Abstract
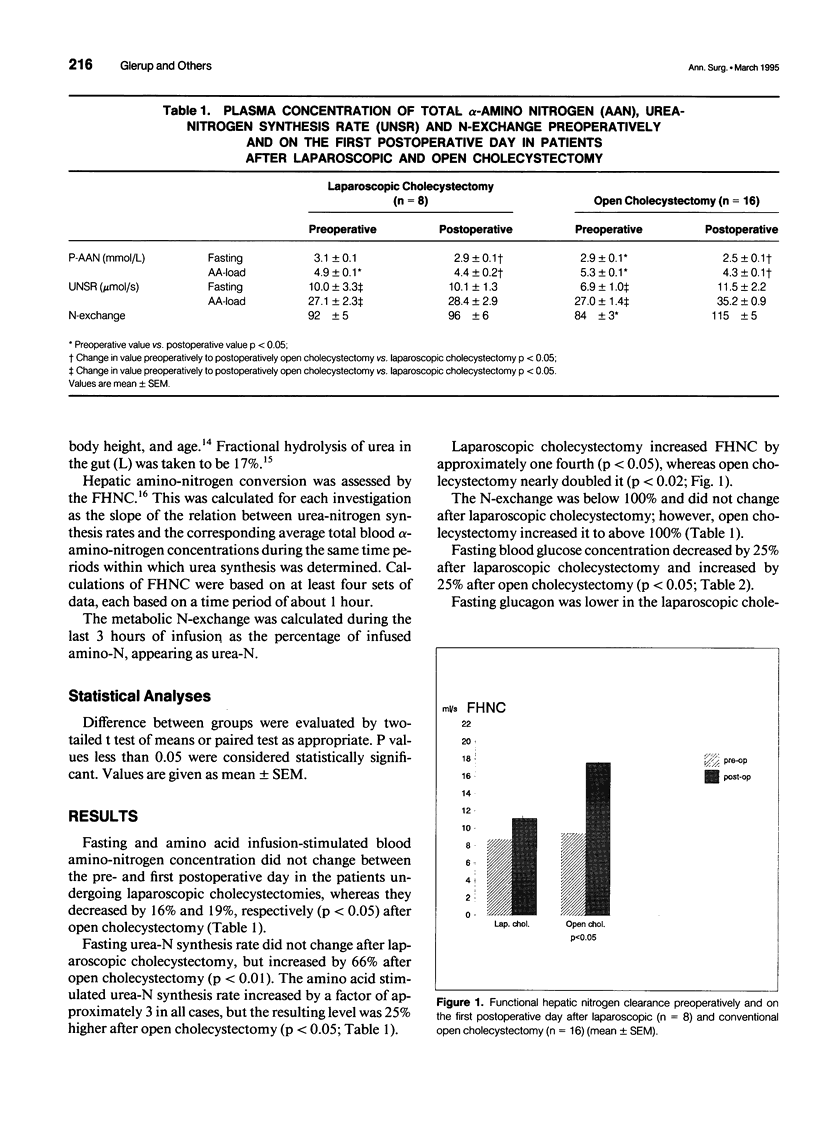
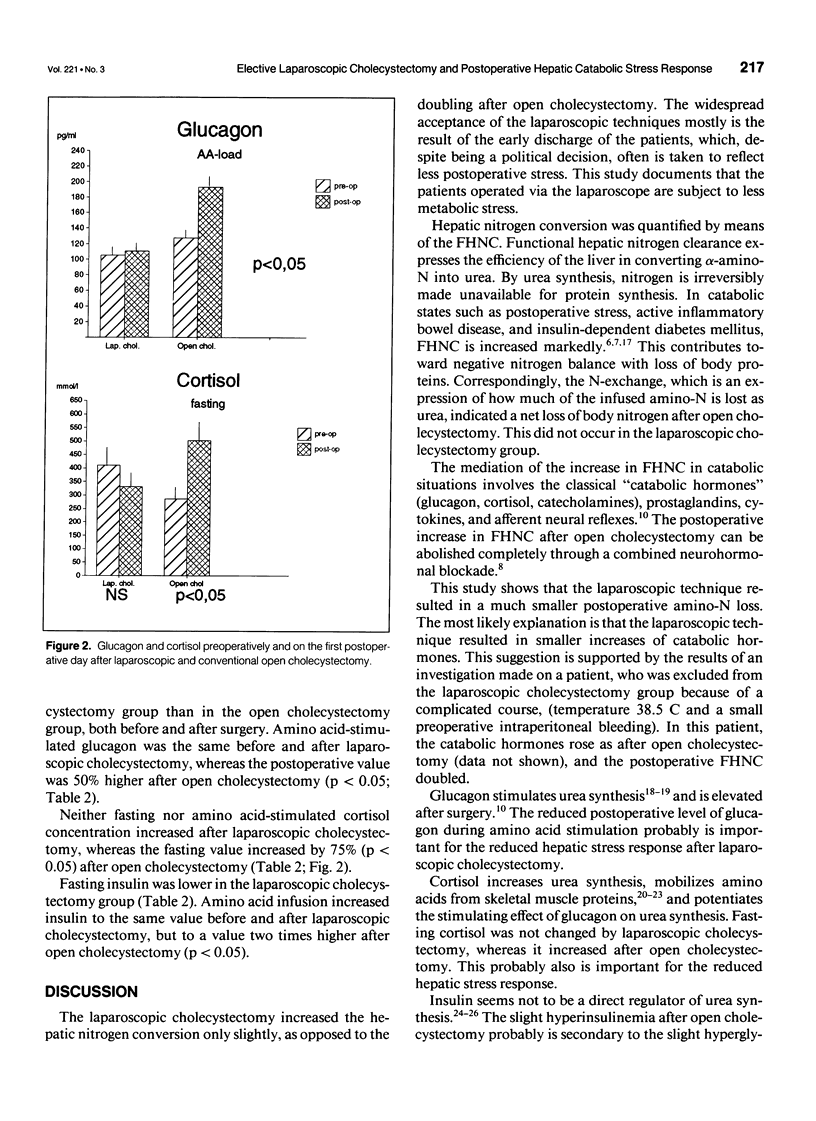
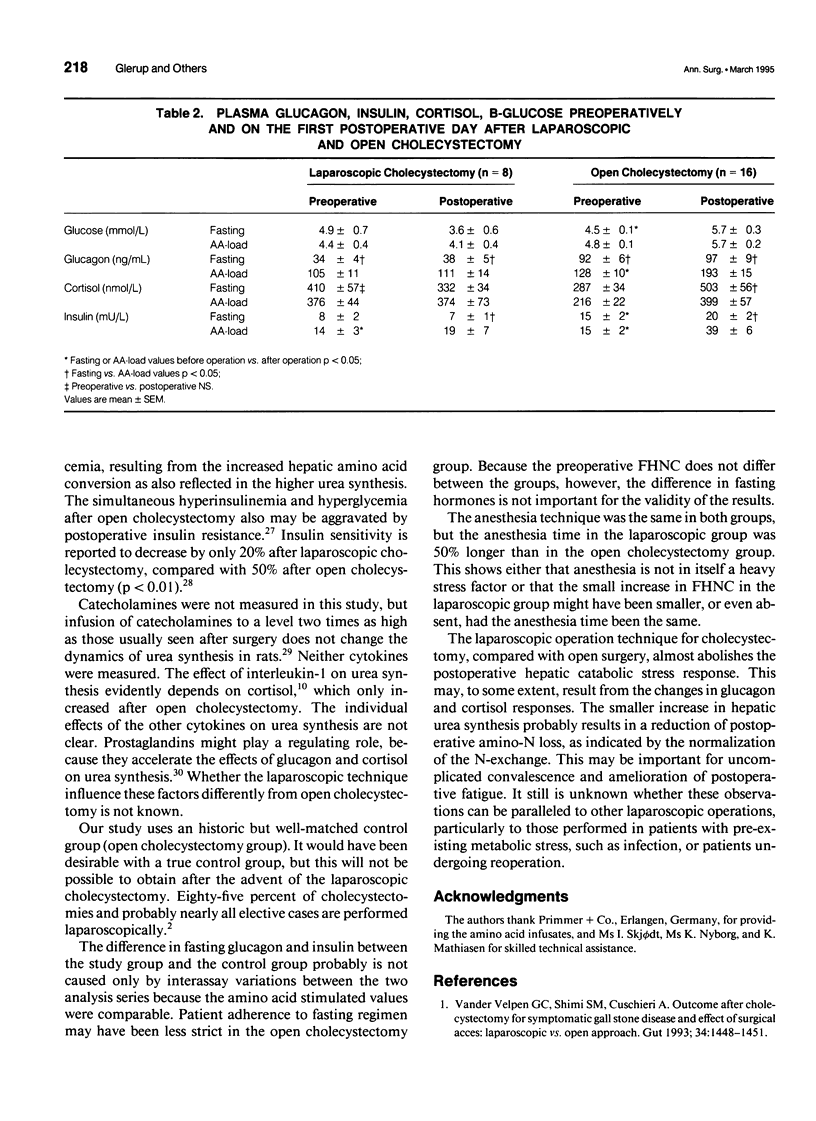
OBJECTIVE: Surgery results in a catabolic state of postoperative stress, where the efficiency of the liver to convert amino acids to urea is increased. This study measured the metabolic consequences of the less traumatic laparoscopic surgery in elective cholecystectomy compared with traditional open surgery technique. SUMMARY BACKGROUND DATA: The authors previously have shown that open cholecystectomy doubles the urea synthesis measured by the means of the functional hepatic nitrogen clearance. Glucagon and cortisol increased by 50% (p < 0.05) and 75% (p < 0.05), respectively, after open cholecystectomy. METHODS: Patients undergoing uncomplicated elective laparoscopic cholecystectomies were included. Preoperatively and on the first postoperative day, blood and urine samples were drawn every hour under basal conditions and during amino acid infusion. The urea synthesis rate was calculated from the urea excreted in urine and accumulated in total body water. Functional hepatic nitrogen clearance was quantified as the slope of the linear relation between blood amino-N concentration and the urea synthesis rate. The results were compared with an historic matched group of patients who underwent open cholecystectomies and were studied by the same protocol. RESULTS: The laparoscopic cholecystectomy increased the functional hepatic nitrogen clearance by only 25% (from 8.7 +/- 0.9 to 11.1 +/- 1.5 mL/sec [mean +/- SEM; p < 0.05]), compared with a doubling after open cholecystectomy (from 9.4 +/- 0.9 to 17.6 +/- 3.3 mL/sec [p < 0.05]). The difference between the groups was significant (p < 0.05). Neither glucagon nor cortisol increased significantly after laparoscopic cholecystectomy. CONCLUSIONS: The laparoscopic technique results in a much smaller postoperative hepatic catabolic stress response and probably reduced tissue loss of amino-N. This may be important for the more rapid convalescence and reduced postoperative fatigue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almdal T. P., Jensen T., Vilstrup H. Increased hepatic efficacy of urea synthesis from alanine in insulin-dependent diabetes mellitus. Eur J Clin Invest. 1990 Feb;20(1):29–34. doi: 10.1111/j.1365-2362.1990.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Coelho J. C., de Araujo R. P., Marchesini J. B., Coelho I. C., de Araujo L. R. Pulmonary function after cholecystectomy performed through Kocher's incision, a mini-incision, and laparoscopy. World J Surg. 1993 Jul-Aug;17(4):544–546. doi: 10.1007/BF01655120. [DOI] [PubMed] [Google Scholar]
- Hamberg O., Vilstrup H. Effects of glucose on hepatic conversion of aminonitrogen to urea in patients with cirrhosis: relationship to glucagon. Hepatology. 1994 Jan;19(1):45–54. [PubMed] [Google Scholar]
- Hansen B. A., Vilstrup H. Increased intestinal hydrolysis of urea in patients with alcoholic cirrhosis. Scand J Gastroenterol. 1985 Apr;20(3):346–350. doi: 10.3109/00365528509091662. [DOI] [PubMed] [Google Scholar]
- Heindorff H. A. The hepatic catabolic stress response. Hormonal regulation of urea synthesis after surgery. Dan Med Bull. 1993 Apr;40(2):224–234. [PubMed] [Google Scholar]
- Heindorff H., Almdal T., Vilstrup H. Effects of epinephrine on urea synthesis in vivo in rats. Liver. 1992 Feb;12(1):46–49. doi: 10.1111/j.1600-0676.1992.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Heindorff H., Schulze S., Mogensen T., Almdal T., Kehlet H., Vilstrup H. Hormonal and neural blockade prevents the postoperative increase in amino acid clearance and urea synthesis. Surgery. 1992 May;111(5):543–550. [PubMed] [Google Scholar]
- Heindorff H., Vilstrup H., Almdal T., Harvald T., Nielsen J., Dalsgaard S. Elective cholecystectomy increases plasma amino-acid clearance and hepatic capacity for urea synthesis for one week. Clin Nutr. 1991 Feb;10(1):10–17. doi: 10.1016/0261-5614(91)90075-n. [DOI] [PubMed] [Google Scholar]
- Heindorff H., Vilstrup H., Bucher D., Billesbølle P., Thygesen V. Increased hepatic amino nitrogen conversion after elective cholecystectomy in man. Clin Sci (Lond) 1988 May;74(5):539–545. doi: 10.1042/cs0740539. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Snodgrass P. J., Rabier D. Induction of urea cycle enzymes by glucagon and dexamethasone in monolayer cultures of adult rat hepatocytes. J Biol Chem. 1982 May 10;257(9):5061–5067. [PubMed] [Google Scholar]
- Muhlbacher F., Kapadia C. R., Colpoys M. F., Smith R. J., Wilmore D. W. Effects of glucocorticoids on glutamine metabolism in skeletal muscle. Am J Physiol. 1984 Jul;247(1 Pt 1):E75–E83. doi: 10.1152/ajpendo.1984.247.1.E75. [DOI] [PubMed] [Google Scholar]
- Nordenström J., Sonnenfeld T., Arner P. Characterization of insulin resistance after surgery. Surgery. 1989 Jan;105(1):28–35. [PubMed] [Google Scholar]
- Orskov H., Thomsen H. G., Yde H. Wick chromatography for rapid and reliable immunoassay of insulin, glucagon and growth hormone. Nature. 1968 Jul 13;219(5150):193–195. doi: 10.1038/219193b0. [DOI] [PubMed] [Google Scholar]
- SWETTENHAM K. The buffered performic-acid-alcian-blue-periodic-acid-Schiff method for the differentiation of basophils in the human and rat pituitary. J Clin Pathol. 1960 May;13:256–260. doi: 10.1136/jcp.13.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalfi L., Coltorti A., Sapio C., Di Biase G., Borrelli R., Contaldo F. Predicted and measured resting energy expenditure in healthy young women. Clin Nutr. 1993 Feb;12(1):1–7. doi: 10.1016/0261-5614(93)90137-s. [DOI] [PubMed] [Google Scholar]
- Schippers E., Ottinger A. P., Anurov M., Polivoda M., Schumpelick V. Laparoscopic cholecystectomy: a minor abdominal trauma? World J Surg. 1993 Jul-Aug;17(4):539–543. doi: 10.1007/BF01655118. [DOI] [PubMed] [Google Scholar]
- Sigsgaard I., Almdal T., Hansen B. A., Vilstrup H. Dexamethasone increases the capacity of urea synthesis time dependently and reduces the body weight of rats. Liver. 1988 Aug;8(4):193–197. doi: 10.1111/j.1600-0676.1988.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Simmons P. S., Miles J. M., Gerich J. E., Haymond M. W. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984 Feb;73(2):412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper N. J., Brunt L. M., Kerbl K. Laparoscopic general surgery. N Engl J Med. 1994 Feb 10;330(6):409–419. doi: 10.1056/NEJM199402103300608. [DOI] [PubMed] [Google Scholar]
- Steiner C. A., Bass E. B., Talamini M. A., Pitt H. A., Steinberg E. P. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994 Feb 10;330(6):403–408. doi: 10.1056/NEJM199402103300607. [DOI] [PubMed] [Google Scholar]
- Vander Velpen G. C., Shimi S. M., Cuschieri A. Outcome after cholecystectomy for symptomatic gall stone disease and effect of surgical access: laparoscopic v open approach. Gut. 1993 Oct;34(10):1448–1451. doi: 10.1136/gut.34.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilstrup H. Effects of glucose on alanine-derived urea synthesis. Clin Physiol. 1984 Dec;4(6):495–507. doi: 10.1111/j.1475-097x.1984.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Vilstrup H., Hansen B. A., Almdal T. P. Glucagon increases hepatic efficacy for urea synthesis. J Hepatol. 1990 Jan;10(1):46–50. doi: 10.1016/0168-8278(90)90072-y. [DOI] [PubMed] [Google Scholar]
- Vilstrup H. On urea synthesis--regulation in vivo. Dan Med Bull. 1989 Oct;36(5):415–429. [PubMed] [Google Scholar]
- Vilstrup H. Synthesis of urea after stimulation with amino acids: relation to liver function. Gut. 1980 Nov;21(11):990–995. doi: 10.1136/gut.21.11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. M., Culebras J. M., Aoki T. T., O'Connor N. E., Finley R. J., Kaczowka A., Moore F. D. The effects of glucagon on protein metabolism in normal man. Surgery. 1979 Aug;86(2):248–257. [PubMed] [Google Scholar]
- Woolfson A. M., Heatley R. V., Allison S. P. Insulin to inhibit protein catabolism after injury. N Engl J Med. 1979 Jan 4;300(1):14–17. doi: 10.1056/NEJM197901043000104. [DOI] [PubMed] [Google Scholar]


