. [DOI] [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Steiger E., Zinno R. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology. 1974 Nov;67(5):975–982. [PubMed] [Google Scholar]
- Lichtman S., Sherman P., Forstner G. Production of secretory immunoglobulin A in rat self-filling blind loops. Local secretory immunoglobulin A immune response to luminal bacterial flora. Gastroenterology. 1986 Dec;91(6):1495–1502. doi: 10.1016/0016-5085(86)90207-6. [DOI] [PubMed] [Google Scholar]
- Lim G. M., Sheldon G. F., Alverdy J. Biliary secretory IgA levels in rats with protein-calorie malnutrition. Ann Surg. 1988 May;207(5):635–640. doi: 10.1097/00000658-198805000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Meurling S., Roos K. A. Gut structure changes in rats on continuous and intermittent complete parenteral nutrition. Acta Chir Scand. 1981;147(6):451–457. [PubMed] [Google Scholar]
- Nagura H., Sumi Y. Immunological functions of the gut--role of the mucosal immune system. Toxicol Pathol. 1988;16(2):154–164. doi: 10.1177/019262338801600208. [DOI] [PubMed] [Google Scholar]
- Rhodes R. S., Karnovsky M. J. Loss of macromolecular barrier function associated with surgical trauma to the intestine. Lab Invest. 1971 Sep;25(3):220–229. [PubMed] [Google Scholar]
- Sherman P., Forstner J., Roomi N., Khatri I., Forstner G. Mucin depletion in the intestine of malnourished rats. Am J Physiol. 1985 Apr;248(4 Pt 1):G418–G423. doi: 10.1152/ajpgi.1985.248.4.G418. [DOI] [PubMed] [Google Scholar]
- Shou J., Redmond H. P., Leon P., Hofmann K. P., Daly J. M. Elemental diet alters macrophage function in mice. J Surg Res. 1991 Sep;51(3):192–196. doi: 10.1016/0022-4804(91)90093-2. [DOI] [PubMed] [Google Scholar]
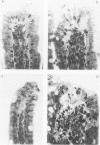
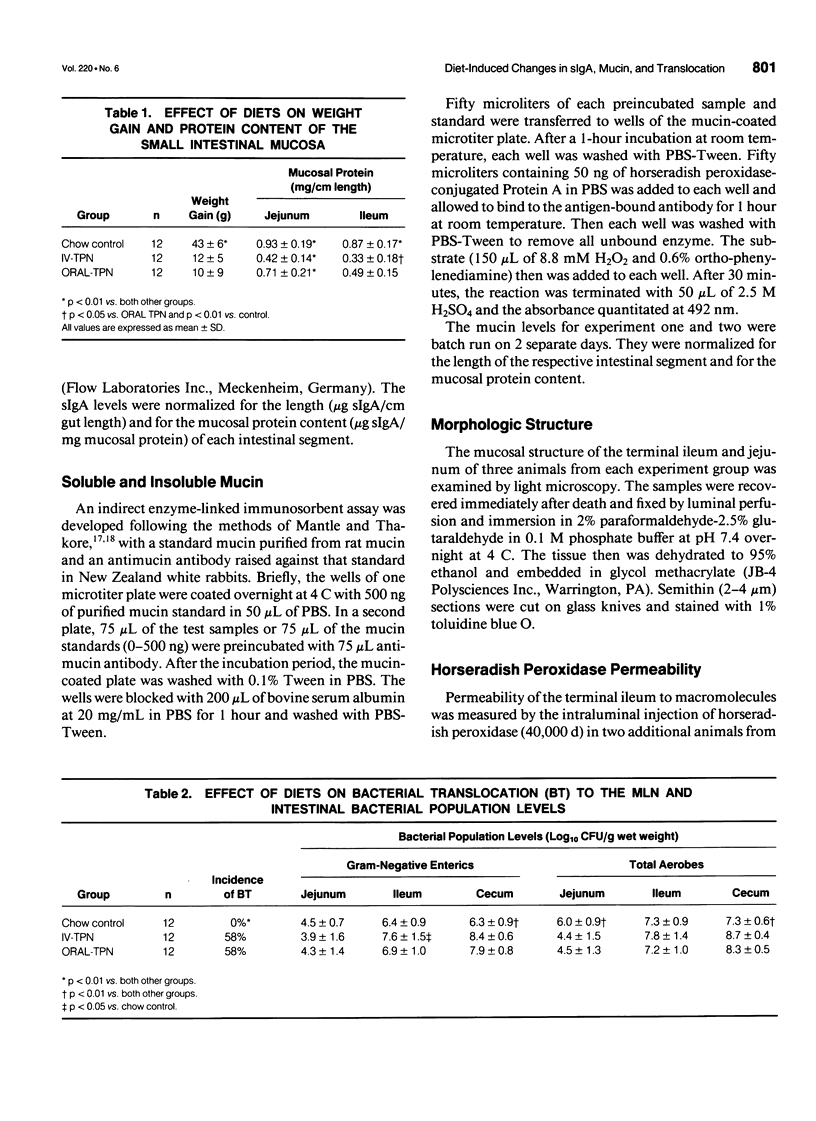
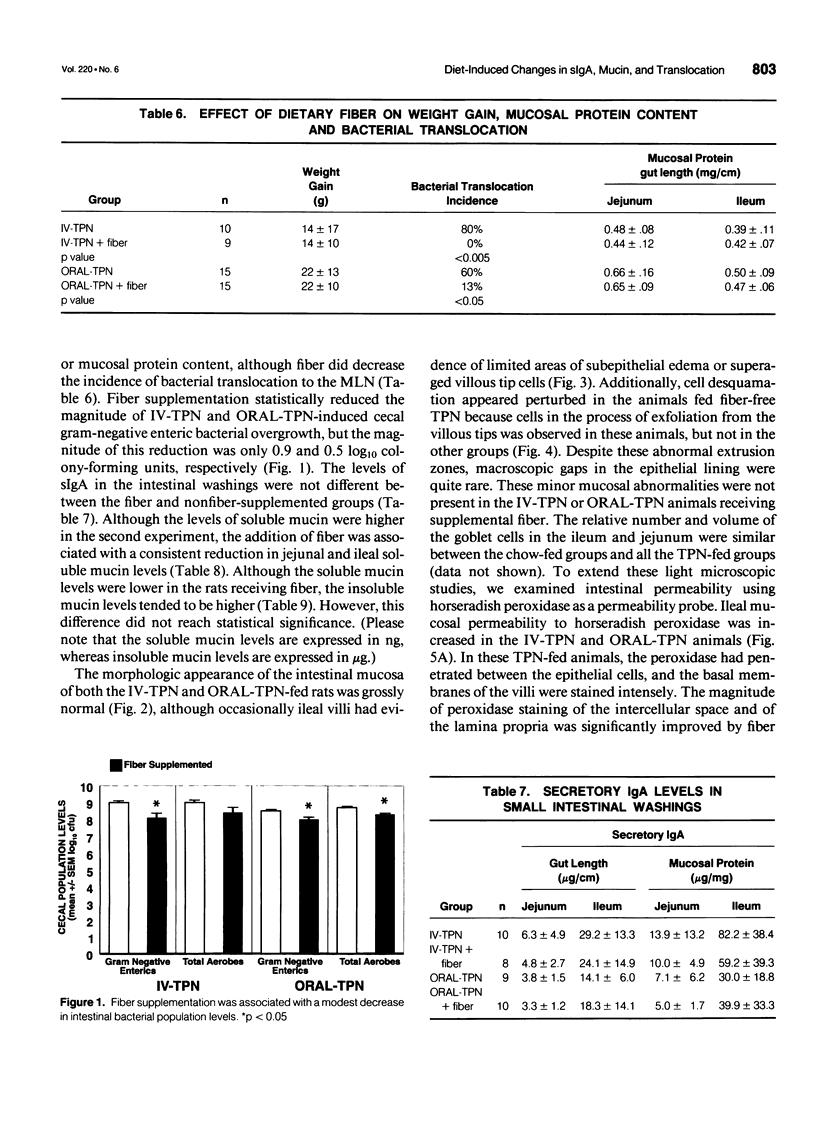
- Spaeth G., Berg R. D., Specian R. D., Deitch E. A. Food without fiber promotes bacterial translocation from the gut. Surgery. 1990 Aug;108(2):240–247. [PubMed] [Google Scholar]
- Spaeth G., Specian R. D., Berg R. D., Deitch E. A. Bulk prevents bacterial translocation induced by the oral administration of total parenteral nutrition solution. JPEN J Parenter Enteral Nutr. 1990 Sep-Oct;14(5):442–447. doi: 10.1177/0148607190014005442. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Le T., Ifrim I., Satchithanandam S., Cassidy M. M. Stimulation of intestinal cytokinetics and mucin turnover in rats fed wheat bran or cellulose. Am J Clin Nutr. 1985 May;41(5):895–900. doi: 10.1093/ajcn/41.5.895. [DOI] [PubMed] [Google Scholar]