Abstract
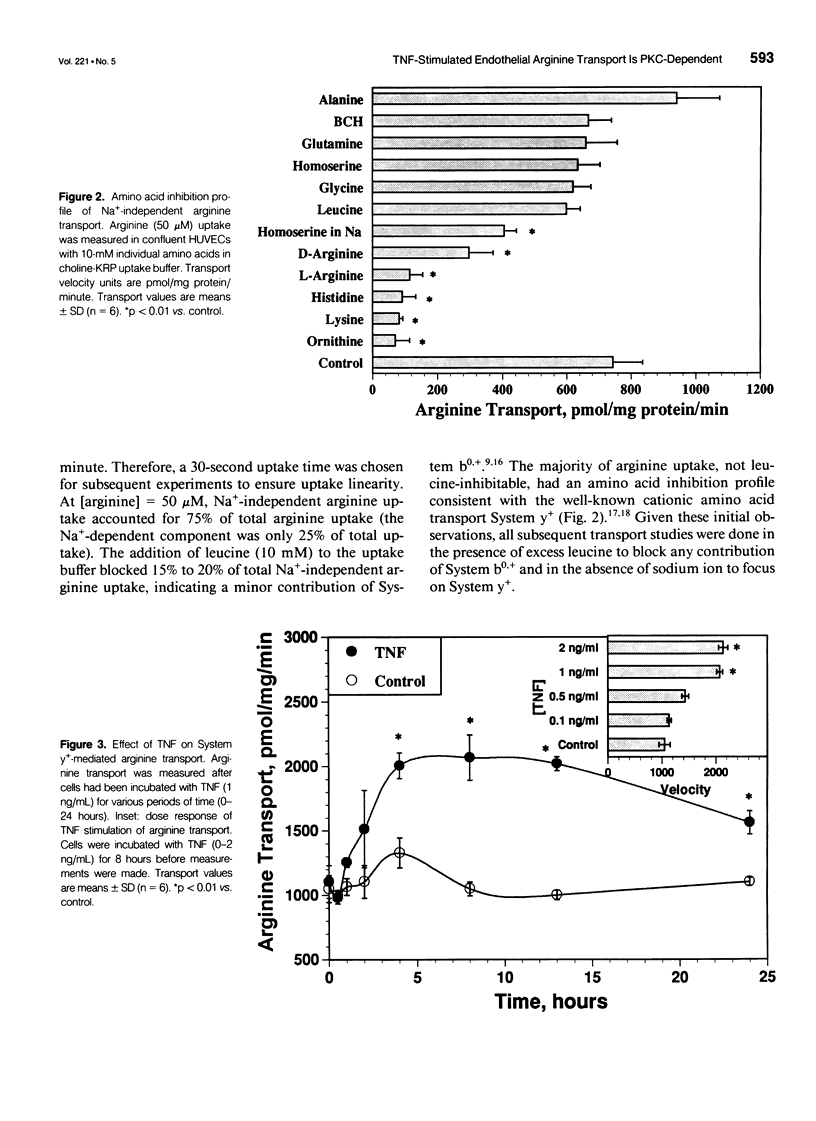
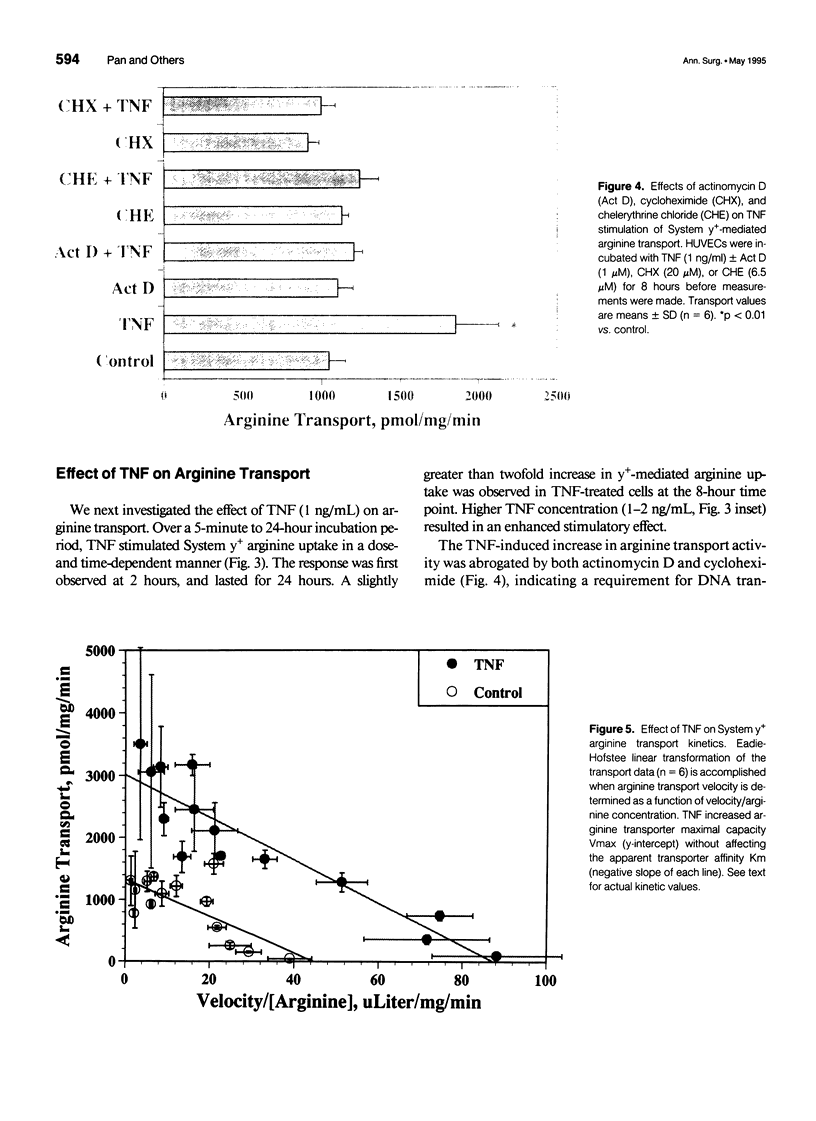
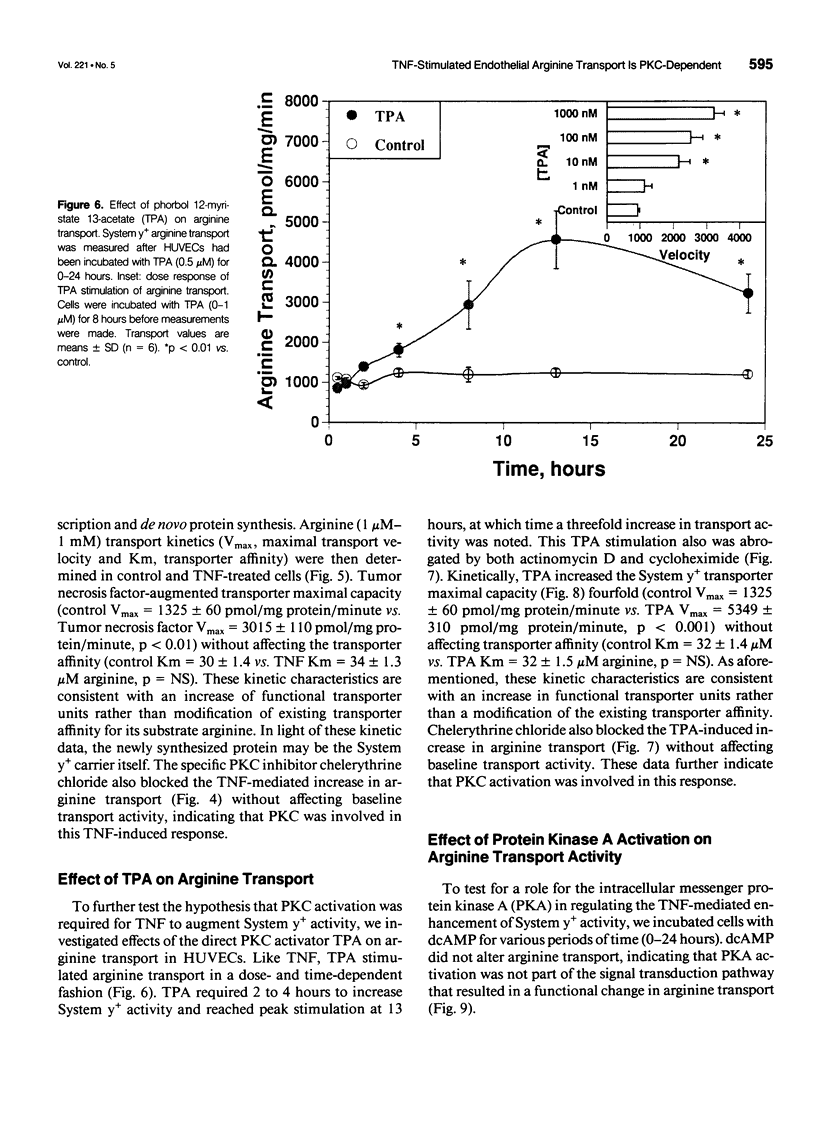
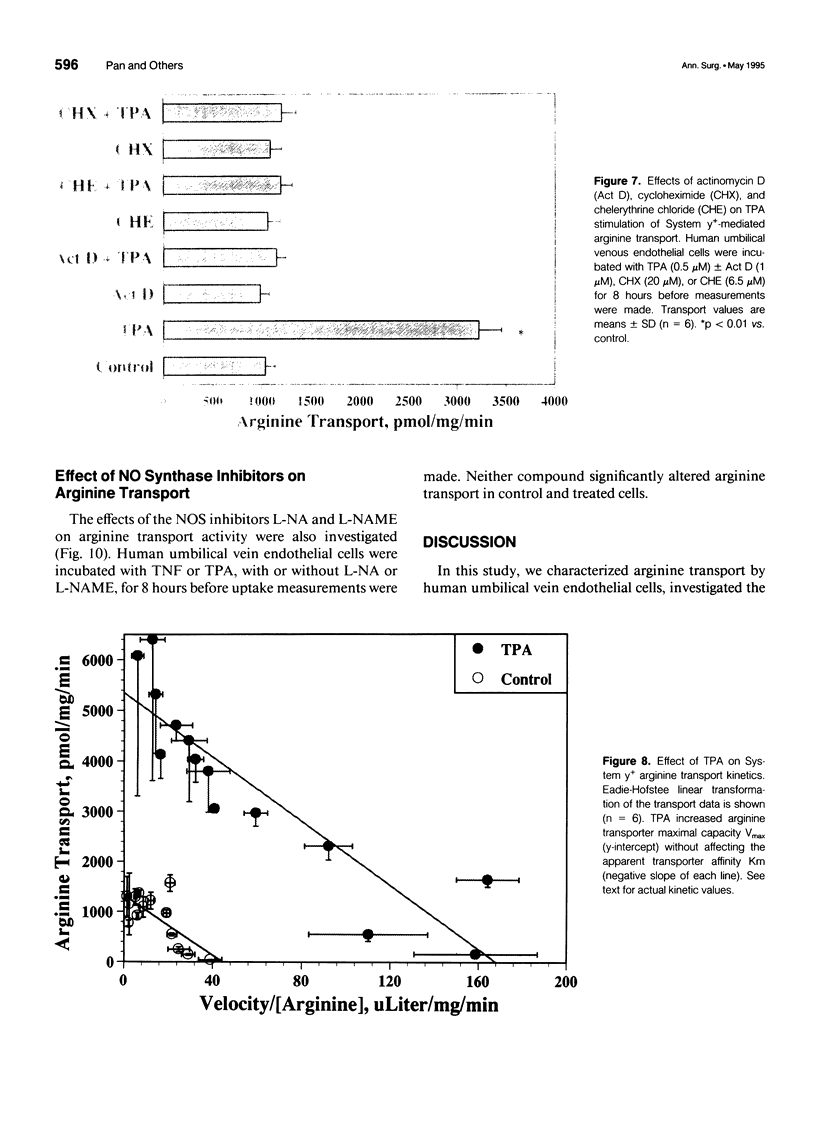
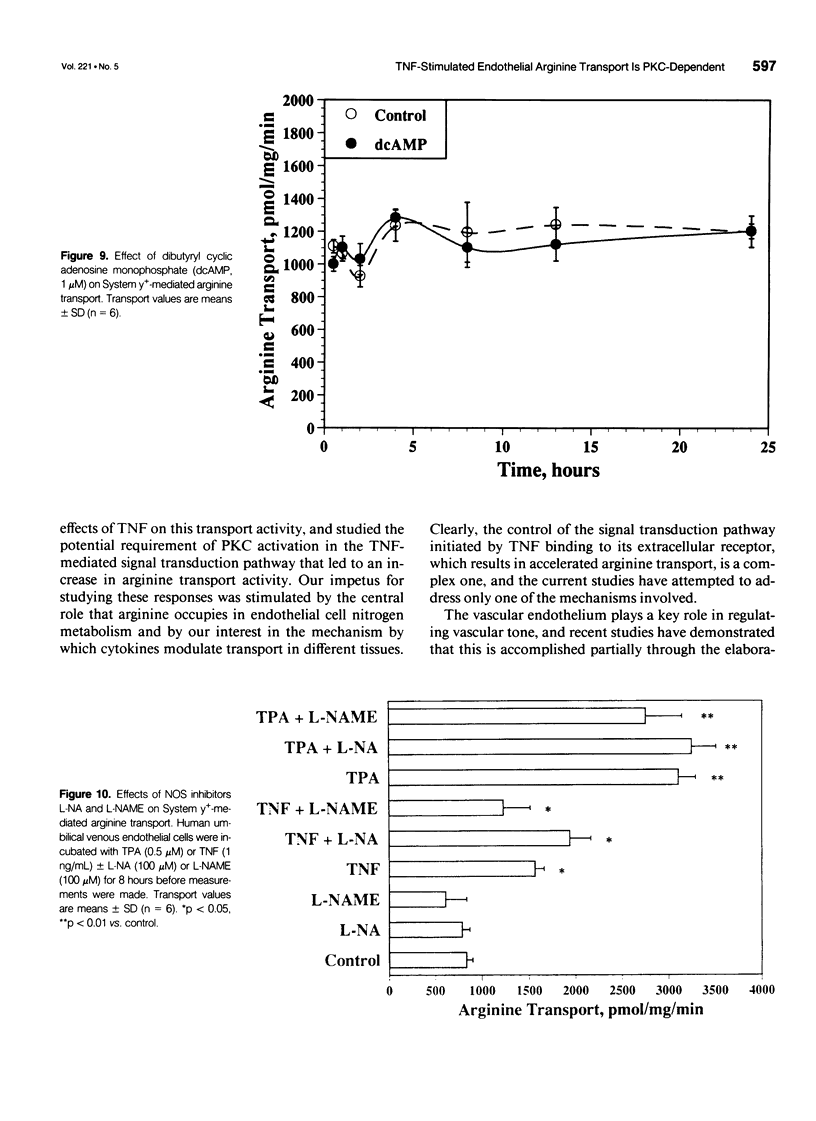
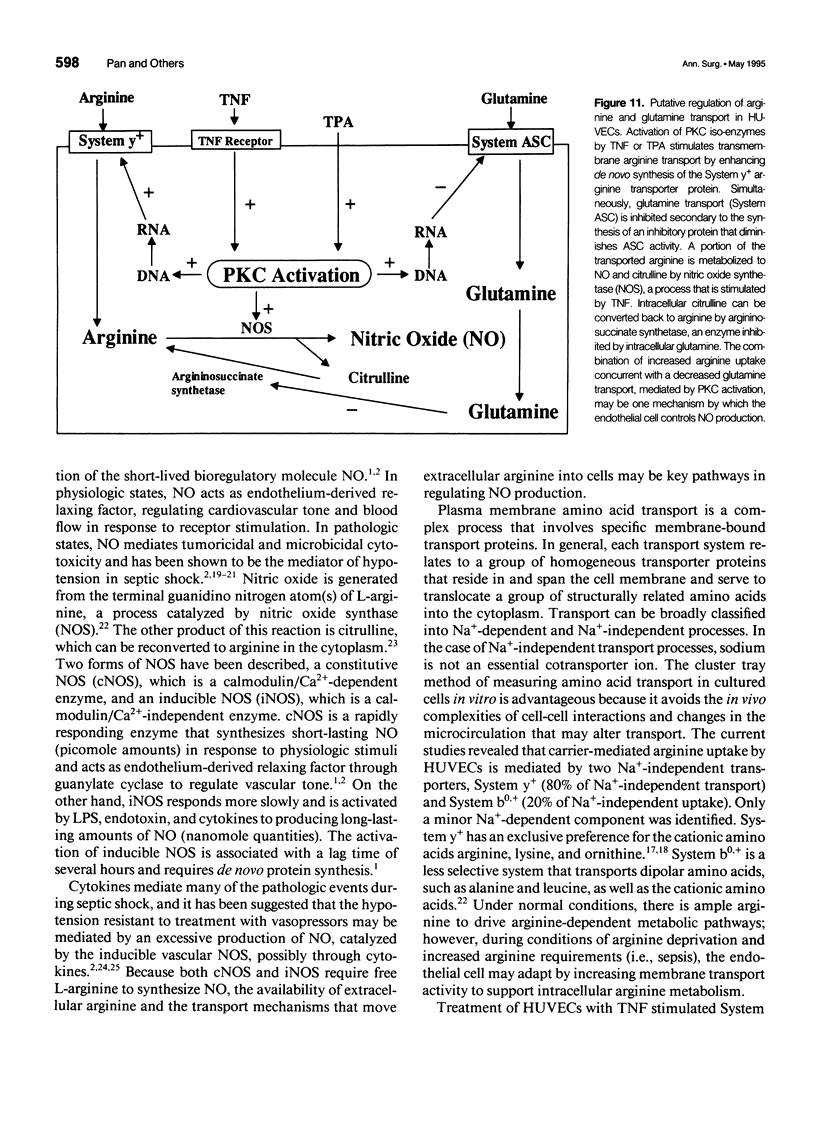
OBJECTIVE: The authors determined the endothelial arginine transport mechanism and the potential role of a tumor necrosis factor (TNF)-alpha-mediated signal transduction pathway involving protein kinase C (PKC) in regulating this transport in cultured endothelial cells. SUMMARY BACKGROUND DATA: The vascular endothelium metabolizes arginine to generate nitric oxide (NO), and an increase in NO production can be stimulated by several cytokines. The mechanism(s) responsible for the accelerated arginine transport are poorly understood. METHODS: Arginine transport was assayed in confluent human umbilical vein endothelial cells in the presence of TNF +/- the PKC inhibitor chelerythrine chloride. RESULTS: Carrier-mediated arginine transport was accomplished by two Na(+)-independent transporters, System y+ (80% of total transport) and System b0,+ (20% of transport). Tumor necrosis factor (0.1-2 ng/mL) increased System y(+)-mediated arginine transport in a time- and dose-dependent manner by augmenting System y+ transport maximal capacity (control Vmax = 1325 +/- 60 pmol/mg protein/minute vs. TNF Vmax = 3015 +/- 110 pmol/mg protein/minute, p < 0.01) without affecting transporter affinity (control Km = 30 +/- 1.4 microM vs. 34 +/- 1.3 microM arginine, p = NS). Stimulation was maximal at the 8-hour time point and was inhibited by both actinomycin D and cycloheximide. In addition, inhibition of PKC with chelerythrine abrogated the TNF-augmented arginine transport. Similarly, incubation of cells with the direct PKC activator TPA (phorbol ester 12-myristate 13-acetate) stimulated System y(+)-mediated arginine transport nearly fivefold, secondary to an increase in transporter Vmax (TPA Vmax = 5349 +/- 310 pmol/mg protein/minute, p < 0.001 vs. control), with no change in Km. This TPA-induced stimulation of arginine transport also was blocked by chelerythrine CI, actinomycin D, and cycloheximide. Incubation of TNF-stimulated cells with two NO synthase inhibitors did not reduce transport activity, suggesting that the arginine transporter and the NO synthase enzyme may, in part, be independently regulated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cendan J. C., Souba W. W., Copeland E. M., 3rd, Lind D. S. Cytokines regulate endotoxin stimulation of endothelial cell arginine transport. Surgery. 1995 Feb;117(2):213–219. doi: 10.1016/s0039-6060(05)80088-1. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Liang M. Transport of diamino acids into the Ehrlich cell. J Biol Chem. 1966 Dec 10;241(23):5542–5551. [PubMed] [Google Scholar]
- Clemens M. J., Trayner I., Menaya J. The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci. 1992 Dec;103(Pt 4):881–887. doi: 10.1242/jcs.103.4.881. [DOI] [PubMed] [Google Scholar]
- Closs E. I., Lyons C. R., Kelly C., Cunningham J. M. Characterization of the third member of the MCAT family of cationic amino acid transporters. Identification of a domain that determines the transport properties of the MCAT proteins. J Biol Chem. 1993 Oct 5;268(28):20796–20800. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Franchi-Gazzola R., White M. F. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981 Aug;115(2):368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz K., Bode B. P., Block E. R., Souba W. W. Characterization of L-glutamine transport by pulmonary artery endothelial cells. Am J Physiol. 1991 Apr;260(4 Pt 1):L241–L246. doi: 10.1152/ajplung.1991.260.4.L241. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian C. A., Ward N. E. Biology of the protein kinase C family. Cancer Metastasis Rev. 1989 Dec;8(3):199–214. doi: 10.1007/BF00047337. [DOI] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Sessa W. C., Hecker M., Mitchell J. A., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: L-glutamine inhibits the generation of L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8607–8611. doi: 10.1073/pnas.87.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem. 1988 Mar 5;263(7):3150–3163. [PubMed] [Google Scholar]
- Westenberger U., Thanner S., Ruf H. H., Gersonde K., Sutter G., Trentz O. Formation of free radicals and nitric oxide derivative of hemoglobin in rats during shock syndrome. Free Radic Res Commun. 1990;11(1-3):167–178. doi: 10.3109/10715769009109680. [DOI] [PubMed] [Google Scholar]


