Summary
Background
Long COVID, characterized by persistent multi-organ symptoms post-SARS-CoV-2 infection, poses a substantial global health burden. While diverse therapeutic interventions have been proposed, their comparative efficacy remains uncertain due to fragmented evidence and methodological heterogeneity in prior studies. Therefore, we conducted a meta-analysis to comprehensively explore the effectiveness of diverse therapeutic interventions in long COVID.
Methods
In this meta-analysis, we searched PubMed, Cochrane Library, Embase, Web of Science, SPORTDiscus (EBSCO), CINAHL (EBSCO), and Rehabilitation & Sports medicine source (EBSCO) from inception to July 20, 2025, for randomized controlled trials (RCTs) evaluating exercise training, respiratory muscle training, telerehabilitation, transcranial direct current stimulation (tDCS), olfactory training, palmitoylethanolamide with luteolin (PEA-LUT), and steroid sprays in adults with Long COVID. Primary outcomes included cardiopulmonary function, exercise capacity, fatigue, and olfactory recovery. Data were pooled using random-effects models, with sensitivity analyses (leave-one-out method) and Egger's test to assess robustness and publication bias. GRADE criteria evaluated evidence certainty. The study was registered with PROSPERO (CRD42024591704).
Findings
We identified a total of 51 eligible trials, comprising 4026 participants. Significant differences were observed in the following outcomes in the context of exercise training: 6MWT (MD, 83.20; 95% CI 52.04–114.37), 30sSTS (MD, 3.05; 95% CI 1.96–4.13), SF-12 Mental Component Summary (SF-12-MCS) (MD, 3.10; 95% CI 0.78–5.43), VO2 peak (% predicted) (MD, 6.00; 95% CI 0.45–11.54), VO2 peak (L/kg/min) (MD, 1.61; 95% CI 0.40–2.81), VO2 peak (L/min) (MD, 0.14; 95% CI 0.03–0.25), mMRC dyspnea scale (MD, −1.04; 95% CI −1.73 to −0.35), the Multidimensional Functional Assessment of Daily Living Scale (MBDS) (MD, −4.61; 95% CI −8.19 to −1.03), and Visual Analogue Fatigue Scale (VAFS) (MD −1.69; 95% CI −3.07 to −0.31). Furthermore, significant differences were also found in the following key outcomes: 6MWT (MD, 89.54; 95% CI 9.86–169.23), MIP (% predicted) (MD, 15.79; 95% CI 2.73–28.84), MIP (cm H2O) (MD, 19.69; 95% CI 10.14–29.24), and mMRC (MD, −1.02; 95% CI −1.86 to −0.18) in respiratory muscle training; 6MWT (MD 34.14; 95% CI 2.54–65.74), 30sSTS (MD 1.41; 95% CI 0.67–2.15), and FSS (MD −1.59; 95% CI −2.64 to −0.53) in telerehabilitation; MFIS-physical (MD, −2.29; 95% CI −4.36 to −0.22) in tDCS; and TDI Score (MD, 4.66; 95% CI 2.16–7.15) in PEA-LUT.
Interpretation
Exercise training should be prioritized for improving cardiopulmonary function and exercise capacity in Long COVID, supported by high-certainty evidence. Respiratory muscle training and PEA-LUT offer targeted benefits for respiratory strength and anosmia, while tDCS may address fatigue. Telerehabilitation, as a form of supervision, also improved the effectiveness of the intervention. In contrast, steroid sprays and olfactory training lack efficacy, highlighting the need for personalized, symptom-specific approaches. These findings advocate for updated clinical guidelines integrating multimodal therapies and underscore the urgency of large-scale trials to optimize dosing and long-term outcomes.
Funding
This study was supported by the Hunan Provincial Natural Foundation of China (2021JJ30040), the National Key Research and Development Plan (2022YFC3601900, 2022YFC2505500), the National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University (2021KFJJ06), and the National Natural Science Foundation of China (No. 81672225).
Keywords: Long COVID, Therapeutic interventions, Exercising training, Respiratory muscle training, Meta-analysis
Research in context.
Evidence before this study
Prior meta-analyses evaluating therapeutic interventions for Long COVID have predominantly focused on exercise training and pulmonary rehabilitation, demonstrating efficacy in improving exercise capacity and respiratory parameters. However, these reviews often excluded emerging interventions such as tDCS, respiratory muscle training, and therapy of olfactory disfunction. Existing studies were limited by small sample sizes, methodological heterogeneity (e.g., varying rehabilitation protocols), and insufficient exploration of non-physical symptoms such as olfactory dysfunction. No prior comprehensive meta-analysis had directly compared the efficacy of diverse interventions, leaving clinicians uncertain about optimal treatment hierarchies for Long COVID. We systematically searched PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of Science, SPORTDiscus (EBSCO), CINAHL (EBSCO), and Rehabilitation & Sports medicine source (EBSCO) from database inception to July 20, 2025. Our search strategy combined terms for long COVID, therapy for long COVID, and RCTs. Key search terms included combinations of “Post-Acute COVID-19 Syndrome”, “exercise”, “respiratory muscle training”, “rehabilitation”, “olfactory training”, “palmitoylethanolamide with luteolin”, “transcranial direct current stimulation”, and “cortisol”. Searches were limited to randomized trials using filters such as “randomized controlled trial”, “controlled clinical trial”, “placebo”, and “randomization”. The inclusion criteria were restricted to RCTs investigating exercise training, respiratory muscle training, tele-rehabilitation, pharmacotherapy, olfactory training, and tDCS in patients with Long COVID, with reported key outcomes encompassing the 6-min walk test (6MWT) and modified Medical Research Council (mMRC) dyspnea scale—validated metrics for assessing exercise endurance and dyspnea severity.
Added value of this study
This systematic review and meta-analysis was the first to comprehensively evaluate seven therapeutic interventions for Long COVID, including exercise training, respiratory muscle training, telerehabilitation, tDCS, olfactory training, PEA-LUT, and steroid sprays. By synthesizing data from 51 RCTs (4026 participants), we provide robust head-to-head comparisons across critical outcomes such as cardiopulmonary function, fatigue, dyspnea, and olfactory recovery. First meta-analysis to quantify the effects of tDCS on fatigue and PEA-LUT on olfactory recovery, revealing statistically significant benefits. This study resolves uncertainties about the comparative efficacy of interventions, identifying exercise training as the most effective for improving exercise capacity, while PEA-LUT and tDCS show promise for specific symptoms.
Implications of all the available evidence
The findings underscore that exercise training should be prioritized in clinical practice due to its broad benefits on cardiopulmonary function and exercise tolerance. Respiratory muscle training and PEA-LUT offer targeted advantages for respiratory strength and olfactory recovery, respectively, and may complement exercise programs. tDCS emerges as a viable non-invasive option for alleviating physical fatigue, though further trials are needed to confirm its role. Telerehabilitation, functioning as a supervisory approach, contributed to improved intervention outcomes. Conversely, steroid sprays, and olfactory training alone lack sufficient evidence for routine use. Clinicians should tailor interventions to individual symptom profiles—for example, combining exercise with PEA-LUT in patients with concurrent fatigue and anosmia. Future research should address gaps in long-term outcomes, optimal dosing, and mechanistic studies to refine personalized therapies.
Introduction
After infection with the novel coronavirus, patients may experience not only acute symptoms in the short term but also a significant likelihood of long-term complications affecting multiple organs and systems, potentially leading to a substantial decline in quality of life. The World Health Organization (WHO) defines long COVID, or post-COVID-19 condition (PCC), as the persistence or emergence of new symptoms three months after the initial SARS-CoV-2 infection.1 Common manifestations include extreme fatigue, dyspnea, anxiety, depression, anosmia, and cognitive impairment.2,3 A systematic review reported that the incidence of long COVID ranges from 50.9% to 87.4%, with fatigue being the most prevalent symptom (44%–63%) after 6–12 months of follow-up, followed by sleep disturbances (24%–46%).4
To address long COVID, clinical strategies such as exercise, respiratory training, pharmacotherapy, olfactory rehabilitation, aromatherapy, tele-rehabilitation, dietary adjustments, transcranial stimulation, and hyperbaric oxygen therapy have been explored.5, 6, 7, 8 Among these, exercise training and respiratory training fall under the category of physical therapy, and personalized physical therapy rehabilitation programs are particularly emphasized as the cornerstone for managing both physical and psychological symptoms.9 While previous meta-analyses have primarily focused on exercise training and pulmonary rehabilitation,10, 11, 12, 13, 14, 15, 16, 17, 18 emerging interventions such as transcranial direct current stimulation (tDCS), respiratory muscle training, and therapy for olfactory dysfunction have not been systematically evaluated. Existing studies are limited by small sample sizes, methodological heterogeneity (e.g., varying rehabilitation protocols), and insufficient exploration of non-physical symptoms such as olfactory dysfunction and fatigue. Moreover, no prior comprehensive meta-analysis has directly compared the efficacy of diverse interventions, leaving clinicians uncertain about optimal treatment hierarchies for long COVID.
We conducted a meta-analysis of RCTs to comprehensively evaluate the effects of several therapeutic interventions on Long COVID. Prior to the formal analysis, we performed a preliminary search and screening to identify interventions with sufficient data for meta-analysis. Consequently, we focused on exercise training, respiratory muscle training, tele-rehabilitation, steroid nasal spray, Palmitoylethanolamide and Luteolin (PEA-LUT), olfactory training, and transcranial direct current stimulation (tDCS). Our goal is to offer evidence-based guidance for personalized treatment of long COVID by clarifying the relative efficacy and symptom-specific benefits of these therapeutic options to support clinical decision-making.
Methods
This meta-analysis was performed within the framework of a systematic review and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines. The study protocol was registered on PROSPERO (CRD42024591704).
Search strategy and selection criteria
A preliminary search conducted on May 1, 2024, revealed that although a wide range of interventions had been reported in the literature, several lacked a sufficient number of randomized controlled trials or did not report outcome measures suitable for meta-analysis. As a result, only selected interventions were included in the formal analysis. Then, a comprehensive systematic literature search was conducted to identify studies on the treatment of long COVID, with the last search performed on July 20, 2025, across five electronic databases: PubMed, Cochrane Library, Embase, Web of Science, SPORTDiscus (EBSCO), CINAHL (EBSCO), and Rehabilitation & Sports medicine source (EBSCO). We also manually searched the reference lists of relevant review articles. After removing duplicate records, two independent reviewers screened the titles and abstracts to identify eligible RCTs. Subsequently, the same reviewers independently assessed the full texts of the selected articles. The inclusion criteria were defined according to the PICO framework: the population included patients diagnosed with Long COVID; interventions comprised exercise training, respiratory muscle training, tele-rehabilitation, pharmacotherapy, olfactory training, and transcranial direct current stimulation (tDCS); comparators included no intervention, standard primary care, or placebo; and outcomes focused on changes or improvements in clinical parameters such as pulmonary function, exercise capacity, fatigue, dyspnea, and olfactory function following treatment. Studies were excluded if they were not randomized controlled trials; were published in languages other than English; if the full text was not accessible; or if the outcome measures were assessed using tools that were not comparable with those used in the majority of included studies, making quantitative synthesis infeasible. Any disagreements regarding study eligibility were resolved through discussion with a senior author.
Data extraction
Two independent reviewers extracted the relevant data from the included studies using a standardized form. The extracted information included the first author, year of publication, country, number of patients, patient characteristics, various assessment scales (MFIS, FSS, SGRQ, SF-36, mMRC, TDI Score, VAS-smell score, and UPSIT), pulmonary function tests, cardiopulmonary exercise test (CPET), exercise capacity, and olfactory recovery rate. In cases of missing data, we contacted the original authors to obtain the raw data whenever possible. If direct retrieval was unsuccessful, values were extracted from figures using Plot Digitizer (https://plotdigitizer.com/). Any discrepancies were resolved through discussion.
Quality assessment
The same two reviewers independently evaluated the risk of bias in the included studies using the Cochrane Collaboration's Risk of Bias Tool. This tool assesses seven domains of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each domain was classified as having a low, unclear, or high risk of bias. Any discrepancies were resolved through discussion.
Primary and secondary outcomes
The primary outcomes of this study include pulmonary function tests (FEV1, FEV1% predicted, FVC, FVC % predicted and FEV1/FVC), CPET parameters (VO2 peak, VO2 peak % predicted, VE, VE/VO2, and RER), respiratory muscle function (maximal inspiratory pressure [MIP], MIP % predicted and MEP), the modified Medical mMRC scale, and exercise capacity assessments (6MWT and 30-s sit-to-stand test [30sSTS]). The mMRC scale evaluates the severity of dyspnea on a scale from 0 to 4. The 6MWT measures the distance a patient can walk on a flat surface within 6 min, providing an assessment of cardiopulmonary function. The 30sSTST requires participants to complete as many sit-to-stand repetitions as possible within 30 s using a 40 cm-high seat without arm support, with the total number of stands recorded.
Secondary outcomes include the Modified Fatigue Impact Scale (MFIS), Fatigue Severity Scale (FSS), St. George's Respiratory Questionnaire (SGRQ), Short Form-12 (SF-12), rate of olfactory recovery, visual analog scale (VAS) smell score, University of Pennsylvania Smell Identification Test (UPSIT-40), and Threshold-Detection-Identification (TDI) score (Table 1).
Table 1.
Table of abbreviation.
| Abbreviation | Full term | Definition |
|---|---|---|
| 6MWT | 6-Minute Walk Test | A clinical test used to assess an individual's cardiorespiratory function and exercise tolerance, in which the subject is instructed to walk as quickly as possible within 6 min, and the distance covered is recorded. |
| 30sSTS | 30-s Sit-to-Stand Test | A clinical functional test designed to assess lower limb muscle strength, balance capacity, and endurance, in which the subject is instructed to perform as many complete sit-to-stand movements as possible within 30 s, with the number of repetitions recorded. |
| mMRC | Modified Medical Research Council Dyspnea Scale | A clinical assessment scale used to evaluate the severity of dyspnea, with higher scores indicating greater severity of breathing difficulty. |
| MBDS | Modified Borg Dyspnea Scale | An assessment scale used to quantify a patient's subjective degree of dyspnea, with higher scores indicating more severe dyspnea. |
| HADS | Hospital Anxiety and Depression Scale | A self-rated screening scale designed to assess symptoms of anxiety and depression, comprising two subscales (anxiety and depression), with higher scores indicating greater severity of symptoms. |
| FSS | Fatigue Severity Scale | A standardized self-assessment instrument designed to evaluate the severity of fatigue and its impact on daily living functioning, with higher scores indicating greater fatigue severity. |
| VAFS | Visual Analogue Fatigue Scale | A unidimensional subjective measurement instrument utilizing a linear scale to quantify fatigue severity in clinical populations, with elevated scores reflecting a higher degree of fatigue intensity. |
| MFIS | Modified Fatigue Impact Scale | MFIS is a validated tool designed to evaluate the multidimensional impact of fatigue on patients' daily living, wherein higher scores indicate a more pronounced adverse effect of fatigue on functional domains of life. |
| SF-12 | 12-Item Short Form Health Survey | A brief rapid-assessment instrument for evaluating health-related quality of life (HRQoL), developed as an abbreviated version of the 36-Item Short Form Health Survey (SF-36), comprising two validated subscales: physical health component (PCS) and mental health component (MCS), with elevated scores indicating superior health status-associated quality of life. |
| VO2 peak | Peak Oxygen Uptake | VO2 peak is operationally defined as the highest measurable oxygen uptake rate achieved during symptom-limited graded exercise testing, serving as a key metric for assessing cardiorespiratory functional capacity and determining critical thresholds of aerobic performance. |
| FVC | Forced Vital Capacity | FVC is defined as the maximum volume of air that can be forcibly exhaled from the lungs after a maximal inhalation, performed with the greatest effort and speed which is utilized to evaluate the effects of pulmonary diseases on respiratory function. |
| FEV1 | Forced Expiratory Volume in the first second | FEV1 is defined as the maximum volume of air an individual can exhale within the first second following a deep inhalation, achieved through maximal forced and rapid exhalation effort. |
| FEV1/FVC | Forced Expiratory Volume in the first second to Forced Vital Capacity Ratio | FEV1/FVC is a quantitative assessment parameter for the degree of airflow limitation, primarily utilized in the clinical diagnosis of obstructive pulmonary diseases |
| VE | Minute Ventilation | Minute Ventilation is defined as the total volume of air inhaled or exhaled per minute by an individual, measured at rest or during physical activity. |
| VE/VO2 | Ventilatory Equivalent for Oxygen | VE/VO2 is defined as the ratio of minute ventilation to oxygen uptake, which assesses the ventilatory efficiency of oxygen utilization in individuals during exercise or at rest. |
| RER | Respiratory Exchange Ratio | RER is defined as the ratio of carbon dioxide output to oxygen uptake, utilized to assess the proportional contribution of carbohydrates and lipids to energy metabolism during substrate utilization. |
| MIP | Maximum Inspiratory Pressure | MIP is defined as the peak negative pressure generated within the oral cavity or airway during a maximal inspiratory effort, which serves to evaluate the contractile strength of respiratory muscles, with specific emphasis on diaphragmatic function. |
| MEP | Maximum Expiratory Pressure | MEP is defined as the peak positive pressure generated within the oral cavity or airway during a maximal expiratory effort, which serves to evaluate the contractile strength of expiratory muscles, including the abdominal muscles and internal intercostal muscles. |
| UPSIT | University of Pennsylvania Smell Identification Test | The UPSIT is a standardized and versatile olfactory function assessment tool, primarily employed to detect hyposmia or anosmia. A score below 25 points on this test indicates clinically significant olfactory dysfunction. |
| VAS-smell score | Visual Analog Scale for Smell Score | The VAS-smell score is a patient-reported instrument designed to subjectively assess olfactory function, where higher scores indicate more severe olfactory dysfunction. |
| TDI Score | threshold-Discrimination-Identification Score | The TDI Score is a standardized composite tool for the comprehensive assessment of olfactory function, wherein a total score ≤15 points is indicative of severe olfactory loss. |
Statistical analysis
All outcomes were treated as continuous variables and measured using consistent scales across studies; therefore, the mean difference (MD) was used as the effect size. The inverse variance method was employed to pool the results, which were reported as MDs with 95% confidence intervals (CIs). Considering potential heterogeneity, a random-effects model was applied for all analyses. Heterogeneity was assessed using the I2 statistic, with the following thresholds for interpretation: 0%–40% might not be important; 30%–60% may represent moderate heterogeneity; 50%–90% may represent substantial heterogeneity; and 75%–100% indicates considerable heterogeneity.19 Sensitivity analysis using the leave-one-out method was conducted to evaluate the robustness of the pooled estimates. In addition, given the potentially small number of studies included in all comparisons, fixed-effects models were also applied to assess the consistency of the results. Publication bias was evaluated using Egger's test, and for outcomes with ten or more included studies, funnel plots were generated for visual inspection of asymmetry. The quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, and the overall certainty across the five GRADE domains was categorized as high, moderate, low, or very low.20 All statistical analyses were performed using Review Manager (version 5.4.1) and Stata (version 17).
Role of funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Results
Literature search
Fig. 1 shows the flow chart of literature search and study selection. After removing duplicate articles, database searching reported 17,949 articles among different databases. After screening for title and abstract and removing duplicates, 137 studies were assessed for eligibility. Then, 137 studies were reviewed by full-text and 86 studies were removed based on the inclusion criteria. Eventually, 51 RCTs21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50,51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 with a total of 4026 patients were included for final analyses (Fig. 1).
Fig. 1.
Search and selection of studies for inclusion.
Baseline characteristics
The characteristics of the included studies are shown in Table 2. A total of twenty-five studies on exercise training programs, eight studies on respiratory muscle training, nine studies on telerehabilitation, two studies on olfactory training, two studies on tDCS, two studies on steroid nasal spray, and five studies on PEA-LUT were included in our study. Two studies were categorized under two different interventions. One was Demir 2025,71 a three-arm trial comparing exercise training, rehabilitation, and no intervention. The other was Mila 2024,37 which compared respiratory muscle training combined with olfactory training versus no intervention. When this study was classified under respiratory muscle training, only respiratory function–related outcomes were analyzed; when classified under olfactory training, only smell-related outcomes—specifically the UPSIT—were considered. Most exercise-training programs included aerobic and strength training, with a few incorporating respiratory muscle training (RMT) or additional health education. In our analysis, exercise training focuses on overall fitness, while RMT targets respiratory muscles using specialized devices and was treated as a separate intervention. Although two studies combined RMT with exercise, we classified them under the “respiratory muscle training” group, as the core objective was to improve respiratory muscle function, ensuring consistency in the classification. Olfactory-related treatments included olfactory training, cortisol spray and PEA-LUT. The same intervention, PEA 700 mg and Luteolin 70 mg per day, was used in all five RCTS on PEA-LUT. However, the two RCTs of cortisol nasal spray were slightly different, one was given once every day and the other was given once every two days, with a dose of 100 μg. Both tDCS included in the studies revolved around the effects of the technique on relieving fatigue. Telerehabilitation-related studies were classified into two categories: those in which telerehabilitation was combined with a specific intervention (e.g., exercise training), and those comparing the same intervention delivered via telerehabilitation versus non-telerehabilitation methods. Studies in the first category were grouped under the corresponding specific intervention, while those in the second category were classified as telerehabilitation. The follow-up period of the studies ranged from 14 to 180 days, with most focusing on 4–12 weeks. The 8-week and 6-week studies were the most common, with 12 and 8 studies, respectively.
Table 2.
Characteristic of included studies.
| Study | Location | Patient characteristics | Mean age (SD),years |
Sex (Female) (n, %) |
Treatment |
Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Exercise training: physical training programs aimed at improving physical fitness, cardiopulmonary endurance, or muscle strength, including aerobic exercise, resistance training, or a combination of both. | |||||||||
| 1.Bai 2024 | China | Long-COVID symptoms for more than 2 months after the diagnosis of COVID-19 | 46.85 (15.26) | 43.42 (14.96) | 4 (33.3%) | 10 (83.3%) | 4-week exercise plan consisting of 12 times aerobic training | follow the guideline-based recommendations for a healthy lifestyle and WHO guideline | 4 weeks |
| 2.Barz 2024 | Germany | fatigue symptoms for more than 3 months after mild to moderate course of COVID-19 | 53.5 (11.9) | 53.5 (12.3) | NA | NA | resistance and aerobic training 1–3 times per week for 8 weeks | NA | 8 weeks |
| 3.Besnier 2024 | Canada | dyspnea and/or fatigue symptoms for more than 3 months after the diagnosis of COVID-19 | 53.89 (12.13) | 52.53 (11.29) | 13 (72%) | 11 (65%) | resistance and aerobic training 3 times per week for 8 weeks | maintained their daily habits | 8 weeks |
| 4.Calvo-Paniagua 2024 | Spain | moderate respiratory and/or functional impairments after the acute SARS-CoV-2 infection | 50.8 (8.4) | 49.4 (10.0) | 18 (56.3%) | 22 (68.8%) | a comprehensive program (consisting of sanitary education sessions, respiratory therapy, aerobic exercise, active mobilizations and motor control exercises) in alternate days for 7 weeks | conventional medical care recommendations | 3 month |
| 5.Demir 2025 | Turkey | patients diagnosed with COVID-19 | 49.5 (12.8) | 38.33 (14.83) | 9 (75%) | 10 (83.3%) | supervised exercises programme 3 times per week for 6 weeks | the same exercises programme without supervision | 6 weeks |
| 6.de Araujo Furtado 2023 | Brazil | recovery from COVID-19 for more than 20 days | 47.50 (12) | 49.20 (13) | 8 (50%) | 10 (62.5%) | An 8-week exercise plan consisting of 24 times exercise training and remote lectures (each 15 days) on health education | remote lectures for 8 weeks (each 15 days) on health education | 8 weeks |
| 7.Elyazed 2024 | Egypt | fatigue, dyspnea, and exercise intolerance symptoms more than 1 month after the diagnosis of COVID-19 | 56.9 (6.7) | 55.5 (7.1) | 14 (46.7%) | 13 (43.3%) | exercise program and usual medical care for 12 weeks | usual medical care for 12 weeks | 12 weeks |
| 8.Ibrahim 2023 | Saudi Arabia | Long-COVID symptoms | 62.55 (4.57) | 62.7 (4.3) | 25 (52%) | 16 (66.7%) | aerobic exercises 4 times per week for 10 weeks | medical care and advice | 10 weeks |
| 9.Kaczmarczyk 2024 | Poland | one or more of the post-COVID signs and symptoms after the diagnosis of COVID-19 | 67.1 (5.6) | 74.2 (7.2) | 11 (42.3%) | 12 (60%) | resistive training 2 times per week for 8 weeks | NA | 8 weeks |
| 10.Kaddoussi 2024 | Tunisia | dyspnea symptom for three months after the diagnosis of COVID-19 | 53 (14) | 52 (14) | 10 (50%) | 6 (60%) | an exercise training 3 times per week for 6 weeks | maintain their usual level of sedentary physical activities | 6 weeks |
| 11.Kerling 2024 | Germany | a continuing impairment of physical or mental health after the diagnosis of COVID-19 | 47.1 (12.5) | 46.9 (10.1) | 22 (73.3%) | 20 (62.5%) | an exercise plan 150 min per week for 3 months | continue with their current lifestyle and everyday activities | 3 months |
| 12.Keskin 2023 | Turkey | Being diagnosed with COVID-19 | 38.65 (11.56) | 36.36 (10.97) | 15 (39.47%) | 15 (39.47) | an exercise programme 3 days per week for 6 weeks | NA | 6 weeks |
| 13.Lai 2024 | Taiwan | Persistent Long-COVID symptoms after the diagnosis of COVID-19 | 38.9 (11.1) | 40.8 (14.0) | 37 (41%) | 33 (36%) | A telerehabilitation training programme 3 times per week for 12 weeks | maintain their usual lifestyles | 12 weeks |
| 14.Li 2022 | China | mMRC dyspnea score of 2–3 after the diagnosis of COVID-19 | 49.17 (10.75) | 52.03 (11.10) | 32 (54.2%) | 34 (56.7%) | An exercise programme 3–4 times per week for 6 weeks | short educational instructions | 6 weeks |
| 15.Longobardi 2023 | Brazil | Being diagnosed with COVID-19 | 60.8 (7.1) | 61.2 (7.7) | 13 (52%) | 12 (48%) | a home-based exercise training programme 3 times per week for 16 weeks | standard of care | 16 weeks |
| 16.McGregor 2024 | UK | ongoing substantial covid-19 related physical and/or mental health sequelae after the diagnosis of COVID-19 | 56.1 (12.1) | 56.2 (12.3) | 162 (54%) | 143 (50%) | A rehabilitation programme for 8 weeks | best practice usual care | 8 weeks |
| 17.Oliveira 2023 | Brazil | Long-COVID symptoms after the diagnosis of COVID-19 | 53.74 (11.21) | 50.75 (10.14) | 17 (54.8%) | 17 (60.7%) | Multicomponent exercise 2 times per week for 12 weeks | educational orientation and performed activities of daily living | 12 weeks |
| 18.Paneroni 2024 | Italy | unable to walk >70% of the predicted distance during a 6MWT after a diagnosis of COVID-19 | 66.7 (10.2) | 67.6 (10.6) | 14 (35%) | 8 (20%) | home-based exercise program and regular nurse teleconsultation 6 times per week for 4 weeks | a remote teleconsultation from nursing staff | 4 weeks |
| 19.Pleguezuelos 2023 | Spain | Long-COVID symptoms after the diagnosis of COVID-19 | 54.6 (11.7) | 54.5 (10.9) | 28 (21.4%) | 28 (21.4%) | a telerehabilitation program combined with aerobic and strength exercises 3 times per week for 15 weeks | no telerehabilitation program and carrying out their routine daily life activities. | 15 weeks |
| 20.Pleguezuelos 2024 | Spain | post-COVID sequelae more than 3 months after the diagnosis of COVID-19 | 65.0 (5.2) | 64.3 (5.0) | 15 (14.2%) | 26 (24.5%) | a telerehabilitation program combined with aerobic and strength exercises 3 times per week for 12 weeks | no telerehabilitation programme and carrying out their routine activities of daily living. | 12 weeks |
| 21.Rodariguez-Blanco 2022 | Spain | Being diagnosed with COVID-19 | 34.81 (11.82) | 42.36 (11.84) | 12 (46.2%) | 12 (54.5%) | Strength exercise program once a day for 14 days | NA | 14 days |
| 22.Rodariguez-Blanco 2023 | Spain | COVID-19 symptom more than 40 days after the diagnosis of COVID-19 | 38.75 (15.40) | 42.58 (11.40) | 13 (27.08%) | 13 (27.08%) | therapeutic exercise telerehabilitation protocol for 14 days | relative rest at home | 14 days |
| 23.Romanet 2023 | France | Dyspnea symptom after a diagnosis of COVID-19 | 57 (14.28) | 59 (9.94) | 11 (40%) | 12 (36%) | exercise training rehabilitation 2 times per week for 10 weeks | standard physiotherapy | 10 weeks |
| 24.Senen 2024 | Spain | Long-COVID symptoms after the diagnosis of COVID-19 | 48.83 (7.0) | 45.17 (6.9) | 14 (77%) | 13 (69%) | a therapeutic physical exercise program for 8 weeks | recommendations on physical exercise and healthy habits based on recommendations for the general population | 8 weeks |
| 25.Sick 2025 | Austria | a laboratory-confirmed SARS coV-2 infection at more than 12 weeks |
41.6 (14.7) | 40.3 (10.8) | 10 (71.4%) | 11 (78.6%) | resistance and endurance exercise 3 times per week for 12 weeks | Maintaining current physical activities without an exercise programme | 12 weeks |
| Respiratory muscle training: targeted interventions aimed at strengthening the respiratory muscles, especially the diaphragm and inspiratory muscles. These programs may include incentive spirometry, diaphragmatic breathing, inspiratory muscle training (IMT), or comprehensive cardiopulmonary rehabilitation. Training may be conducted with or without resistance, and often includes structured frequency and duration. | |||||||||
| 1.Abo Elyazed 2024 | Egypt | presented easy fatiguability and/or shortness of breath and/or cough after mild-to-moderate COVID-19 | 39.25 (4.43) | 40.4 (5.4) | 16 (40%) | 10 (50%) | incentive spirometry, diaphragmatic breathing and standard care for 8 weeks | Standard care | 8 weeks |
| 2.del Corrala 2023 | Spain | fatigue and dyspnea for at least 3 months after the COVID-19 diagnosis | 47.7 (8.95) | 45.15 (11.4) | 33 (75%) | 30 (68.2%) | a home-based respiratory muscle training programme, 40 min/day, split into two 20-min sessions (morning and afternoon), 6 times per week, for 8 weeks. | Sham respiratory muscle training (without resistance) | 8 weeks |
| 3.Gomes Dos Santos 2024 | Brazil | respiratory or/and functional symptoms after the diagnosis of COVID-19 | 50.76 (11.28) | 44 (11.28) | 10 (58.82%) | 10 (62.5%) | cardiopulmonary rehabilitation (respiratory, aerobic, and resistance muscle training) for 6 weeks | remote lectures on health education for 6 weeks | 6 weeks |
| 4.McNarry 2022 | UK | dyspnea symptoms after the diagnosis of COVID-19 | 46.76 (12.03) | 46.13 (12.73) | 95 (86%) | 35 (95%) | inspiratory muscle training for 8 weeks | usual care | 8 weeks |
| 5.Mila 2024 | Spain | Long-COVID symptoms (dyspnea, loss of smell and taste) after the diagnosis of COVID-19 | 23 (14) | 22 (13) | 59 (59%) | 42 (42%) | A rehabilitation programme (including inspiratory muscle training and aerobic exercise) 2–3 times per week for 31 days | usual care | 31 days |
| 6.Pietranis 2024 | Poland | systemic post-COVID-19 complications or dyspnea after the diagnosis of COVID-19 | 65.41 (11.23) | 57.90 (16.02) | 67 (26%) | 60 (13%) | Respiratory muscle training and exercise training for 6 weeks | aerobic exercise and sham respiratory muscle training (without resistance) | 6 weeks |
| 7.Sari 2022 | Turkey | pulmonary involvement after the diagnosis of COVID-19 | 53.5 (5.39) | 59 (7.63) | NA | NA | Breathing exercise, resistance training and inspiratory muscle training for 6 weeks | Breathing exercise and resistance training | 6 weeks |
| 8.Spiesshoefer 2024 | Germany | persistent exertional dyspnea with diaphragm muscle weakness after the diagnosis of COVID-19 | 59.6 (14.08) | 60 (20.28) | 3 (33.3%) | 4 (4.44%) | inspiratory muscle training for 6 weeks | Sham inspiratory muscle training (without resistance) | 6 weeks |
| Telerehabilitation: delivery of rehabilitation training, guidance, and supervision to patients remotely through online platforms, mobile applications, video conferencing, or telephone. The interventions typically include multimodal exercise, respiratory training, and health education. | |||||||||
| 1.Carpallo-porcar 2023 | Spain | Long-COVID symptoms after the diagnosis of COVID-19 | 58.00 (2.00) | 59.00 (2.00) | 11 (55.00) | 9 (45.00) | multimodal program via a telerehabilitation platform accessible through a website or through a mobile app for 12 weeks | the same multimodal program but through a home rehabilitation paper explanatory booklet |
12 weeks |
| 2.da silva 2023 | Brazil | after the diagnosis of COVID-19 | 57 (9.0) | 54.0 (13.0) | 10 (35.7%) | 13 (44.8%) | a physical therapy session and some sessions were supervised by the physical therapist via video-conference for 8 weeks |
The same physical therapy session via video-conference but without supervision | 8 weeks |
| 3.Demir 2025 | Turkey | patients diagnosed with COVID-19 | 49.5 (12.8) | 38.33 (14.83) | 9 (75%) | 10 (83.3%) | supervised exercises programme 3 times per week for 6 weeks | the same exercises programme without supervision | 6 weeks |
| 4.Jorge 2025 | Brazil | long-COVID symptoms for more than 12 weeks after the diagnosis of COVID-19 | 49.2 (18.6) | 43.2 (15.41) | 11 (55%) | 16 (84.2%) | supervised exercises programme 2 times per week for 8 weeks | a guidebook containing home exercises and health care instructions without supervision |
8 weeks |
| 5.Okan 2022 | Turkey | Dyspnea symptom after the diagnosis of COVID-19 | 48.85 (10.85) | 52.19 (14.84) | 11 (42.3%) | 14 (53.8%) | breathing exercises under the supervision of the researchers for 5 weeks | a brochure explaining breathing control, pursed lip breathing, and diaphragmatic breathing exercise. | 5 weeks |
| 6.Pehlivan 2022 | Turkey | diagnosed with COVID-19 and discharged after treatment, still in the first 4 weeks after discharging, | 53.88 (13.92) | 42.15 (13.36) | 3 (18%) | 6 (35%) | an exercise program 3 times per week for 6 weeks with the supervision of the physiotherapist in all exercise. | one session of exercise training and a brochure including similar exercises as the intervention group by smartphone without supervision of the physiotherapist | 6 weeks |
| 7.Sahin 2023 | Turkey | being diagnosed with COVID-19 | 57.67 (8.42) | 63.67 (7.90) | 8 (38%) | 6 (28.6%) | a home-based pulmonary rehabilitation programme for 8 weeks with phone calls from a physiotherapist once a week. | The same home-based pulmonary rehabilitation programme without phone calls from a physiotherapist | 8 weeks |
| 8.Samper 2023 | Spain | Persistent long-COVID symptoms more than 12 weeks after the diagnosis of COVID-19 | 48.25 (10.36) | 48.31 (8.01) | 44 (84.5%) | 48 (68.75%) | the treatment as usual methods established by their general practitioner via Recovery APP for 3 months | the same treatment without using Recovery APP | 3 months |
| 9.Sarmento 2024 | Canada | mild to severe persistent respiratory symptoms more than 3 months after confirmed or suspected COVID-19 infection | 50 (9) | 49 (9) | 7 (87%) | 5 (83) | an exercise program (aerobic, strengthening, and breathing exercises) three times per week for 8 weeks led by a physiotherapist via video conference | The same exercise program following a pre-recorded video | 8 weeks |
| Transcranial direct current stimulation (tDCS): non-invasive brain stimulation technique that delivers a low-intensity direct current via electrodes placed on the scalp to modulate cortical excitability. It is used to target specific brain regions for therapeutic purposes. | |||||||||
| 1.Oliver-Mas 2023 | Spain | Symptoms of fatigue and Diagnosis of COVID-19 with positive RT-PCR results at least 6 months before | 47.26 (9.05) | 44.12 (9.83) | 15 (65.21%) | 22 (91.66%) | anodal transcranial direct current stimulation (2 mA, 20 min/time) on the left dorsolateral prefrontal cortex 4 times per week for 2 weeks | electrodes were placed in the same regions without the current during the 20 min session. | 2 weeks |
| 2.Santana 2023 | Brazil | diagnosis of PASC-related fatigue and three to 12 months after acute confirmed SARS-CoV-2 infection | 51.63 (15.87) | 54.46 (19.01) | 24 (69%) | 21 (60%) | high-definition transcranial direct current stimulation (3 mA, 30 min/time) targeting the left primary motor cortex program. 2 times per week for 5 weeks. | the device targeting the same place provided a 30-s ramp-up period to the full 3 mA, followed immediately by a 30-s ramp down. | 5 weeks |
| Olfactory training: repeated exposure to a set of specific odors over a defined period to stimulate and potentially restore olfactory function. | |||||||||
| 1.Berube 2023 | Canada | Olfactory dysfunction after the diagnosis of COVID-19 | 44.9 (7.4) | 44.5 (10.1) | 16 (64%) | 17 (68%) | Patients exposed themselves to four odors (floral, fruity, aromatic resinous) and 2 time per day for 12 weeks | Patients were asked to sniff four glass vials that were identical in appearance to the ones distributed to the intervention group, but odorants were odorless propylene glycol | 12 weeks |
| 2.Mila 2024 | Spain | Long-COVID symptoms (dyspnea, loss of smell and taste) after the diagnosis of COVID-19 | 23 (14) | 22 (13) | 59 (59%) | 42 (42%) | A rehabilitation programme (including inspiratory muscle training and aerobic exercise) 2–3 times per week for 31 days | usual care | 31 days |
| Steroid nasal spray: Use of intranasal corticosteroid sprays, such as mometasone furoate, often combined with olfactory training, aimed at reducing nasal inflammation and improving olfactory function. | |||||||||
| 1.Abdelalim 2021 | Egypt | sudden recent anosmia or hyposmia after the diagnosis of COVID-19 | 28.83 (13.36) | 27.5 (5.72) | 26 (52%) | 28 (56%) | mometasone furoate nasal spray once daily (100 μg) in each nostril for 3 weeks and olfactory training | only olfactory training | 3 weeks |
| 2.Kasiri 2021 | Iran | olfactory dysfunction for two weeks and after the diagnosis of COVID-19 | 35.4 (9) | 33.2 (8.5) | 19 (48.7%) | 19 (50%) | mometasone furoate nasal spray twice daily (100 μg) in each nostril for 4 weeks and olfactory training | saline spray in each nostril twice daily and olfactory training. | 4 weeks |
| Palmitoylethanolamide and Luteolin (PEA-LUT): use of ultramicronized Palmitoylethanolamide combined with Luteolin (PEA-LUT) as an oral supplement, often combined with olfactory training, aimed at reducing neuroinflammation and supporting olfactory recovery. | |||||||||
| 1.Cantone 2024 | Italy | smell disturbances | 44.8 (11.81) | 52.1 (11.8) | 11 (65%) | 13 (57%) | ultramicronized Palmitoylethanolamide and Luteolin once daily (Glialia 700 + 70; Epitech) and olfactory training for 180 days | only olfactory training | 180 days |
| 2.D'Ascanio 2021 | Italy | post-infection olfactory impairment that persisted ≥90 days after SARS-CoV-2 negative testing | NA | NA | 5 (71.4%) | 3 (50%) | weekly olfactory rehabilitation plus daily oral supplement with PEA and Luteolin for 30 days | two times a day olfactory rehabilitation alone | 30 days |
| 3.Di Stadio 2022 | Italy | olfactory disturbances after SARS-CoV-2 infections | 36.7 (11.8) | 50.5 (12.7) | 49 (52%) | 21 (58%) | daily treatment with co-ultra-micronized PEA 700 mg and Luteolin 70 mg and olfactory training for 90 days | a placebo supplement therapy and olfactory training | 90 days |
| 4.Di Stadio 2023 (1) | Italy | prior COVID-19 and persistent olfactory impairment more than 6 months after follow-up SARS-CoV-2 negative testing | 42.1 (14.5) | 47 (14.6) | 83 (63.8%) | 38 (69%) | daily co-ultra-micronized PEA 700 mg and Luteolin 70 mg and olfactory training for 90 days | daily treatment with placebo and olfactory training | 90 days |
| 5.Di Stadio 2023 (2) | Italy | presence of persistent anosmia or persistent hyposmia after the diagnosis of COVID-19 | 42.7 (13.5) | 40.9 (11.7) | 40 (71.4%) | 26 (68.4%) | Daily co-ultra-micronized Palmitoylethanolamide 700 mg and Luteolin 70 mg and olfactory training for 90 days | daily placebo supplement therapy and olfactory training | 90 days |
6MWT, 6-Minute Walk Test; mMRC, Modified Medical Research Council Dyspnea Scale; NA, not addressed; PASC, Post-Acute Sequelae of SARS-CoV-2 infection; PEA-LUT, Palmitoylethanolamide and Luteolin; SD, standard deviations; tDCS, Transcranial direct current stimulation.
Risk of bias
The risk of bias of RCTs ranged from low to high, with 3 studies with low risk of bias, 12 with some concerns, and 33 with high risk. Lack of blinding or unclear description of blinding caused more bias. In more than half of the studies, there was a loss to follow-up rate exceeding 10%, which was the main source of risk of bias and affected up to 28 articles. The problem with randomization and blinding is primarily that the methods of implementation were not described in detail in the literature, so we do not know with certainty whether randomization and blinding were actually performed. Selective outcome reporting was the domain with better scores (eFigure 2).
Efficiency of treatment in long COVID
Exercise training
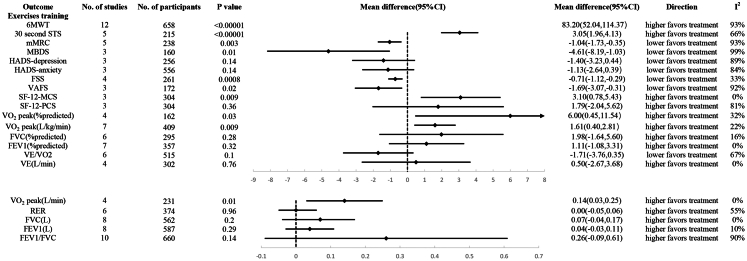
Compared to the control group, the exercise training group demonstrated statistically significant better performance in the 6MWT (MD, 83.20; 95% CI 52.04–114.37), 30sSTS (MD, 3.05; 95% CI 1.96–4.13), SF-12-MCS (MD, 3.10; 95% CI 0.78–5.43), VO2 peak (% predicted) (MD, 6.00; 95% CI 0.45–11.54), VO2 peak (L/kg/min) (MD, 1.65; 95% CI 0.30–3.01), and VO2 peak (L/min) (MD, 0.14; 95% CI 0.03–0.25). Additionally, the exercise training group had statistically significantly lower scores in mMRC (MD, −1.04; 95% CI −1.73 to −0.35), FSS (MD, −0.71; 95% CI −1.12 to −0.29), MBDS (MD, −4.61; 95% CI −8.19 to −1.03), and VAFS (MD −1.69; 95% CI −3.07 to −0.31). However, there was no statistically significant difference between the exercise training group and the control group in terms of HADS-depression, HADS-anxiety, SF-12-PCS, FVC (% predicted), FEV1 (L), FEV1 (% predicted), VE/VO2, VE (L/min), RER, FVC (L), and FEV1/FVC (Fig. 2 and eFigure 3).
Fig. 2.
Efficiency of exercise training in long COVID. (6MWT, 6-Minute Walk Test; STS, Sit-to-Stand Test; mMRC, Modified Medical Research Council Dyspnea Scale; MBDS, Modified Borg Dyspnea Scale; HADS, Hospital Anxiety and Depression Scale; FSS, Fatigue Severity Scale; VAFS, Visual Analogue Fatigue Scale; SF-12-MCS, 12-Item Short Form Health Survey-mental health component; SF-12-PCS, 12-Item Short Form Health Survey-physical health component; VO2 peak, Peak Oxygen Uptake; FVC, Forced Vital Capacity; VE, Minute Ventilation; FEV1, Forced Expiratory Volume in the first second; RER, Respiratory Exchange Ratio).
Respiratory muscle training
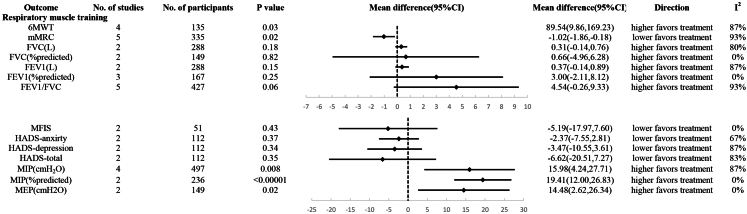
Compared to the control group, the respiratory muscle training group demonstrated statistically significant better performance in the 6MWT (MD, 89.54; 95% CI 9.86–169.23), MIP (% predicted) (MD, 15.79; 95% CI 2.73–28.84), and MIP (cm H2O) (MD, 19.69; 95% CI 10.14–29.24). Additionally, the respiratory muscle training group had statistically significantly lower scores in mMRC (MD, −1.02; 95% CI −1.86 to −0.18). However, there was no statistically significant difference between the respiratory muscle training group and the control group in terms of FVC (L), FVC predicted %, FEV1 (L), FEV1 predicted %, FEV1/FVC, MFIS, HADS-anxiety, HADS-depression, HADS-total, and MEP (cm H2O) (Fig. 3 and eFigure 3).
Fig. 3.
Efficiency of Respiratory muscle training in long COVID. (6MWT, 6-Minute Walk Test; mMRC, Modified Medical Research Council Dyspnea Scale; FVC, Forced Vital Capacity; FEV1, Forced Expiratory Volume in the first second; MFIS, Modified Fatigue Impact Scale; HADS, Hospital Anxiety and Depression Scale; MIP, Maximum Inspiratory Pressure; MEP, Maximum Expiratory Pressure).
Telerehabilitation
Compared to the control group, the telerehabilitation group demonstrated statistically significant better performance in the 6MWT (MD, 34.14; 95% CI 2.54–65.74) and 30sSTS (MD, 1.41; 95% CI 0.67–2.15). Additionally, the telerehabilitation group had statistically significantly lower scores in FSS (MD, −1.59; 95% CI −2.64 to −0.53). However, there was no statistically significant difference between the telerehabilitation group and the control group in terms of 6MWT, FEV1 (% predicted), FVC (% predicted), and FEV1/FVC (Fig. 4 and eFigure 3).
Fig. 4.
Efficiency of Telerehabilitation, Transcranial direct current stimulation (tDCS), Olfactory training, Steroid sprays, Percutaneous electrical acupuncture-lumbar traction (PEA-LUT) in long COVID. (6MWT, 6-Minute Walk Test; mMRC, Modified Medical Research Council Dyspnea Scale; FEV1, Forced Expiratory Volume in the first second; FVC, Forced Vital Capacity; STS, Sit-to-Stand Test; FSS, Fatigue Severity Scale; tDCS, Transcranial direct current stimulation; MFIS, Modified Fatigue Impact Scale; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analogue scale; PEA-LUT, Palmitoylethanolamide and Luteolin; TDI, threshold-Discrimination-Identification).
tDCS
tDCS showed a statistically significant lower score in MFIS-physical compared to the control group (MD, −2.29; 95% CI −4.36 to −0.22). However, there was no statistically significant difference between tDCS and the control group in terms of MFIS-cognitive, MFIS-psychosocial, and MFIS-total (Fig. 4 and eFigure 3).
Olfactory training
Two studies investigated the effect of olfactory training on long-COVID, and the results found that there was no statistically significant difference between the olfactory training and control groups in UPSIT (MD, 3.73; 95% CI −1.75 to 9.21) (Fig. 4 and eFigure 3).
Steroid sprays
Two studies investigated the effect of steroid sprays on smell recovery, and the results found that there was no statistically significant difference between steroid sprays and the control group in VAS-smell score at one week (MD, 0.45; 95% CI −1.15 to 2.04), two weeks (MD, 0.89; 95% CI −1.78 to 3.57), and three weeks (MD, 0.35; 95% CI −1.45 to 2.14) (Fig. 4 and eFigure 3).
PEA-LUT
Five studies investigated the effect of PEA-LUT on long-COVID, and the results found that PEA-LUT showed a statistically significant higher score in TDI Score compared to the control group (MD, 4.66; 95% CI 2.16–7.15) (Fig. 4 and eFigure 3).
Sensitivity analysis
We performed a sensitivity analysis on all outcomes using the leave-one-out method. The results indicated that only some outcomes were robust. Specifically, thirty-one outcomes exhibited changes in statistical significance—either shifting from significance to non-significance or vice versa—when a single study was excluded (eTable 1).
We also conducted analyses using a fixed-effects model for all outcomes. In the exercise training group, HADS-depression, HADS-anxiety, and SF-12-PCS all showed statistically significant differences, favoring exercise training. In the respiratory muscle training group, FVC (L), FEV1 (L), and FEV1/FVC also showed statistically significant differences, all supporting respiratory muscle training. In the telerehabilitation group, mMRC showed a statistically significant difference, favoring the control group. In the tDCS group, MFIS-cognitive, MFIS-psychosocial, and MFIS-total all showed statistically significant differences, supporting tDCS. In the olfactory training group, UPSIT also showed a statistically significant difference, favoring olfactory training (eFigure 4). However, it should be noted that the fixed-effects model is more aggressive, while the results from the random-effects model are more conservative.
Publication bias
We performed Egger's test for all outcomes, and the results indicated that four outcomes—focusing on 6MWT (p = 0.013), VO2 peak (L/kg/min) (p = 0.016) and VE/VO2 (p < 0.001) in the exercise training group, and FEV1/FVC (p = 0.010) in telerehabilitation —may be at potential risk of publication bias. Additionally, we generated funnel plots for 6MWT and FEV1/FVC in the exercise training group. The funnel plots for both outcomes showed asymmetry around the vertical axis, with a predominant left-side deficiency (indicating the absence of negative results), suggesting a significant likelihood of publication bias. However, Egger's test for FEV1/FVC (p = 0.056) did not detect publication bias (eFigure 5).
GRADE assessment
The GRADE assessments indicated the following quality ratings for all outcomes: 27% of the evidence was rated as very low, 57% as low, 11% as moderate, and 5% as high. Only two outcomes—MFIS-physical in tDCS and MIP in respiratory muscle training (% predicted)—received a high-quality rating (eTable 2).
Discussion
As mentioned earlier, the improvement in cardiopulmonary function and exercise capacity through exercise training is one of the most frequently discussed outcomes. Our results indicated that exercise training led to improvements in cardiopulmonary function and exercise capacity. Our investigation demonstrated thematic alignment with prior meta-analyses in focusing on rehabilitative interventions for Long COVID, prioritizing improvement in core symptomatology including cardiopulmonary function, exercise capacity, fatigue, and dyspnea. The findings corroborated existing consensus regarding exercise training's significant efficacy in enhancing exercise capacity and cardiopulmonary parameters in post-COVID patients.10,12,15 However, divergent conclusions emerge concerning psychological manifestations such as fatigue and anxiety/depression, potentially attributable to heterogeneity in assessment methodologies across studies.10, 11, 12,15 This discrepancy underscores the need for standardized evaluation protocols when investigating neuropsychiatric sequelae of Long COVID. Notably, fatigue is increasingly recognized as a multidimensional symptom with both physical and psychological components, and its complexity warrants more detailed discussion and standardized evaluation in Long COVID research.72 Overall, exercise training appears to be a reasonable option for many patients with long COVID, but there are still limitations to consider. For instance, the RCTs included in the analysis employed widely varied exercise programs, incorporating different types of aerobic and anaerobic exercises, as well as varying rehabilitation periods and frequencies. Future research may focus on identifying the optimal training program for these patients. However, it is important to recognize that a subset of Long COVID patients may experience post-exertional malaise (PEM), a condition characterized by the worsening of symptoms following physical or mental exertion. In such cases, exercise interventions may pose a risk of symptom exacerbation rather than improvement. Therefore, clinicians should carefully assess patients' baseline fatigue patterns and tolerance before recommending exercise-based rehabilitation. Individualized programs with gradual progression and close monitoring are essential to minimize potential harm.
Respiratory muscle training is a form of exercise designed to enhance respiratory muscle strength and endurance. Several factors contribute to its potential as an effective treatment option. First, the virus itself can damage respiratory muscles and lung tissue.73,74 Second, prolonged bed rest or mechanical ventilation may lead to muscle atrophy due to reduced usage.75,76 Third, compromised respiratory muscle and lung function can limit exercise capacity in the post-acute phase, complicating rehabilitation efforts.49 Our study demonstrated the effectiveness of respiratory muscle training in improving respiratory muscle function. However, there was no beneficial effect of respiratory muscle training on lung function, with a previous meta-analysis on the effect of respiratory muscle training showing the same results as ours.77 This discrepancy underscores the need for additional studies to further validate its effectiveness.
tDCS is a non-invasive therapeutic technique that works by modulating cortical excitability and neuronal activity through the application of a direct current to targeted brain regions.78 Previous studies have suggested that anodal tDCS, when applied to the primary motor and/or sensory cortex (M1/S1) and the left dorsolateral prefrontal cortex (DLPFC), may help alleviate fatigue from various causes.79,80 Our meta-analysis results indicated that tDCS had a positive effect on physical fatigue, although the change in the total MFIS score was not statistically significant. Due to its non-invasive nature and ease of use, tDCS may emerge as a promising treatment option in the future. However, further research is needed to firmly establish its efficacy before it can be widely implemented in clinical settings.
Olfactory dysfunction is a prevalent symptom of long-COVID, with a European study reporting that over 80% of COVID-19 patients experience this condition.81 Our study examined three interventions for olfactory dysfunction: olfactory training, topical corticosteroid sprays, and a combination of ultramicronized palmitoylethanolamide (PEA) and luteolin (LUT) (PEA-LUT). To the best of our knowledge, no previous meta-analysis has specifically addressed olfactory dysfunction in the context of long-COVID, making our study the first to evaluate the effectiveness of these treatment modalities. Our findings indicated that both olfactory training and PEA-LUT were effective in improving olfactory function, while corticosteroids did not demonstrate a significant benefit. Olfactory dysfunction is thought to result from olfactory neuroinflammation, and it is likely that PEA-LUT exerts its effects by suppressing this inflammation.82,83 Furthermore, combining olfactory training with PEA-LUT may lead to even more favorable outcomes, suggesting the potential for synergistic effects in the treatment of olfactory dysfunction in long-COVID patients.65 Although our findings did not show significant efficacy for intranasal corticosteroid sprays and olfactory training, this should not be taken as definitive evidence of their ineffectiveness. The current evidence base remains constrained by limited RCT data. Subsequent investigations may yield divergent outcomes through protocol optimization (e.g., extended treatment duration, dose Adjustment) and enhancing compliance of subjects.
The key distinction between the telerehabilitation program and the control group was that the telerehabilitation sessions were supervised by a staff member, while the control group only had a manual to guide them through the rehabilitation exercises without supervision.52 Our study found that telerehabilitation combined with exercise produced better outcomes in the 6-min walk test (6MWT), Fatigue Severity Scale (FSS), and 30-s sit-to-stand test (30sSTS) compared to exercise alone. This may be attributed to the ability of telerehabilitation to improve adherence and provide more professional guidance for interventions such as exercise.
Our study demonstrates several notable strengths. Firstly, this represents the inaugural meta-analysis to compare seven distinct therapeutic modalities for Long COVID patients, with tDCS, telerehabilitation, and therapeutic interventions for olfactory dysfunction being quantitatively investigated for the first time in this context. Secondly, we implemented an exhaustive search strategy employing multiple databases to minimize potential omission of relevant literature, employing no language filters and continuously incorporating newly published literature throughout the study period. And to ensure methodological rigor, we exclusively included RCTs meeting predefined quality criteria in our analytical framework. Third, we implemented the GRADE framework to stratify evidence quality into four certainty levels (high, moderate, low, and very low), thereby enhancing the credibility and clinical applicability of findings. Concurrent leave-one-out sensitivity analysis revealed methodological fragility across 31 outcome measures, highlighting the imperative for future studies to address critical discrepancies in trial design parameters. These strengths position this study as a pivotal reference for guideline development and personalized Long COVID management strategies.
Our study has several limitations. Firstly, not all relevant RCTs were included due to various factors such as the unavailability of original articles, non-English language publications, or the use of different scales or experimental methods to assess the same symptoms, which limited the number of studies available for analysis. Secondly, the quality of the included literature was variable, with some studies exhibiting a high risk of bias. Thirdly, the included studies demonstrated limited longitudinal outcome assessments, with maximum follow-up durations capped at 180 days and the majority restricted to 4–12week observation periods. This collectively constitutes a notable methodological limitation regarding sustained therapeutic effect evaluation. Fourthly, the limited number of studies included for some outcomes resulted in a limited ability of sensitivity analyses to assess the robustness of those outcomes. Fifthly, although individualized multimodal interventions may be promising, our current analysis was unable to evaluate their effectiveness as a distinct category due to the limited number of relevant studies. The potential benefits of combining multiple interventions warrant further investigation in future clinical trials. Additionally, certain experiments were difficult to blind, which may have introduced subjective factors that influenced the original results, making the findings of this study susceptible to bias. Lastly, there was high heterogeneity across studies, likely due to differences in intervention methods, duration, frequency, and follow-up periods, which may have contributed to variability in the results.
Despite these limitations, this meta-analysis provides valuable insights into the efficacy and safety of various Long COVID management approaches. For patients with dyspnea, exercise training or respiratory muscle training is recommended. tDCS may be considered as a novel therapeutic option for persistent fatigue, while PEA-LUT could be selected for olfactory dysfunction rehabilitation. Regarding exercise interventions, individuals may choose aerobic exercise, resistance training, or a combination regimen based on personal requirements and baseline physical conditions. Supervised implementation appears non-mandatory for exercise training. Clinical management should implement multimodal combination therapies tailored to patients' specific clinical manifestations, with emphasis on developing personalized therapeutic regimens through comprehensive symptom management strategies. This integrated approach aims to holistically enhance quality of life by addressing multidimensional pathophysiological sequelae of Long COVID.
Future research should prioritize large-scale RCTs to validate preliminary positive findings from small-sample studies such as tDCS. Furthermore, optimal dosing regimens and long-term therapeutic effects of tDCS and PEA-LUT need to be systematically established. Regarding exercise rehabilitation, subsequent investigations should focus on optimizing three core parameters: exercise modalities, intervention duration, and frequency, with the ultimate goal of maximizing clinical benefits through evidence-based prescription refinement.
Current evidence indicates that exercise training is the most promising therapeutic intervention, showing significant efficacy in improving exercise capacity and cardiorespiratory parameters. However, no substantial improvements were observed in fatigue, anxiety/depression symptoms, or pulmonary function metrics. Respiratory muscle training demonstrated comparable effectiveness in enhancing both exercise performance and respiratory muscle strength. PEA-LUT administration was associated with a notable increase in TDI scores, suggesting its potential clinical utility. Telerehabilitation, as a means of supervising interventions such as exercise, helps enhance their effectiveness in improving physical function and alleviating fatigue. Additionally, tDCS showed measurable effects on MFIS-physical, though the limited existing evidence warrants further investigation through large-scale RCTs. In contrast, olfactory training, and corticosteroid nasal sprays showed no therapeutic efficacy across the evaluated outcomes in our meta-analytic analysis.
Contributors
CT and SG developed the initial idea for the study, drafted the manuscript, and SG is the guarantor. CT, JM, and SG drafted the initial study protocol. CT and JM conducted the screening, extraction, and risk of bias assessment. CT and JM performed the statistical analyses. CT and SG provided supervision and mentorship. CT and JM had access to and verified the underlying data. CT, JM, and SG had full access to the underlying data and independently verified its accuracy. All authors provided critical revision of the manuscript for important intellectual content and gave approval of the submission of the manuscript for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The full dataset used in this study is available for viewing and replication in additional files accompanying this article (see Supplement).
Declaration of interests
We declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103412.
Appendix A. Supplementary data
References
- 1.Pouliopoulou D.V., Macdermid J.C., Saunders E., et al. Rehabilitation interventions for physical capacity and quality of life in adults with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(9):e2333838. doi: 10.1001/jamanetworkopen.2023.33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramakrishnan R.K., Kashour T., Hamid Q., Halwani R., Tleyjeh I.M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner-Scott J., Levy M., Hawkes C., Yeh A., Giovannoni G. Long COVID or post COVID-19 syndrome. Mult Scler Relat Disord. 2021;55 doi: 10.1016/j.msard.2021.103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang H.J., Lin C.W., Hsiao M.Y., Wang T.G., Liang H.W. Long COVID and rehabilitation. J Formos Med Assoc. 2024;123(Suppl 1):S61–s69. doi: 10.1016/j.jfma.2023.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins J., Hires C., Keenan L., Dunne E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: a randomized, double-blinded, placebo controlled clinical trial. Complement Ther Med. 2022;67 doi: 10.1016/j.ctim.2022.102823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrea L., Grant W.B., Frias-Toral E., et al. Dietary recommendations for post-COVID-19 syndrome. Nutrients. 2022;14(6) doi: 10.3390/nu14061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorenshtein A., Liba T., Leibovitch L., Stern S., Stern Y. Intervention modalities for brain fog caused by long-COVID: systematic review of the literature. Neurol Sci. 2024;45(7):2951–2968. doi: 10.1007/s10072-024-07566-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Lim K.H., Lim K.T., et al. Complementary and alternative medicine for long COVID: a systematic review of randomized controlled trials. Ther Adv Chronic Dis. 2023;14 doi: 10.1177/20406223231204727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-García J.C., Reinoso-Cobo A., Piqueras-Sola B., et al. Long COVID and physical therapy: a systematic review. Diseases. 2023;11(4) doi: 10.3390/diseases11040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng C., Chen X.K., Sit C.H.P., et al. Effect of physical exercise-based rehabilitation on long COVID: a systematic review and meta-analysis. Med Sci Sports Exerc. 2024;56(1):143–154. doi: 10.1249/MSS.0000000000003280. [DOI] [PubMed] [Google Scholar]
- 11.Meléndez-Oliva E., Martínez-Pozas O., Cuenca-Zaldívar J.N., Villafañe J.H., Jiménez-Ortega L., Sánchez-Romero E.A. Efficacy of pulmonary rehabilitation in post-COVID-19: a systematic review and meta-analysis. Biomedicines. 2023;11(8) doi: 10.3390/biomedicines11082213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira M.R., Hoffman M., Jones A.W., Holland A.E., Borghi-Silva A. Effect of pulmonary rehabilitation on exercise capacity, dyspnea, fatigue, and peripheral muscle strength in patients with post-COVID-19 syndrome: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2024;105(8):1559–1570. doi: 10.1016/j.apmr.2024.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mhanna S.B., Wan Ghazali W.S., Mohamed M., et al. Effectiveness of pulmonary rehabilitation among COVID-19 patients: a systematic review and meta-analysis. Healthcare (Basel) 2022;10(11) doi: 10.3390/healthcare10112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Pozas O., Meléndez-Oliva E., Rolando L.M., Rico J.A.Q., Corbellini C., Sánchez Romero E.A. The pulmonary rehabilitation effect on long covid-19 syndrome: a systematic review and meta-analysis. Physiother Res Int. 2024;29(2) doi: 10.1002/pri.2077. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X., Cao M., Yeung W.F., Cheung D.S.T. The effectiveness of exercise in alleviating long COVID symptoms: a systematic review and meta-analysis. Worldviews Evid Based Nurs. 2024;21(5):561–574. doi: 10.1111/wvn.12743. [DOI] [PubMed] [Google Scholar]
- 16.Nantakool S., Sa-Nguanmoo P., Konghakote S., Chuatrakoon B. Effects of exercise rehabilitation on cardiorespiratory fitness in long-COVID-19 survivors: a meta-analysis. J Clin Med. 2024;13(12) doi: 10.3390/jcm13123621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvache-Mateo A., Heredia-Ciuró A., Martín-Núñez J., et al. Efficacy and safety of respiratory telerehabilitation in patients with long COVID-19: a systematic review and meta-analysis. Healthcare (Basel) 2023;11(18) doi: 10.3390/healthcare11182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira A., Pinto A.C.P.N., Garcia B.M.S.P., Eid R.A.C., Mól C.G., Nawa R.K. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: a systematic review. J Physiother. 2022;68(2):90–98. doi: 10.1016/j.jphys.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai B., Xu M., Zhou H., et al. Effects of aerobic training on cardiopulmonary fitness in patients with long COVID-19: a randomized controlled trial. Trials. 2024;25(1):649. doi: 10.1186/s13063-024-08473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barz A., Berger J., Speicher M., et al. Effects of a symptom-titrated exercise program on fatigue and quality of life in people with post-COVID condition - a randomized controlled trial. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-82584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besnier F., Malo J., Mohammadi H., et al. Effects of cardiopulmonary rehabilitation on cardiorespiratory fitness and clinical symptom burden in long COVID: results from the COVID-rehab randomized controlled trial. Am J Phys Med Rehabil. 2025;104(2):163–171. doi: 10.1097/PHM.0000000000002559. [DOI] [PubMed] [Google Scholar]
- 24.Calvo-Paniagua J., Díaz-Arribas M.J., Valera-Calero J.A., et al. Educational, exercise, and occupational therapy-based telerehabilitation program versus "Wait-and-See" for improving self-perceived exertion in patients with post-COVID fatigue and dyspnea: a randomized clinical trial. Am J Phys Med Rehabil. 2024;103(9):797–804. doi: 10.1097/PHM.0000000000002441. [DOI] [PubMed] [Google Scholar]
- 25.de Araújo Furtado P.L., do Socorro Brasileiro-Santos M., de Mello B.L.C., et al. The effect of telerehabilitation on physical fitness and depression/anxiety in post-COVID-19 patients: a randomized controlled trial. Int J Telerehabil. 2023;15(1) doi: 10.5195/ijt.2023.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elyazed T.I.A., Alsharawy L.A., Salem S.E., Helmy N.A., El-Hakim A.A.E.M.A. Effect of home-based pulmonary rehabilitation on exercise capacity in post COVID-19 patients: a randomized controlled trail. J NeuroEng Rehabil. 2024;21(1):40. doi: 10.1186/s12984-024-01340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes Dos Santos E.G., Vieira da Costa K., Cordeiro de Souza I.T., et al. Effects of a cardiopulmonary rehabilitation protocol on functional capacity, dyspnea, fatigue, and body composition in individuals with post-COVID-19 syndrome: a randomized controlled trial. Physiother Res Int. 2024;29(2) doi: 10.1002/pri.2086. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim A.A., Hussein H.M., Ali M.S., et al. A randomized controlled trial examining the impact of low vs. moderate-intensity aerobic training in post-discharge COVID-19 older subjects. Eur Rev Med Pharmacol Sci. 2023;27(9):4280–4291. doi: 10.26355/eurrev_202305_32338. [DOI] [PubMed] [Google Scholar]
- 29.Kaczmarczyk K., Matharu Y., Bobowik P., Gajewski J., Maciejewska-Skrendo A., Kulig K. Resistance exercise program is feasible and effective in improving functional strength in post-COVID survivors. J Clin Med. 2024;13(6) doi: 10.3390/jcm13061712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaddoussi R., Rejeb H., Kalai A., et al. Effects of a cardiopulmonary rehabilitation programme on submaximal exercise in Tunisian patients with long-COVID19: a randomized clinical trial. Biol Sport. 2024;41(4):197–217. doi: 10.5114/biolsport.2024.139072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerling A., Beyer S., Dirks M., et al. Effects of a randomized-controlled and online-supported physical activity intervention on exercise capacity, fatigue and health related quality of life in patients with post-COVID-19 syndrome. BMC Sports Sci Med Rehabil. 2024;16(1):33. doi: 10.1186/s13102-024-00817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keskin B., Saka S. The effect of strengthening and relaxation exercises on musculoskeletal pain, anxiety, and sleep quality in COVID-19 survivors. Eurasian J Pulmonol. 2023;25(2):125–132. [Google Scholar]
- 33.Lai C.Y., Lin C.H., Chao T.C., et al. Effectiveness of a 12-week telerehabilitation training in people with long COVID: a randomized controlled trial. Ann Phys Rehabil Med. 2024;67(5) doi: 10.1016/j.rehab.2024.101853. [DOI] [PubMed] [Google Scholar]
- 34.Li J., Xia W., Zhan C., et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. 2022;77(7):697–706. doi: 10.1136/thoraxjnl-2021-217382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longobardi I., Goessler K., de Oliveira Júnior G.N., et al. Effects of a 16-week home-based exercise training programme on health-related quality of life, functional capacity, and persistent symptoms in survivors of severe/critical COVID-19: a randomised controlled trial. Br J Sports Med. 2023;57(20):1295–1303. doi: 10.1136/bjsports-2022-106681. [DOI] [PubMed] [Google Scholar]
- 36.McGregor G., Sandhu H., Bruce J., et al. Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial. BMJ. 2024;384 doi: 10.1136/bmj-2023-076506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez Milá Z., Rodríguez Sanz D., Martín Nieto A., et al. Effects of a respiratory and neurological rehabilitation treatment plan in post Covid-19 affected university students. Randomized clinical study. Chron Respir Dis. 2024;21 doi: 10.1177/14799731241255967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira K.C.V.d., Ferreira A.P.d.L., Silva D.d.A., et al. The impact of post-COVID multicomponent rehabilitation. Fisioterapia em Movimento. 2023;36 [Google Scholar]
- 39.Paneroni M., Scalvini S., Perger E., et al. Home-based exercise program for people with residual disability following hospitalization for COVID-19: randomized control trial. Ann Phys Rehabil Med. 2024;67(2) doi: 10.1016/j.rehab.2023.101815. [DOI] [PubMed] [Google Scholar]
- 40.Pleguezuelos E., Del Carmen A., Moreno E., Miravitlles M., Serra M., Garnacho-Castaño M.V. Effects of a telerehabilitation program and detraining on cardiorespiratory fitness in patients with post-COVID-19 sequelae: a randomized controlled trial. Scand J Med Sci Sports. 2024;34(1) doi: 10.1111/sms.14543. [DOI] [PubMed] [Google Scholar]
- 41.Pleguezuelos E., Del Carmen A., Moreno E., Serra-Prat M., Serra-Payá N., Garnacho-Castaño M.V. Telerehabilitation improves cardiorespiratory and muscular fitness and body composition in older people with post-COVID-19 syndrome. J Cachexia Sarcopenia Muscle. 2024;15(5):1785–1796. doi: 10.1002/jcsm.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Blanco C., Bernal-Utrera C., Anarte-Lazo E., Gonzalez-Gerez J.J., Saavedra-Hernandez M. A 14-day therapeutic exercise telerehabilitation protocol of physiotherapy is effective in non-hospitalized post-COVID-19 conditions: a randomized controlled trial. J Clin Med. 2023;12(3) doi: 10.3390/jcm12030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez-Blanco C., Bernal-Utrera C., Anarte-Lazo E., et al. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: a randomized controlled trial. Clin Rehabil. 2022;36(4):486–497. doi: 10.1177/02692155211061221. [DOI] [PubMed] [Google Scholar]
- 44.Romanet C., Wormser J., Fels A., et al. Effectiveness of exercise training on the dyspnoea of individuals with long COVID: a randomised controlled multicentre trial. Ann Phys Rehabil Med. 2023;66(5) doi: 10.1016/j.rehab.2023.101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berenguel Senén A., Gadella Fernández A., Godoy López J., et al. Functional rehabilitation based on therapeutic exercise training in patients with postacute COVID syndrome (RECOVER) Rev Esp Cardiol. 2024;77(2):167–175. doi: 10.1016/j.rec.2023.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Abo Elyazed T.I., Abd El-Hakim A.A.E.M., Saleh O.I., et al. Diaphragmatic strengthening exercises for patients with post COVID-19 condition after mild-to-moderate acute COVID-19 infection: a randomized controlled study. J Rehabil Med. 2024;56:jrm25491. doi: 10.2340/jrm.v56.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Corral T., Fabero-Garrido R., Plaza-Manzano G., Fernández-de-Las-Peñas C., Navarro-Santana M., López-de-Uralde-Villanueva I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: randomized controlled trial. Ann Phys Rehabil Med. 2023;66(1) doi: 10.1016/j.rehab.2022.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNarry M.A., Berg R.M.G., Shelley J., et al. Inspiratory muscle training enhances recovery post-COVID-19: a randomised controlled trial. Eur Respir J. 2022;60(4) doi: 10.1183/13993003.03101-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietranis K.A., Izdebska W.M., Kuryliszyn-Moskal A., et al. Effects of pulmonary rehabilitation on respiratory function and thickness of the diaphragm in patients with post-COVID-19 syndrome: a randomized clinical trial. J Clin Med. 2024;13(2) doi: 10.3390/jcm13020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sari F., Bayram S., Pala G.G., Çömçe F., Küçük H., Oskay D. Effects of inspiratory muscle training in patients with post-COVID-19. J Harran Univ Med Fac. 2022;19(3):581–588. [Google Scholar]
- 51.Spiesshoefer J., Regmi B., Senol M., et al. Potential diaphragm muscle weakness-related dyspnea persists 2 years after COVID-19 and could be improved by inspiratory muscle training: results of an observational and an interventional clinical trial. Am J Respir Crit Care Med. 2024;210(5):618–628. doi: 10.1164/rccm.202309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carpallo-Porcar B., Calvo S., Alamillo-Salas J., Herrero P., Gómez-Barrera M., Jiménez-Sánchez C. An opportunity for management of fatigue, physical condition, and quality of life through asynchronous telerehabilitation in patients after acute coronavirus disease 2019: a randomized controlled pilot study. Arch Phys Med Rehabil. 2024;105(8):1439–1448. doi: 10.1016/j.apmr.2024.04.014. [DOI] [PubMed] [Google Scholar]
- 53.da Silva M.M., Viana D.R., Colucci M.G., et al. Effects of a cardiopulmonary telerehabilitation using functional exercises in individuals after COVID-19 hospital discharge: a randomized controlled trial. J Telemed Telecare. 2025;31(3):311–319. doi: 10.1177/1357633X231188394. [DOI] [PubMed] [Google Scholar]
- 54.Okan F., Okan S., Duran Yücesoy F. Evaluating the efficiency of breathing exercises via telemedicine in post-covid-19 patients: randomized controlled study. Clin Nurs Res. 2022;31(5):771–781. doi: 10.1177/10547738221097241. [DOI] [PubMed] [Google Scholar]
- 55.Pehlivan E., Palalı İ., Atan S.G., Turan D., Çınarka H., Çetinkaya E. The effectiveness of POST-DISCHARGE telerehabilitation practices in COVID-19 patients: tele-COVID study-randomized controlled trial. Ann Thorac Med. 2022;17(2):110–117. doi: 10.4103/atm.atm_543_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samper-Pardo M., León-Herrera S., Oliván-Blázquez B., Méndez-López F., Domínguez-García M., Sánchez-Recio R. Effectiveness of a telerehabilitation intervention using ReCOVery APP of long COVID patients: a randomized, 3-month follow-up clinical trial. Sci Rep. 2023;13(1):7943. doi: 10.1038/s41598-023-35058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Şahın H., Naz İ., Karadeniz G., Süneçlı O., Polat G., Ediboğlu O. Effects of a home-based pulmonary rehabilitation program with and without telecoaching on health-related outcomes in COVID-19 survivors: a randomized controlled clinical study. J Bras Pneumol. 2023;49(1):e20220107. doi: 10.36416/1806-3756/e20220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarmento A., Adodo R., Hodges G., Webber S.C., Sanchez-Ramirez D.C. Virtual pulmonary rehabilitation approaches in patients with post COVID syndrome: a pilot study. BMC Pulm Med. 2024;24(1):139. doi: 10.1186/s12890-024-02965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliver-Mas S., Delgado-Alonso C., Delgado-Álvarez A., et al. Transcranial direct current stimulation for post-COVID fatigue: a randomized, double-blind, controlled pilot study. Brain Commun. 2023;5(2):fcad117. doi: 10.1093/braincomms/fcad117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santana K., França E., Sato J., et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC) Brain Stimul. 2023;16(1):100–107. doi: 10.1016/j.brs.2023.01.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bérubé S., Demers C., Bussière N., et al. Olfactory training impacts olfactory dysfunction induced by COVID-19: a pilot study. ORL J Otorhinolaryngol Relat Spec. 2023;85(2):57–66. doi: 10.1159/000528188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdelalim A.A., Mohamady A.A., Elsayed R.A., Elawady M.A., Ghallab A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021;42(2) doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasiri H., Rouhani N., Salehifar E., Ghazaeian M., Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: a randomized, double blind clinical trial. Int Immunopharmacol. 2021;98 doi: 10.1016/j.intimp.2021.107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantone E., D'Ascanio L., De Luca P., et al. Persistent COVID-19 parosmia and olfactory loss post olfactory training: randomized clinical trial comparing central and peripheral-acting therapeutics. Eur Arch Otorhinolaryngol. 2024;281(7):3671–3678. doi: 10.1007/s00405-024-08548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D'Ascanio L., Vitelli F., Cingolani C., Maranzano M., Brenner M.J., Di Stadio A. Randomized clinical trial "olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin": preliminary results. Eur Rev Med Pharmacol Sci. 2021;25(11):4156–4162. doi: 10.26355/eurrev_202106_26059. [DOI] [PubMed] [Google Scholar]
- 66.Di Stadio A., Cantone E., De Luca P., et al. Parosmia COVID-19 related treated by a combination of olfactory training and ultramicronized PEA-LUT: a Prospective randomized controlled trial. Biomedicines. 2023;11(4) doi: 10.3390/biomedicines11041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Stadio A., D'Ascanio L., Vaira L.A., et al. Ultramicronized palmitoylethanolamide and luteolin supplement combined with olfactory training to treat post-COVID-19 olfactory impairment: a multi-center double-blinded randomized placebo- controlled clinical trial. Curr Neuropharmacol. 2022;20(10):2001–2012. doi: 10.2174/1570159X20666220420113513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Stadio A., Gallina S., Cocuzza S., et al. Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: a blinded controlled multicenter randomized trial. Eur Arch Otorhinolaryngol. 2023;280(11):4949–4961. doi: 10.1007/s00405-023-08085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sick J., Steinbacher V., Kotnik D., et al. Exercise rehabilitation in post COVID-19 patients: a randomized controlled trial of different training modalities. Eur J Phys Rehabil Med. 2025;61(1):130–140. doi: 10.23736/S1973-9087.24.08487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jorge M.S.G., Nepomuceno P., Schneider R.H., Wibelinger L.M. Eight weeks of Pilates Method improves physical fitness and sleep quality of individuals with post-COVID-19 syndrome: a randomized clinical trial blinded. J Bodyw Mov Ther. 2025;41:238–245. doi: 10.1016/j.jbmt.2024.11.037. [DOI] [PubMed] [Google Scholar]
- 71.Demir C., Aksoy C.C., Gokmen G.Y., Durmaz D. Effect of telerehabilitation on post-COVID-19 individuals with long-term dyspnea: a randomized controlled study. J Telemed Telecare. 2025 doi: 10.1177/1357633X251333903. [DOI] [PubMed] [Google Scholar]
- 72.Margalit I., Yelin D., Sagi M., et al. Risk factors and multidimensional assessment of long coronavirus disease fatigue: a nested case-control study. Clin Infect Dis. 2022;75(10):1688–1697. doi: 10.1093/cid/ciac283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Upadhya S., Rehman J., Malik A.B., Chen S. Mechanisms of lung injury induced by SARS-CoV-2 infection. Physiology. 2022;37(2):88–100. doi: 10.1152/physiol.00033.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Severin R., Franz C.K., Farr E., et al. The effects of COVID-19 on respiratory muscle performance: making the case for respiratory muscle testing and training. Eur Respir Rev. 2022;31(166) doi: 10.1183/16000617.0006-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dirks M.L., Wall B.T., van de Valk B., et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 76.Tobin M.J., Laghi F., Jubran A. Narrative review: ventilator-induced respiratory muscle weakness. Ann Intern Med. 2010;153(4):240–245. doi: 10.1059/0003-4819-153-4-201008170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvache-Mateo A., Reychler G., Heredia-Ciuró A., et al. Respiratory training effects in Long COVID-19 patients: a systematic review and meta-analysis. Expert Rev Respir Med. 2024;18(3–4):207–217. doi: 10.1080/17476348.2024.2358933. [DOI] [PubMed] [Google Scholar]
- 78.Woods A.J., Antal A., Bikson M., et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefaucheur J.P., Chalah M.A., Mhalla A., Palm U., Ayache S.S., Mylius V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol Clin. 2017;47(2):173–184. doi: 10.1016/j.neucli.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Palm U., Ayache S.S., Padberg F., Lefaucheur J.P. Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimul. 2014;7(6):849–854. doi: 10.1016/j.brs.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu B., Gong M., Xiang Y., Qu S., Zhu H., Ye D. Mechanism and treatment of olfactory dysfunction caused by coronavirus disease 2019. J Transl Med. 2023;21(1):829. doi: 10.1186/s12967-023-04719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anastassopoulou C., Davaris N., Ferous S., et al. The molecular basis of olfactory dysfunction in COVID-19 and long COVID. Lifestyle Genom. 2024;17(1):42–56. doi: 10.1159/000539292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.