Abstract
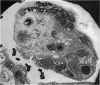
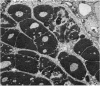
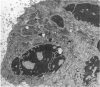
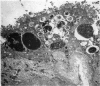
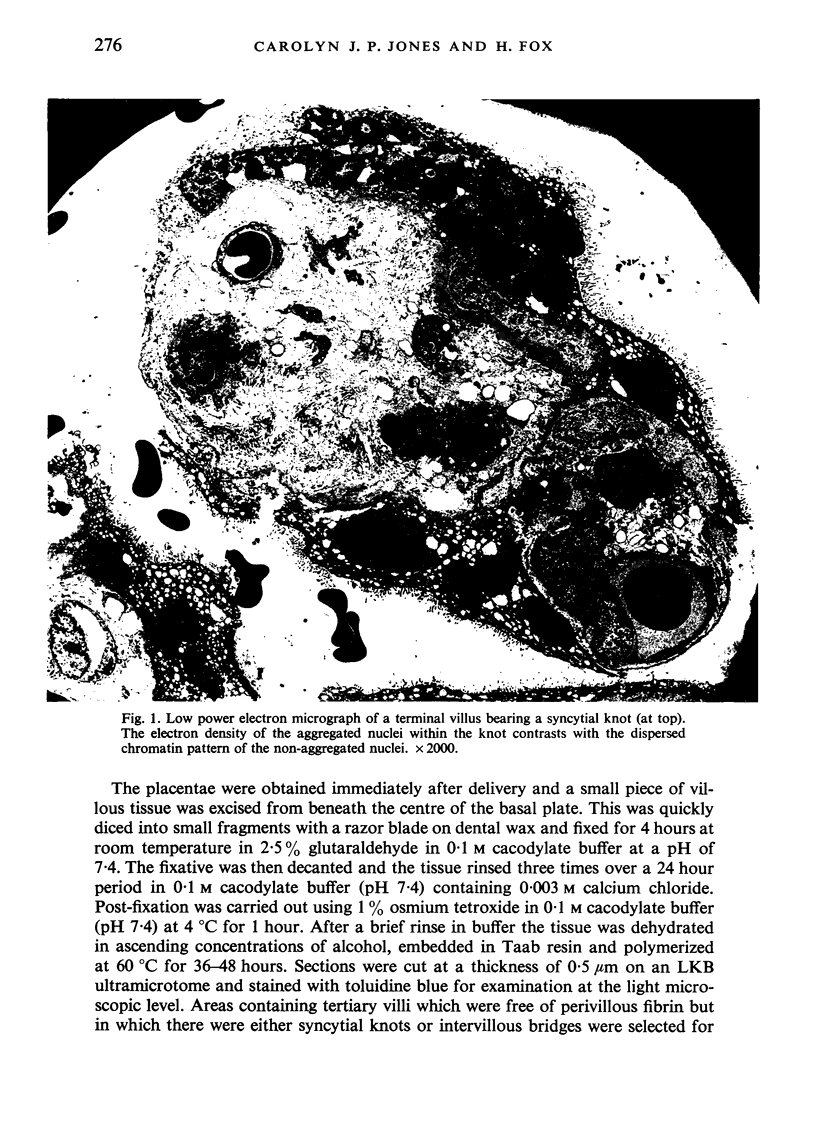
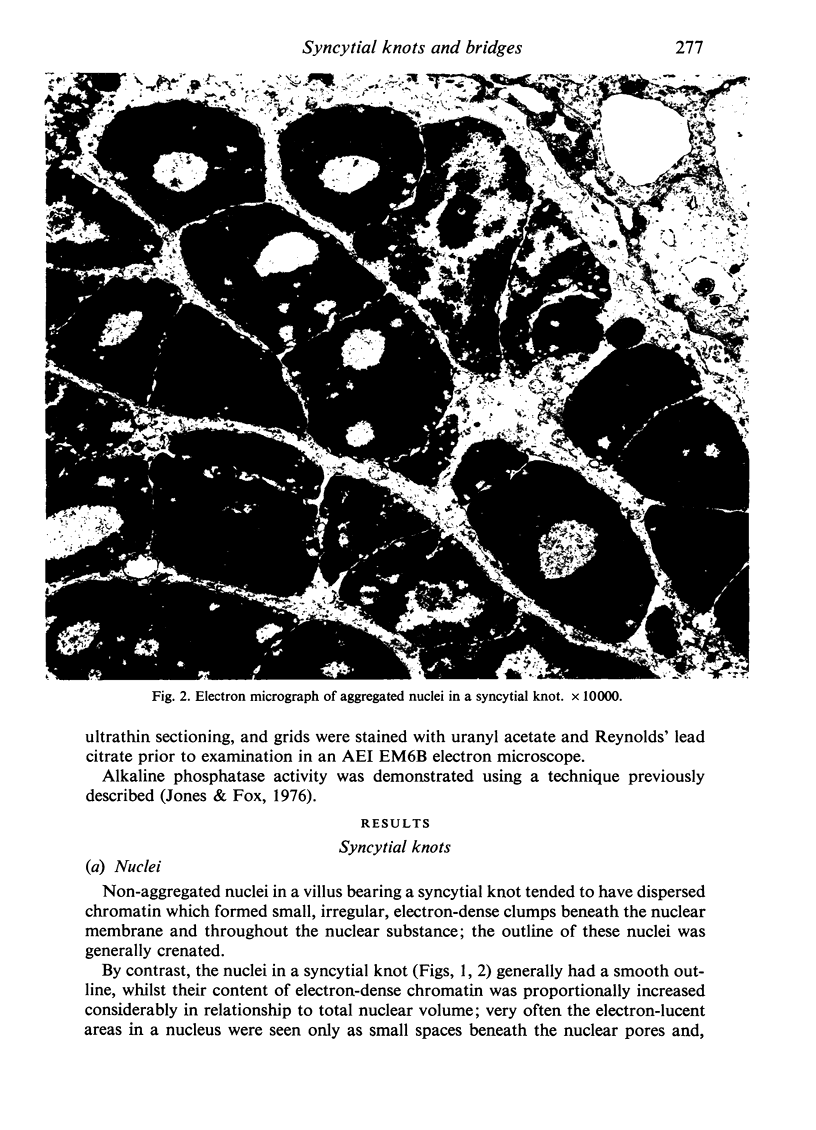
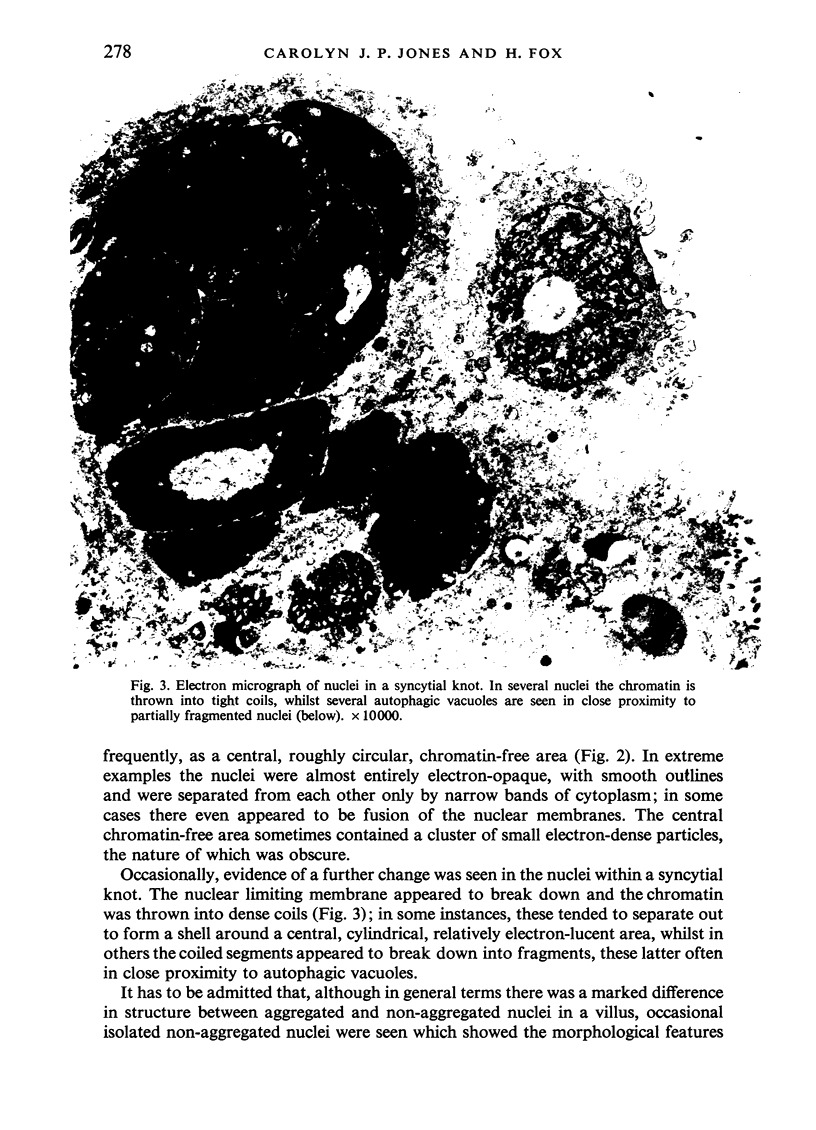
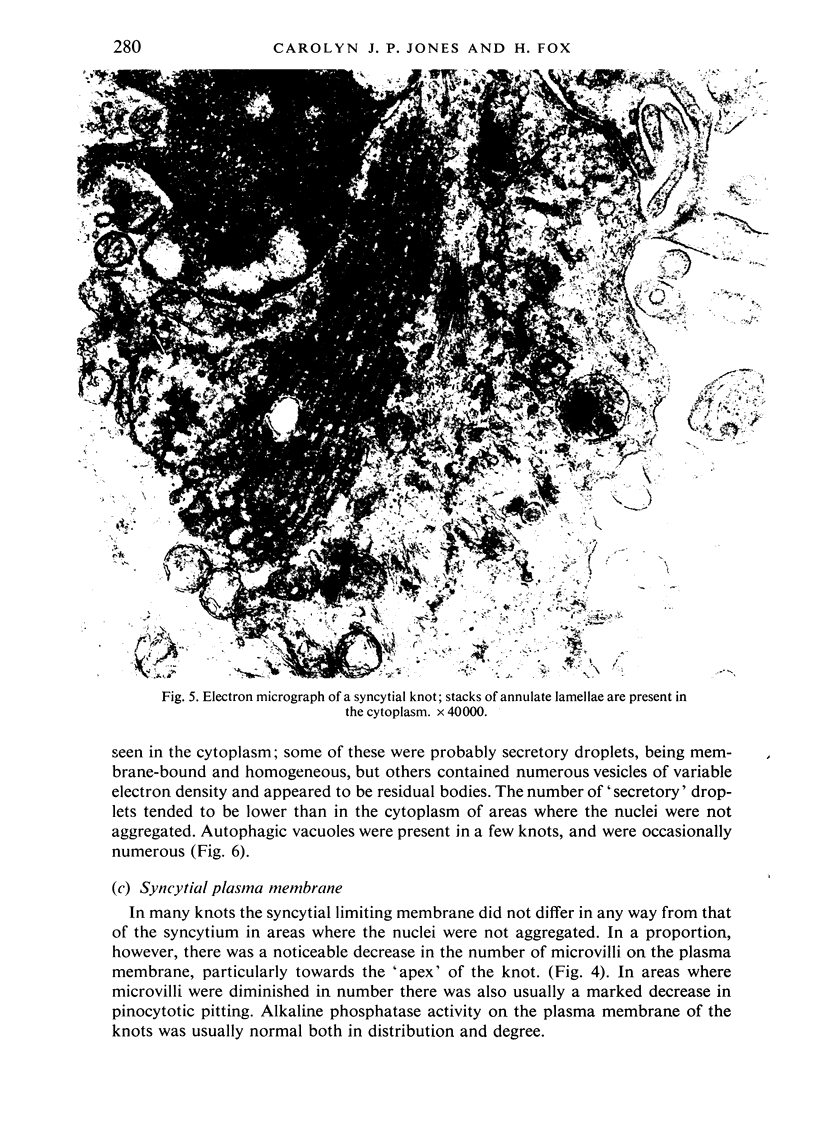
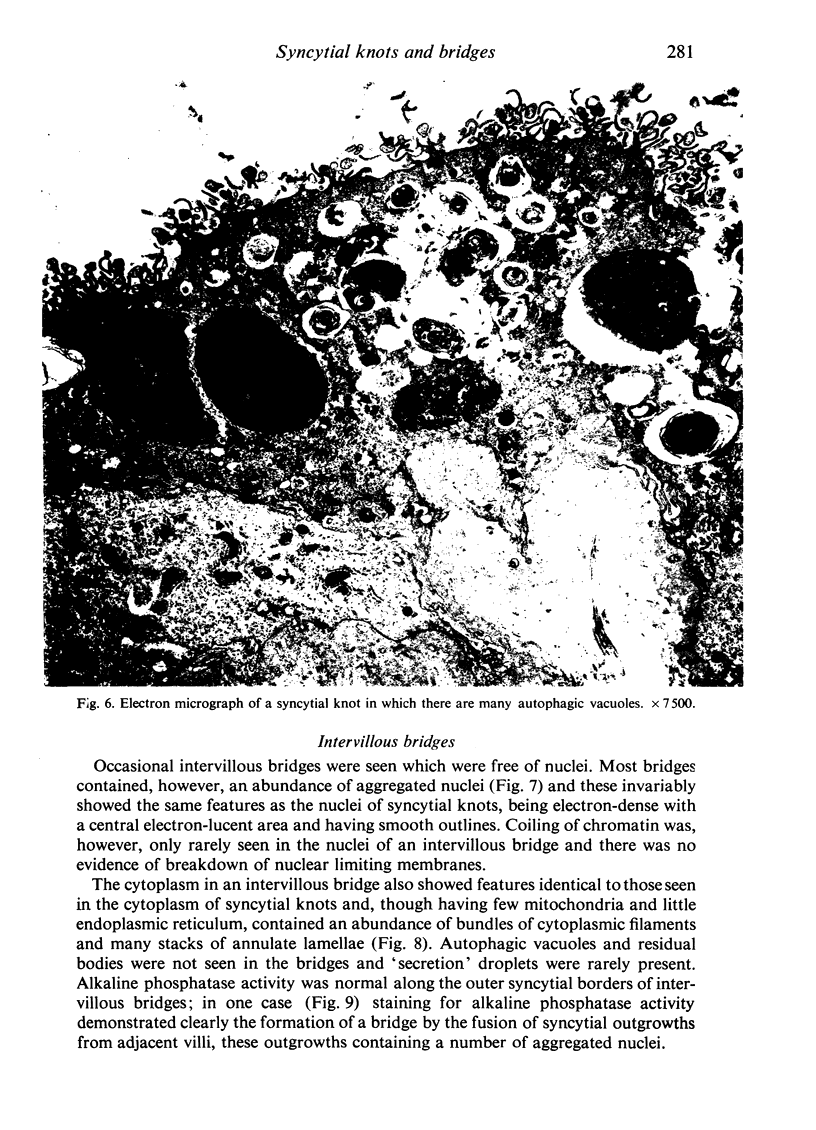
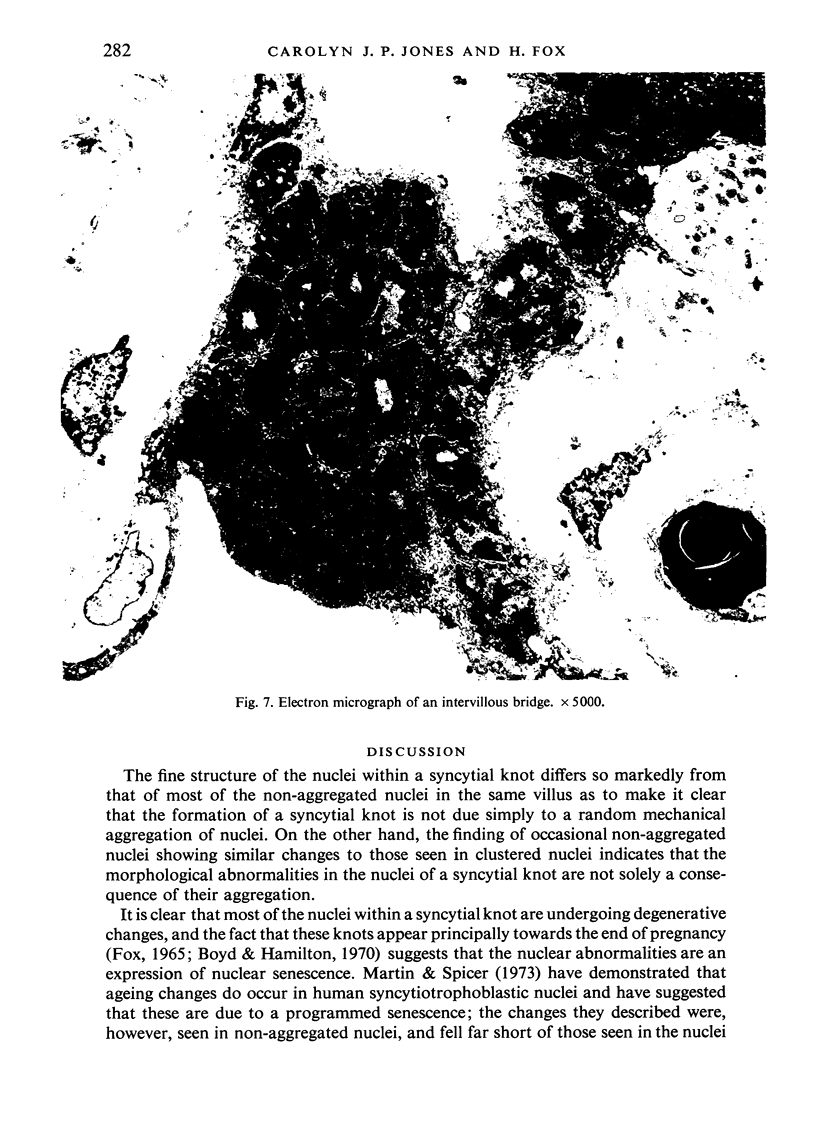
An electron microscopic study has shown that the syncytial knots of the villi of the human placenta contain aggregated nuclei which exhibit marked degenerative changes; within the cytoplasm there is an abundance of cytoplasmic filaments and many stacks of annulate lamellae. It is suggested that syncytial knots are a sequestration phenomenon in which senescent nuclear material is aggregated and removed from metabolically active areas of the syncytiotrophoblast. Intervillous bridges appear to be formed chiefly by fusion of syncytial knots from adjacent villi, and it seems reasonable that the effete material in a syncytial knot should be used for this purpose. The intervillous bridges hava a fine structure which suggests that they have a mechanical function, and this lends support to the theory that they form an internal strut system within the placenta.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aladjem S. The syncytial knot: a sign of active syncytial proliferation. Am J Obstet Gynecol. 1967 Oct 1;99(3):350–358. doi: 10.1016/s0002-9378(16)34542-2. [DOI] [PubMed] [Google Scholar]
- BOYD J. D., HAMILTON W. J. STROMAL TROPHOBLASTIC BUDS. J Obstet Gynaecol Br Commonw. 1964 Feb;71:1–10. doi: 10.1111/j.1471-0528.1964.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Benzo C. A. Annulate lamellae in hepatic and pancreatic beta cells of the chick embryo. 1. Am J Anat. 1974 May;140(1):139–143. doi: 10.1002/aja.1001400111. [DOI] [PubMed] [Google Scholar]
- FOX H. THE SIGNIFICANCE OF VILLOUS SYNCYTIAL KNOTS IN THE HUMAN PLACENTA. J Obstet Gynaecol Br Commonw. 1965 Jun;72:347–355. doi: 10.1111/j.1471-0528.1965.tb01469.x. [DOI] [PubMed] [Google Scholar]
- Gerl D., Eichhorn H., Eichhorn K. H., Franke H. Quantitative Messungen synzytialer Zellkernkonzentrationen der menschlichen Plazenta bei normalen und pathologischen Schwangerschaften. Zentralbl Gynakol. 1973 Feb 23;95(8):263–266. [PubMed] [Google Scholar]
- Jones C. J., Fox H. An ultrahistochemical study of the distribution of acid and alkaline phosphatases in placentae from normal and complicated pregnancies. J Pathol. 1976 Mar;118(3):143–151. doi: 10.1002/path.1711180303. [DOI] [PubMed] [Google Scholar]
- MERRILL J. A. Common pathological changes of the placenta. Clin Obstet Gynecol. 1963 Mar;6:96–109. doi: 10.1097/00003081-196303000-00009. [DOI] [PubMed] [Google Scholar]
- Martin B. J., Spicer S. S. Ultrastructural features of cellular maturation and aging in human trophoblast. J Ultrastruct Res. 1973 Apr;43(1):133–149. doi: 10.1016/s0022-5320(73)90074-9. [DOI] [PubMed] [Google Scholar]
- SHANKLIN D. R. The human placenta: a clinicopathologic study. Obstet Gynecol. 1958 Feb;11(2):129–138. [PubMed] [Google Scholar]
- Tominaga T., Page E. W. Accommodation of the human placenta to hypoxia. Am J Obstet Gynecol. 1966 Mar 1;94(5):679–691. doi: 10.1016/0002-9378(66)90401-7. [DOI] [PubMed] [Google Scholar]
- Wischnitzer S. The annulate lamellae. Int Rev Cytol. 1970;27:65–100. doi: 10.1016/s0074-7696(08)61246-2. [DOI] [PubMed] [Google Scholar]