Abstract
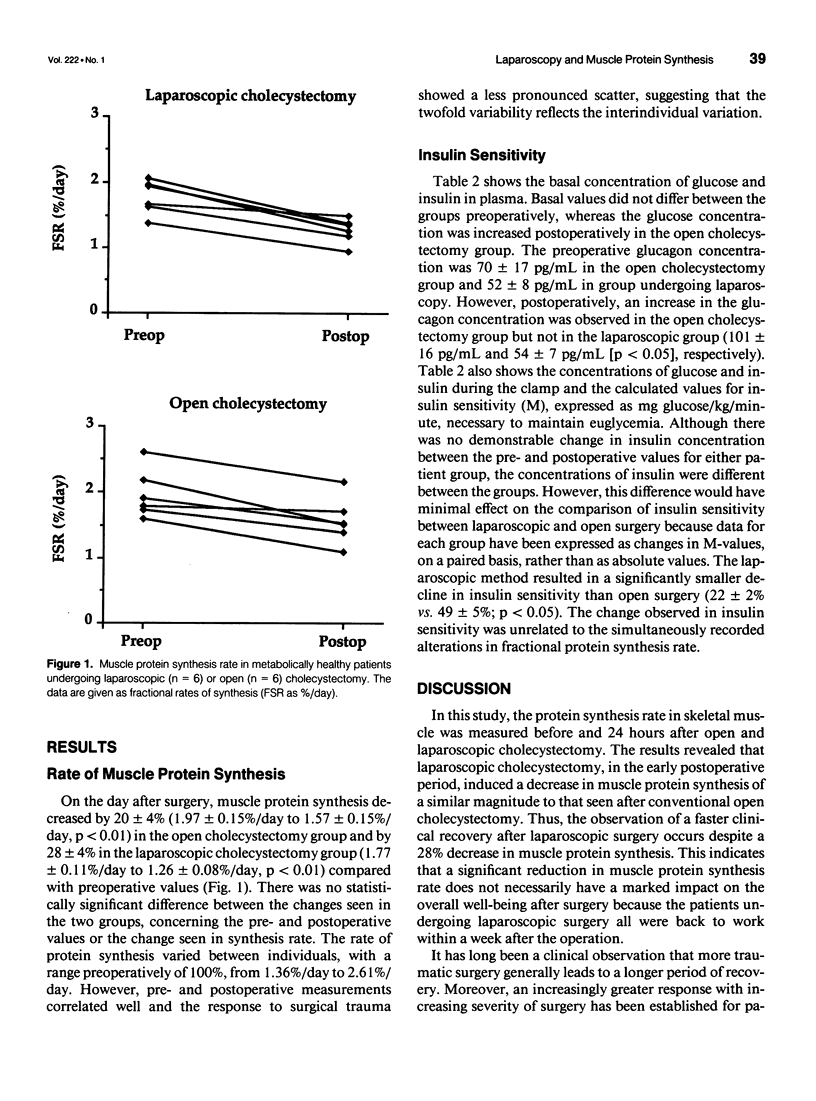
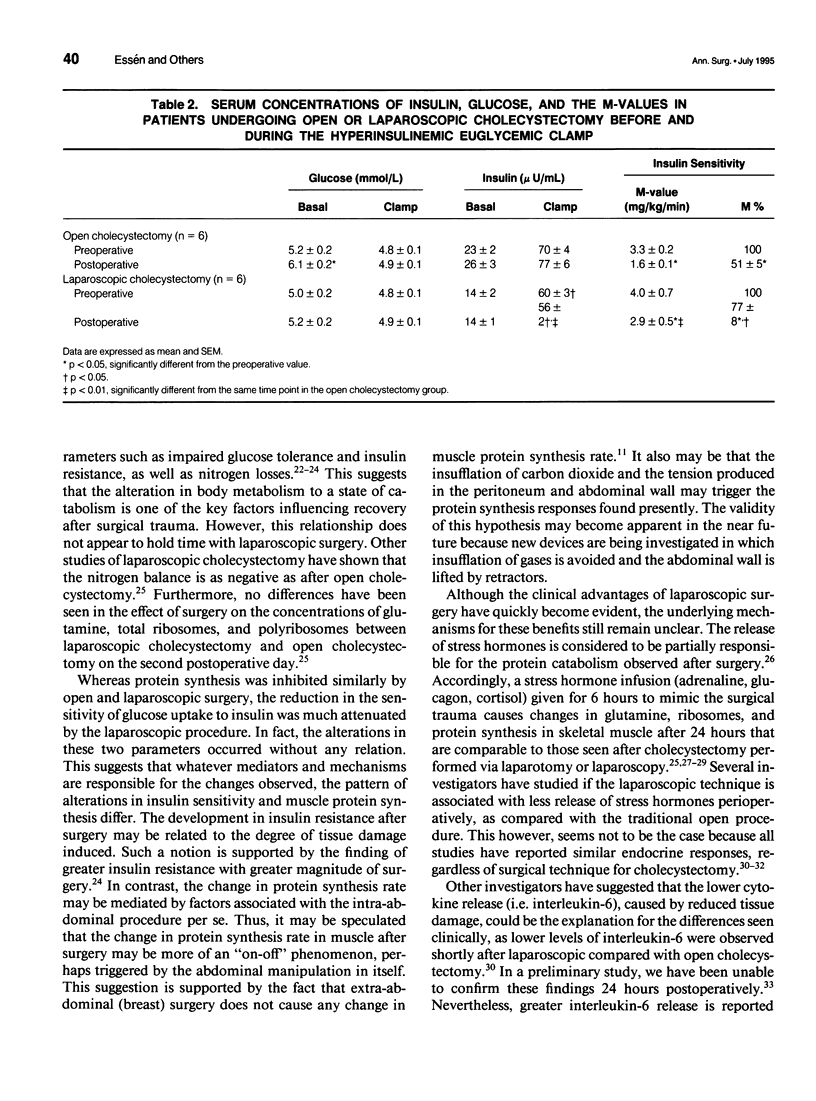
OBJECTIVE: The authors determined the effect of laparoscopic cholecystectomy on protein synthesis in skeletal muscle. In addition to a decrease in muscle protein synthesis, after open cholecystectomy, the authors previously demonstrated a decrease in insulin sensitivity. This study on patients undergoing laparoscopic and open surgery, therefore, included simultaneous measurements of protein synthesis and insulin sensitivity. SUMMARY BACKGROUND DATA: Laparoscopy has become a routine technique for several operations because of postoperative benefits that allow rapid recovery. However, its effect on postoperative protein catabolism has not been characterized. Conventional laparotomy induces a drop in muscle protein synthesis, whereas degradation is unaffected. METHODS: Patients were randomized to laparoscopic or open cholecystectomy, and the rate of protein synthesis in skeletal muscle was determined 24 hours postoperatively by the flooding technique using L-(2H5)phenylalanine, during a hyperinsulinemic normoglycemic clamp to assess insulin sensitivity. RESULTS: The protein synthesis rate decreased by 28% (1.77 +/- 0.11%/day vs. 1.26 +/- 0.08%/day, p < 0.01) in the laparoscopic group and by 20% (1.97 +/- 0.15%/day vs. 1.57 +/- 0.15%/day, p < 0.01) in the open cholecystectomy group. In contrast, the fall in insulin sensitivity after surgery was lower with laparoscopic (22 +/- 2%) compared with open surgery (49 +/- 5%). CONCLUSIONS: Laparoscopic cholecystectomy did not avoid a substantial decline in muscle protein synthesis, despite improved insulin sensitivity. The change in the two parameters occurred independently, indicating different mechanisms controlling insulin sensitivity and muscle protein synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banauch D., Brümmer W., Ebeling W., Metz H., Rindfrey H., Lang H., Leybold K., Rick W., Staudinger H. J. Eine Glucose-Dehydrogenase für die Glucose-Bestimmung in Körperflüssigkeiten. Z Klin Chem Klin Biochem. 1975 Mar;13(3):101–107. [PubMed] [Google Scholar]
- Barkun J. S., Barkun A. N., Sampalis J. S., Fried G., Taylor B., Wexler M. J., Goresky C. A., Meakins J. L. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet. 1992 Nov 7;340(8828):1116–1119. doi: 10.1016/0140-6736(92)93148-g. [DOI] [PubMed] [Google Scholar]
- Calder A. G., Anderson S. E., Grant I., McNurlan M. A., Garlick P. J. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992 Jul;6(7):421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- Cruickshank A. M., Fraser W. D., Burns H. J., Van Damme J., Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990 Aug;79(2):161–165. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D. P. The distribution of nitrogen and sulphur in the urine during conditions of increased catabolism. Biochem J. 1931;25(1):236–244. doi: 10.1042/bj0250236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel P. M. The metabolic homoeostatic role of muscle and its function as a store of protein. Lancet. 1977 Aug 27;2(8035):446–448. doi: 10.1016/s0140-6736(77)90622-5. [DOI] [PubMed] [Google Scholar]
- Davies C. L., Newman R. J., Molyneux S. G., Grahame-Smith D. G. The relationship between plasma catecholamines and severity of injury in man. J Trauma. 1984 Feb;24(2):99–105. doi: 10.1097/00005373-198402000-00002. [DOI] [PubMed] [Google Scholar]
- Essén P., McNurlan M. A., Sonnenfeld T., Milne E., Vinnars E., Wernerman J., Garlick P. J. Muscle protein synthesis after operation: effects of intravenous nutrition. Eur J Surg. 1993 Apr;159(4):195–200. [PubMed] [Google Scholar]
- Essén P., McNurlan M. A., Wernerman J., Vinnars E., Garlick P. J. Uncomplicated surgery, but not general anesthesia, decreases muscle protein synthesis. Am J Physiol. 1992 Mar;262(3 Pt 1):E253–E260. doi: 10.1152/ajpendo.1992.262.3.E253. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Protein turnover and amino acid metabolism in the regulation of gluconeogenesis. Fed Proc. 1974 Apr;33(4):1092–1097. [PubMed] [Google Scholar]
- Garlick P. J., Wernerman J., McNurlan M. A., Essen P., Lobley G. E., Milne E., Calder G. A., Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a 'flooding dose' of [1-13C]leucine. Clin Sci (Lond) 1989 Sep;77(3):329–336. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- HOWARD J. M. Studies of the absorption and metabolism of glucose following injury; the systemic response to injury. Ann Surg. 1955 Mar;141(3):321–326. doi: 10.1097/00000658-195503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. M., Desborough J. P. Interleukin-6 and the metabolic response to surgery. Br J Anaesth. 1992 Oct;69(4):337–338. doi: 10.1093/bja/69.4.337-a. [DOI] [PubMed] [Google Scholar]
- Hamberger B., Järnberg P. O. Plasma catecholamines during surgical stress: differences between neurolept and enflurane anaesthesia. Acta Anaesthesiol Scand. 1983 Aug;27(4):307–310. doi: 10.1111/j.1399-6576.1983.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris J., Cigarini I., Legrand M., Jacquet N., De Groote D., Franchimont P., Lamy M. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br J Anaesth. 1992 Oct;69(4):341–345. doi: 10.1093/bja/69.4.341. [DOI] [PubMed] [Google Scholar]
- Le J. M., Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989 Dec;61(6):588–602. [PubMed] [Google Scholar]
- Lund J., Stjernström H., Jorfeldt L., Wiklund L. Effect of extradural analgesia on glucose metabolism and gluconeogenesis. Studies in association with upper abdominal surgery. Br J Anaesth. 1986 Aug;58(8):851–857. doi: 10.1093/bja/58.8.851. [DOI] [PubMed] [Google Scholar]
- Mansour M. A., Stiegmann G. V., Yamamoto M., Berguer R. Neuroendocrine stress response after minimally invasive surgery in pigs. Surg Endosc. 1992 Nov-Dec;6(6):294–297. doi: 10.1007/BF02498863. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Essén P., Thorell A., Calder A. G., Anderson S. E., Ljungqvist O., Sandgren A., Grant I., Tjäder I., Ballmer P. E. Response of protein synthesis in human skeletal muscle to insulin: an investigation with L-[2H5]phenylalanine. Am J Physiol. 1994 Jul;267(1 Pt 1):E102–E108. doi: 10.1152/ajpendo.1994.267.1.E102. [DOI] [PubMed] [Google Scholar]
- Mealy K., Gallagher H., Barry M., Lennon F., Traynor O., Hyland J. Physiological and metabolic responses to open and laparoscopic cholecystectomy. Br J Surg. 1992 Oct;79(10):1061–1064. doi: 10.1002/bjs.1800791024. [DOI] [PubMed] [Google Scholar]
- Midgley A. R., Jr, Rebar R. W., Niswender G. D. Radioimmunoassays employing double antibody techniques. Acta Endocrinol Suppl (Copenh) 1969;142:247–254. doi: 10.1530/acta.0.062s247. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Bennegård K., Edén E., Emery P. W., Lundholm K. Urinary excretion and efflux from the leg of 3-methylhistidine before and after major surgical operation. Metabolism. 1984 Mar;33(3):250–256. doi: 10.1016/0026-0495(84)90046-5. [DOI] [PubMed] [Google Scholar]
- Rutberg H., Håkanson E., Anderberg B., Jorfeldt L., Mårtensson J., Schildt B. Effects of the extradural administration of morphine, or bupivacaine, on the endocrine response to upper abdominal surgery. Br J Anaesth. 1984 Mar;56(3):233–238. doi: 10.1093/bja/56.3.233. [DOI] [PubMed] [Google Scholar]
- Thorell A., Efendic S., Gutniak M., Häggmark T., Ljungqvist O. Development of postoperative insulin resistance is associated with the magnitude of operation. Eur J Surg. 1993 Nov-Dec;159(11-12):593–599. [PubMed] [Google Scholar]
- Thorell A., Efendic S., Gutniak M., Häggmark T., Ljungqvist O. Insulin resistance after abdominal surgery. Br J Surg. 1994 Jan;81(1):59–63. doi: 10.1002/bjs.1800810120. [DOI] [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Westland R. E., Nelson R. L., Gerich J. E., Service F. J. Glucose clamping using the Biostator GCIIS. Horm Metab Res. 1980 Apr;12(4):133–135. doi: 10.1055/s-2007-996224. [DOI] [PubMed] [Google Scholar]
- Vinnars E., Fürst P., Liljedahl S. O., Larsson J., Schildt B. Effect of parenteral nutrition on intracellular free amino acid concentration. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):184–187. doi: 10.1177/014860718000400223. [DOI] [PubMed] [Google Scholar]
- Wernerman J., von der Decken A., Vinnars E. Protein synthesis in skeletal muscle in relation to nitrogen balance after abdominal surgery: the effect of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1986 Nov-Dec;10(6):578–582. doi: 10.1177/0148607186010006578. [DOI] [PubMed] [Google Scholar]


