Abstract
To investigate the origins and relationships of Australian and Melanesian populations, 611 males from 18 populations from Australia, Melanesia, and eastern/southeastern Asia were typed for eight single-nucleotide polymorphism (SNP) loci and seven short tandem-repeat loci on the Y chromosome. A unique haplotype, DYS390.1del/RPS4Y711T, was found at a frequency of 53%–69% in Australian populations, whereas the major haplotypes found in Melanesian populations (M4G/M5T/M9G and DYS390.3del/RPS4Y711T) are absent from the Australian populations. The Y-chromosome data thus indicate independent histories for Australians and Melanesians, a finding that is in agreement with evidence from mtDNA but that contradicts some analyses of autosomal loci, which show a close relationship between Australian and Melanesian (specifically, highland Papua New Guinean) populations. Since the Australian and New Guinean landmasses were connected when first colonized by humans ⩾50,000 years ago but separated some 8,000 years ago, a possible way to reconcile all the genetic data is to infer that the Y-chromosome and mtDNA results reflect the past 8,000 years of independent history for Australia and New Guinea, whereas the autosomal loci reflect the long preceding period of common origin and shared history. Two Y-chromosome haplotypes (M119C/M9G and M122C/M9G) that originated in eastern/southeastern Asia are present in coastal and island Melanesia but are rare or absent in both Australia and highland Papua New Guinea. This distribution, along with demographic analyses indicating that population expansions for both haplotypes began ∼4,000–6,000 years ago, suggests that these haplotypes were brought to Melanesia by the Austronesian expansion. Most of the populations in this study were previously typed for mtDNA SNPs; population differentiation is greater for the Y chromosome than for mtDNA and is significantly correlated with geographic distance, a finding in agreement with results of similar analyses of European populations.
Introduction
The relationships of aboriginal Australian and Melanesian populations remain enigmatic. The initial occupation of Sahul (the combined Australia–New Guinea landmass) occurred ⩾50,000–60,000 years ago, on the basis of dating of archaeological (Roberts et al. 1990) and fossil (Thorne et al. 1999) remains, and by 30,000–40,000 years ago humans had spread throughout present-day New Guinea and Island Melanesia, including New Britain, New Ireland, and the Solomon Islands (Groube et al. 1986; Allen et al. 1988; Wickler and Spriggs 1988; Allen 1989; Pavlides and Gosden 1994). Australia and New Guinea were separated by rising sea levels during the end of the last glaciation, ∼8,000 years ago (White and O'Connell 1982; Jones 1995), leaving a period of perhaps as much as 50,000 years during which Australian and New Guinean populations potentially had a shared history.
Subsequent migrations influenced island and coastal Melanesian populations; in particular, the Austronesian expansion that lead eventually to the colonization of Polynesia reached coastal New Guinea and island Melanesia about 3,500 years ago (Bellwood 1978, 1987, 1989). These migrations had little (if any) linguistic or cultural impact on highland New Guinea or Australian populations (Bellwood 1978, 1987; Blust 1984/85, 1999; Spriggs 1985; Kirch 1995); moreover, genetic markers associated with the origin of Polynesians are rare or absent in highland Papua New Guinea and/or Australia (Hill et al. 1989; O'Shaughnessy et al. 1990; Lum et al. 1994; Melton et al. 1995; Redd et al. 1995; Sykes et al. 1995; Roberts-Thomson et al. 1996; Kayser et al. 2000a).
According to this scenario, Australian and highland New Guinean populations have a common origin and share a long genetic history that was little influenced by subsequent migrations, and hence they should be more closely related to one another than to other populations, such as those of island/coastal Melanesia. However, the genetic evidence concerning the relationships among Australian, highland New Guinean, and coastal/island Melanesian populations are equivocal. Some mtDNA data support the common-origin hypothesis (van Holst Pellekaan et al. 1997, 1998), but other studies support independent origins for highland Papua New Guineans and Australians (Stoneking et al. 1990; Redd and Stoneking 1999). In particular, Redd and Stoneking (1999) found links between highlanders from Papua New Guinea (PNG) and sub-Saharan Africans and between Australians and southern Indians. Data from autosomal DNA markers are similarly contradictory. For example, one study of α-globin–gene haplotypes supports a common origin of PNG highlanders and Australians (Roberts-Thomson et al. 1996), whereas others report major genetic differences between populations from the two regions, supporting the independent-origin hypothesis (Yenchitsomanus et al. 1986; Tsintsof et al. 1990).
What is missing so far is a comparative study of Y-chromosome markers, to address the origins of Australians and Melanesians. The Y chromosome is haploid, paternally inherited, and (for the most part) nonrecombining. Thus, the use of polymorphic Y-chromosome markers enables one to trace paternal lineages, in the same way as it is possible to trace maternal lineages by using polymorphic mtDNA markers. One advantage of Y-chromosome markers, compared with mtDNA, is that Y-chromosome polymorphisms seem to show a higher degree of population specificity (Seielstad et al. 1998), making them more informative for tracing population relationships. Another advantage of the Y chromosome compared with mtDNA is that different types of markers with different mutation rates are available, offering the possibility of investigation of different evolutionary timescales.
We have therefore used a combined analysis of the slowly evolving Y-chromosome single-nucleotide-polymorphisms (SNPs) and the more rapidly evolving Y-chromosome short-tandem-repeat (STR) polymorphisms, to investigate the origins and relationships of Australians, PNG highlanders, and coastal/island Melanesians. We typed eight Y-chromosome SNPs and seven Y-chromosome STRs in 132 males from highland/coastal PNG and Island Melanesia, as well as in 95 males from two Australian populations. An additional 384 males from 12 populations from eastern/southeastern Asia and Polynesia were studied for the presence of the same markers, to shed further light on the relationships among Melanesian and Australian populations.
Samples and Methods
Samples
The geographic location of DNA samples used in this study are shown in figure 1, whereas the sample sizes are given in table 1. Further description of the population samples can be found elsewhere (Kayser et al. 2000a).
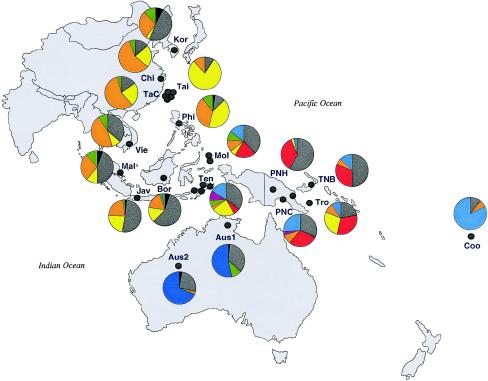
Figure 1.
Y-chromosome haplotypes, as defined by six Y-chromosome SNPs and two specific deletions at the Y-chromosome STR locus DYS390, and their frequency distribution in human populations from eastern/southeastern Asia, Melanesia, Polynesia, and Australia. Color codings are as follows: black, complete ancestral haplotype (with respect to the markers analyzed); gray, M9G; red, M4G/M5T/M9G; yellow, M119C/M9G; orange, M122C/M9G; green, RPS4Y711T; dark blue, DYS390.1del/RPS4Y711T; purple, DYS390.3del/M9G; and light blue, DYS390.3del/RPS4Y711T. Abbreviations are as follows: Aus1 = Australia, Arnhem Land; Aus2 = Australia, Sandy Desert; Bor = southern Borneo; Chi = China; Coo = Cook Islands; Jav = Java; Kor = Korea; Mal = Malaysia; Mol = Moluccas; Phi = Philippines; PNC = PNG coast; PNH = PNG highlands; TaC = Taiwan Chinese; Tai = Taiwan aborigines; Ten = Nusa Tenggara; TNB = Tolai New Britain; Tro = Trobriand Islands; Vie = Vietnam.
Table 1.
Y-Chromosome Haplotype-Diversity Characteristics of 18 Populations from Asia/Oceania
|
No. (Diversity ± SD) of Haplotype |
|||
| Population (n) | Y-Chromosome SNP/DYS390 Deletiona | Y-Chromosome STR Haplotypesb | Y-Chromosome SNP/STRc |
| Korea (25) | 5 (.670 ± .068) | 24 (.997 ± .013) | 24 (.997 ± .013) |
| China (36) | 4 (.605 ± .071) | 34 (.997 ± .008) | 34 (.997 ± .008) |
| Taiwan: | |||
| Chinese (26) | 4 (.612 ± .081) | 24 (.994 ± .013) | 24 (.994 ± .013) |
| Aborigines (43) | 3 (.361 ± .085) | 30 (.980 ± .010) | 31 (.981 ± .010) |
| Philippines (39) | 5 (.699 ± .043) | 26 (.964 ± .017) | 26 (.964 ± .017) |
| Vietnam (11) | 4 (.709 ± .099) | 11 (1.000 ± .038) | 11 (1.000 ± .039) |
| Malay (18) | 5 (.739 ± .076) | 18 (1.000 ± .019) | 18 (1.000 ± .019) |
| Java (53) | 5 (.650 ± .044) | 38 (.980 ± .009) | 38 (.980 ± .009) |
| Southern Borneo (40) | 6 (.628 ± .071) | 39 (.999 ± .006) | 39 (.999 ± .006) |
| Moluccas (34) | 6 (.788 ± .045) | 21 (.961 ± .017) | 21 (.961 ± .017) |
| Nusa Tenggara (31) | 7 (.804 ± .044) | 30 (.998 ± .009) | 30 (.998 ± .009) |
| Tolai New Britain (16) | 4 (.675 ± .085) | 14 (.983 ± .028) | 14 (.983 ± .028) |
| Trobriand Islands (54) | 5 (.772 ± .024) | 38 (.983 ± .008) | 39 (.985 ± .007) |
| PNG: | |||
| Coast (31) | 5 (.759 ± .034) | 27 (.989 ± .012) | 27 (.989 ± .012) |
| Highlands (31) | 4 (.553 ± .060) | 26 (.985 ± .013) | 26 (.985 ± .013) |
| Cook Islands (28) | 3 (.320 ± .106) | 15 (.894 ± .043) | 15 (.894 ± .043) |
| Australia: | |||
| Arnhem Land (60) | 4 (.593 ± .039) | 42 (.962 ± .017) | 42 (.962 ± .017) |
| Sandy Desert (35) | 4 (.476 ± .077) | 25 (.966 ± .020) | 25 (.966 ±. 020) |
| Total (611) | 9 | 413 | 430 |
Based on six SNPs and two specific STR deletions.
Based on seven STRs.
Based on six SNPs and seven STRs.
Genetic Analyses
The Y-chromosome STR loci DYS19 (or DYS394), DYS389I, DYS389II, DYS390, DYS391, DYS392, and DYS393 were analyzed by use of previously described allelic nomenclature, genotyping protocols, and (for DYS390) DNA sequencing protocols (Kayser et al. 1997, 2000a; Redd et al. 1997). The Y-chromosome SNPs M9 (Underhill et al. 1997), RPS4Y711 (Bergen et al. 1999), and M122 (Underhill et al. 2000) were typed as described by Kayser et al. (2000a). The Y-chromosome SNPs M4, M5, M16, and M21 (Underhill et al. 1997) and M119 (Underhill et al. 2000) were amplified according to the following standard PCR conditions, (for additional information, see table 2): 0.4 μM each primer, 1 × GeneAmp PCR buffer II (PE Biosystems), 1.5 mM MgCl2, 1 U of either AmpliTaq Gold DNA polymerase or AmpliTaq DNA polymerase (PE Biosystems), 0.1 mM each dNTP (Pharmacia Biotech), 25 μg bovine serum albumin (Sigma), 10–100 ng of DNA, and a hot-start PCR of 4 min at 95°C, for initial denaturation (11 min for AmpliTaq Gold DNA polymerase), followed by 30–50 cycles of 30 s at 94°C, 30 s at the locus-specific annealing temperature, and 45 s at 72°C, followed by a final step of 10 min at 72°C. Genotypes were determined by either PCR-RFLP or sequence-specific oligonucleotide (SSO) hybridization (for details, see table 3). Restriction-endonuclease digestions were performed overnight, according to the instructions of the manufacturers (see table 2). Fragments were resolved on 3% NuSieve agarose gels and were stained with ethidium bromide. SSO typing and chemiluminescent detection were performed as described elsewhere (Melton et al. 1995). In a subset of samples, a polymorphic 5-bp deletion at the Y-chromosome locus M175 (Underhill et al. 2000) was typed. PCR was performed with the 5′-FAM–labeled forward primer 5′-tctctgaatcaggcacatgc-3′ and the reverse primer 5′-tttgtccaatgctgaaagtaagtat-3′, with the standard PCR conditions as given above, except that 1.8 mM MgCl2, 0.2 μM each primer, an annealing temperature of 57°C, and 30–40 cycles were used. A 2.5-μl aliquot of the labeled PCR product was separated on a 5% Long Ranger (FMC Bioproducts) gel on an ABI PRISM 377 DNA Sequencer (PE Biosystems), under the Y-chromosome STR–typing conditions. The resulting PCR products were 142 bp for the ancestral allele and 137 bp for the mutant allele.
Table 2.
PCR Primers and PCR/RFLP Typing Parameters for Y-Chromosome SNP Analysis
|
Parameter |
||||||
| PCR |
PCR-RFLP |
|||||
| Primer(5′→3′) |
Fragment(s)(bp) |
|||||
| Marker | Forward | Reverse | Tanna(°C) | Enzyme | Ancestral | Mutant |
| M4 A→G | TCCTAGGTTATGATTACAGAGCG | GGCACAAGCTGTTCCAGTACA | 60 | NdeI | 164 | 80 and 84 |
| M5 C→T | GGTTTATACTGACCTGCCAATGT | CTATTACCAAAGGTTTGTGTTAGG | 59b | …c | …c | …c |
| M9 C→G | GCAGCATATAAAACTTTCAGG | GAAATGCATAATGAAGTAAGCG | 54 | HinfI | 100 and 64 | 164 |
| M16 C→A | ATATTGTTATGTCATTTGAACCCAGG | TGTTCTATTAAAAGCTGACAAATCCAA | 59 | …c | …c | …c |
| M21 A→T | AAGCCCTTGATTTTTATTTATC | AACAGCAGATTTGAGCAGG | 51d | …c | …c | …c |
| M119 A→C | CGCAGTGCTATGTGTTTATTTG | GTTATGGGTTATTCCAATTCAGC | 56e | CviTI | 31 and 62 | 93 |
| M122 T→C | GTTGCCTTTTGGAAATGAATAAATC | CACTTGCTCTGTGTTAGAAAAGATAGC | 58 | Hsp92II | 58 and 51 | 109 |
| RPS4Y711 C→T | CTGTACTTACTTTTATCTCCTC | CAGCAACAGTAAGTCGAATG | 54 | BslI | 34 and 57 | 91 |
Annealing temperature.
Touchdown PCR: decrease of Tann, in increments of 0.5°C, starting from 64°C, for the first 10 cycles, followed by 20 cycles at 59°C.
Typed only via SSO hybridization (see table 3).
Touchdown PCR: decrease of Tann, in increments of 0.4°C, starting from 58°C, for the first 18 cycles, followed by 30–40 cycles at 51°C.
Touchdown PCR: decrease of Tann, in increments of 0.4°C, starting from 63°C, for the first 18 cycles, followed by 30–40 cycles at 56°C.
Table 3.
SSO-Typing Probes and Conditions for Y-Chromosome SNP Analysis
|
Ancestral Allele |
Mutant Allele |
|||
| Marker | Probea(5′-3′) | Stringent-Wash Conditions | Probea(5′-3′) | Stringent-Wash Conditions |
| M4 A→G | ATGATATTCATATATAATC | Once at 45°C for 5 min | GATTACATATGAATATCAT | Twice at 50°C for 10 min |
| M5 C→T | AGGAAGAAGCAGAAGGAGA | Twice at 55°C for 10 min | TCTCCTTCTACTTCTTCCT | Once at 55°C for 5 min |
| M9 C→G | GATGGTTGAATCCTCTTTAT | Twice at 55°C for 10 min | ATAAAGAGCATTCAACCATC | Twice at 55°C for 10 min |
| M16 C→A | TCTTCGAACCCTCAGTTTT | Twice at 55°C for 10 min | AAAACTGAGTGTTCGAAGA | Once at 55°C for 5 min |
| M21 A→T | AACTGAAACTATACAGTCT | Once at 55°C for 5 min | AGACTGTATTGTTTCAGTT | Once at 55°C for 10 min |
| M119 A→C | GCATACAGGCTAAAATAGCA | Twice at 55°C for 15 min | GCATACAGGCGAAAATAGCA | Once at 55°C for 10 min |
5′-Biotin labeled, for chemiluminescent detection.
Statistical Analyses
Since the DYS389II PCR product also contains DYS389I, for all statistical analyses a simple subtraction of the DYS389I repeat length from that of DYS389II was done, to avoid double-counting variation at DYS389I. The modified Y-chromosome STR data were then analyzed with respect to haplotype diversity and the associated SD, mean number of pairwise differences between haplotypes, pairwise FST and RST (or ΦST) values, and associated P values based on 10,000 permutations, by means of the software package ARLEQUIN (version 2.000) (Schneider et al. 2000). Principle-coordinate (PC) analysis was performed by means of the software package STATISTICA (Statsoft). Median-joining network analysis (Bandelt et al. 1999) was performed by means of Network 2.0b software (Phylogenetic Network Analysis Shareware Software). Correlation analysis and statistical tests of correlation coefficients by Mantel permutation testing were performed by means of the program PERMUTE! (version 3.4), with 1,000 permutations (Legendre et al. 1994). Coalescence analysis of the data was performed by means of the program BATWING (University of Aberdeen Department of Mathematical Sciences). The principles of the Markov-chain Monte Carlo–based inference method implemented in this program have been described elsewhere (Wilson and Balding 1998). We chose a two-phase population model, in which, in the past, the population size was of constant size N and was followed by a period of exponential growth up to the present. Thus, the demography of the population is defined by three parameters (initial population size, growth rate, and time of expansion). We allow for locus-specific mutation rates, which adds a further seven parameters to our evolutionary model. Since BATWING implements a Bayesian approach to inference, the prior-probability distribution of the model parameters must be specified. We assigned gamma-distributed priors to the mutation rates of the seven loci, adjusted to the corresponding estimates reported by Kayser et al. (2000b). For each locus, we chose a gamma distribution such that the mode was equal to the respective estimate and the SD was inversely related to the number of meioses investigated by Kayser et al. (2000b). To specify our uncertainty about the population size N, we used a lognormal (6,1) prior distribution. This distribution has mode 148, median 403, and mean 665 and represents a rather small initial founder population. The growth-rate prior was an exponential distribution with mean .01, which covers the simple constant-population-size model as well as reasonable growth rates for human populations. The length of the growth period (measured in units of N × generation time) also had an exponential prior, with mean 1. To produce the results, we sampled 20,000 times from the Markov chain after discarding the first 2,000 samples. Between samples, 100 attempts to update the model parameters were performed, and each such attempt was followed by 40 trials to update the genealogy. The resulting 20,000 samples were used to estimate posterior distributions of the model parameters, as well as the posterior of the time back to the most recent common ancestor (TMRCA). We also computed the median, the mode, and a 95% equal-tailed interval for each posterior probability. We checked the robustness of our results by using a variety of prior distributions for mutation rates as well as for population parameters. The results were reasonably stable under different priors. Thus, we conclude that our results reflect the information in the data, rather than the prior distributions.
Results
To investigate their Y-chromosome history, indigenous human populations from several geographic regions of Melanesia (n=132)—including populations from highland PNG, coastal PNG, New Britain, and the Trobriand Islands—and from two regions of Australia (n=95)—including populations from Arnhem Land and the Great Sandy Desert—were studied at eight Y-chromosome SNP loci and at seven Y-chromosome STR loci. An additional 384 individuals from 12 populations of eastern/southeastern Asia and Polynesia were studied for the same markers, to shed further light on the relationships among Melanesian and Australian populations.
Y-Chromosome Haplotypes and Their Diversity
The Y-chromosome SNPs M4, M5, M9, M16, M21, M119, M122, and RPS4Y711 were chosen for analysis, on the basis of previous reports of polymorphism for these markers in Asian/Oceanic populations (Underhill et al. 1997, 2000; Bergen et al. 1999; Karafet et al. 1999; Su et al. 1999; Kayser et al. 2000a). For two markers, M16 and M21, the mutant allele was not observed in the entire sample of 611 individuals from 18 populations. The mutant allele of M16 (M16A) was initially detected in two Melanesians from Bougainville Island (Underhill et al. 1997, 2000). Since it was found neither within the 132 Melanesians analyzed here nor anywhere else, M16A most likely reflects a very recent mutational event of restricted regional distribution. The mutant allele of M21 (M21T) was initially identified in a single Australian (Underhill et al. 1997); however, additional Y-chromosome SNP data indicate probable European ancestry of this individual, since this allele is associated with M170 (Underhill et al. 2000).
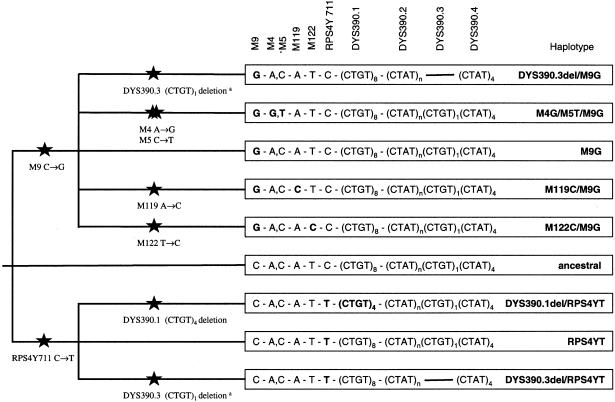
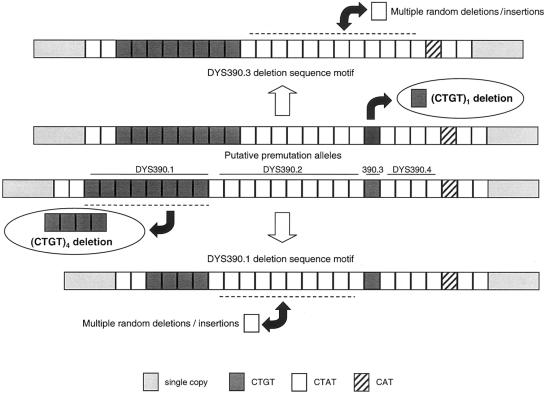
With the remaining six SNPs, six Y-chromosome haplotypes were observed (fig. 2). The mutant alleles for M4, M5, M119, and M122 were observed exclusively on Y chromosomes that carried the mutant M9G allele. Mutations at M4 and M5 were always associated with one another, suggesting the occurrence of two mutations during a relatively short time span. At the Y-chromosome STR locus DYS390, two specific deletions, at the subloci 390.1 and 390.3 (fig. 3), were observed that have been described elsewhere (Forster et al. 1998; Kayser et al. 2000a). The 390.3 deletion was observed not only on RPS4Y711T chromosomes, as reported elsewhere by us (Kayser et al. 2000a), but also was found in four RPS4Y711C/M9G chromosomes from Nusa Tenggara and coastal PNG. This most likely reflects a recurrent mutation event involving the 390.3 deletion. The 390.1 deletion was observed exclusively in association with RPS4Y711T.
Figure 2.
Y-chromosome haplotypes, based on six Y-chromosome SNPs and two specific deletions at the Y-chromosome STR locus DYS390, and their phylogenetic relationship. The superscript “a” denotes a recurrent mutation event.
Figure 3.
Structure of the Y-chromosome STR locus DYS390, and proposed mutational events leading to the DYS390.1 deletion and the DYS390.3 deletion.
Y-chromosome haplotype-diversity values (table 1) were calculated for three classes of haplotypes: (i) those based on six SNPs and the two DYS390 deletions (Y-chromosome SNP/STRdel haplotypes); (ii) those based on seven STRs (Y-chromosome STR haplotypes); and (iii) those based on six SNPs and seven STRs combined (Y-chromosome SNP/STR haplotypes). A total of nine Y-chromosome SNP/STRdel haplotypes were observed among the 611 individuals from 18 populations, and haplotype diversity ranged from .32 in the Cook Islanders to .80 in the Nusa Tenggara sample, with most populations having diversity values >.65. A total of 413 Y-chromosome STR haplotypes were found in the entire sample set. The Y-chromosome STR haplotype diversity ranged from .89 in the Cook Islanders to 1.00 in Malay and Vietnamese, with most of the populations having diversity values >.98. Including the Y-chromosome SNPs with the Y-chromosome STRs increases the number of haplotypes observed, from 413 (for Y-chromosome STRs alone) to 430 (for Y-chromosome SNPs/STRs) (individual haplotypes are available on request from the authors). This means that, in 17 cases, identical Y-chromosome STR haplotypes are found on different Y-chromosome SNP haplotypes, which is evidence of recurrent mutations (most likely at Y-chromosome STR loci).
Y-Chromosome Haplotypes: Frequency Distribution and Demographic Inference
The frequencies and distribution of the nine Y-chromosome SNP/STRdel haplotypes are given in table 4 and figure 1. We have described the DYS390.3del/RPS4Y711T and M122C/M9G haplotypes in detail elsewhere (Kayser et al. 2000a); the remaining seven haplotypes are described and analyzed below. The DYS390.3del/RPS4Y711T haplotype, which was the major Polynesian haplotype, with a frequency of 82% in the Cook Islands (Kayser et al. 2000a), was found at a frequency of 9%–26% in coastal PNG and Island Melanesia and in 1 of 31 PNG Highlanders but was totally absent from Australia (table 4 and fig. 1). In addition, the M122C/M9G haplotype, which was the major eastern Asian haplotype, with a frequency of 28%–58% (Kayser et al. 2000a), was observed at a frequency of 6%–10% in coastal PNG and Island Melanesia but was absent from highland PNG and was nearly absent (1 of 95) from Australia (table 4 and fig. 1).
Table 4.
Y-Chromosome Haplotypes and Their Frequency Distribution, and Y-Chromosome Microsatellite–Based Diversity, in 18 Populations from Asia/Oceania (n=611)
| Population(n) | Ancestral | M9G | M4G/M5T/M9G | M119C/M9G | M122C/M9G | DYS390.3del/M9G | RPS4YT | DYS390.1del/RPS4YT | DYS390.3del/RPS4YT |
| Frequency |
|||||||||
| Korea (25) | 8.0 | 48.0 | 0 | 4.0 | 28.0 | 0 | 12.0 | 0 | 0 |
| China (36) | 0 | 13.9 | 0 | 22.2 | 58.3 | 0 | 5.6 | 0 | 0 |
| Taiwan: | |||||||||
| Chinese (26) | 0 | 15.4 | 0 | 23.1 | 57.7 | 0 | 3.9 | 0 | 0 |
| Aborigines (43) | 0 | 9.3 | 0 | 79.1 | 11.6 | 0 | 0 | 0 | 0 |
| Philippines (39) | 2.6 | 10.3 | 0 | 41.0 | 35.9 | 0 | 10.3 | 0 | 0 |
| Vietnam (11) | 0 | 36.4 | 0 | 9.1 | 45.5 | 0 | 9.1 | 0 | 0 |
| Malay (18) | 5.6 | 44.4 | 0 | 11.1 | 27.8 | 0 | 11.1 | 0 | 0 |
| Java (53) | 1.9 | 50.9 | 0 | 22.6 | 22.6 | 0 | 1.9 | 0 | 0 |
| Southern Borneo (40) | 5.0 | 57.5 | 0 | 15.0 | 17.5 | 0 | 2.5 | 0 | 2.5 |
| Moluccas (34) | 0 | 38.2 | 20.6 | 5.9 | 11.8 | 0 | 8.8 | 0 | 14.7 |
| Nusa Tenggara (31) | 0 | 35.5 | 6.5 | 22.6 | 3.2 | 9.7 | 6.5 | 0 | 16.1 |
| Tolai New Britain (16) | 0 | 50.0 | 31.2 | 0 | 6.3 | 0 | 0 | 0 | 12.5 |
| Trobriand Islands (54) | 0 | 22.2 | 31.5 | 27.8 | 9.3 | 0 | 0 | 0 | 9.3 |
| PNG: | |||||||||
| Coast (31) | 0 | 32.3 | 29.0 | 0 | 9.7 | 3.2 | 0 | 0 | 25.8 |
| Highlands (31) | 0 | 58.1 | 35.5 | 3.2 | 0 | 0 | 0 | 0 | 3.2 |
| Cook Islands (28) | 0 | 10.7 | 0 | 0 | 7.1 | 0 | 0 | 0 | 82.1 |
| Australia: | |||||||||
| Arnhem Land (60) | 1.7 | 35.0 | 0 | 0 | 0 | 0 | 10.0 | 53.3 | 0 |
| Sandy Desert (35) | 2.9 |
25.7 |
0 |
0 |
2.9 |
0 |
0 |
68.6 |
0 |
| No. of Individuals/No. of Y-Chromosome STR Haplotypes (Diversity ± SD) |
|||||||||
| Overall | 9/9 (1.000 ± .052) | 196/154 (.996 ± .001) | 51/41 (.988 ± .007) | 111/59 (.977 ± .006) | 108/81 (.984 ± .006) | 4/4 (1.000 ± .177) | 26/22 (.985 ± .0162) | 56/31 (.932 ± .024) | 50/29 (.958 ± .011) |
M4G/M5T/M9G Haplotype
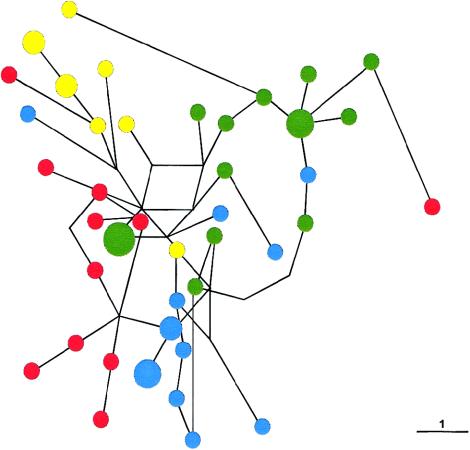
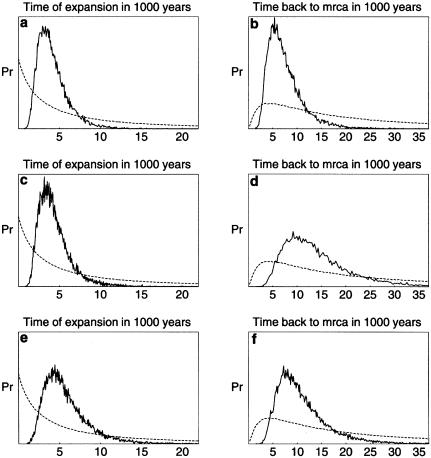
The M4G/M5T/M9G haplotype was found in all Melanesian populations analyzed, at a frequency of 29%–35%, and in the Moluccas and Nusa Tenggara islands from eastern Indonesia, at a frequency of 20.6% and 6.5%, respectively. It was absent from Australia and from all eastern/southeastern Asian populations and from Polynesia (table 4 and fig. 1). Its highest frequency, 35.5%, was in highland PNG. In total, 51 individuals were found carrying this haplotype, in whom 41 Y-chromosome STR haplotypes were observed (h=.996). RST analysis of Y-chromosome STR haplotypes revealed four groups that differed significantly (P<.01) from one another: those from highland PNG, coastal PNG/New Britain, the Trobriand Islands, and eastern Indonesia. The highest Y-chromosome STR haplotype diversity was found in highland PNG (1.00±.04), and lower diversities were found in coastal PNG/New Britain (.96±.05), eastern Indonesia (.94±.07), and the Trobriand Islands (.93±.05). Y-chromosome STR haplotypes were shared only within groups, not between groups. In a median-joining network analysis of the Y-chromosome STR haplotypes associated with the M4G/M5T/M9G haplotype, most Y-chromosome STR haplotypes are connected by single-mutation steps (fig. 4). Clustering of haplotypes according to groups is evident from the network, with the exception of a few haplotypes. Only three reticulations were observed in the network, indicating very close relationships among the Y-chromosome STR haplotypes. When differences in the mutation rate of the Y-chromosome STRs used for haplotype construction (Kayser et al. 2000b) were considered in the network calculation, the same picture was obtained (data not shown). To infer demographic data from associated Y-chromosome STR haplotypes, a coalescence analysis using a Bayesian approach was employed. The TMRCA of all 51 individuals carrying the M4G/M5T/M9G haplotype was estimated to be 9,700 years (table 5 and fig. 5). A signal of moderate population growth (growth rate .014/generation) was detected, dating to the start of a population expansion ∼5,100 years ago.
Figure 4.
Median-joining network of 41 Y-chromosome STR haplotypes found in 51 individuals carrying the M4G/M5T/M9G haplotype. Circles denote the Y-chromosome STR haplotypes, with the area of the circle being proportional to the number of individuals. Lines denote mutation steps (one-step distance is indicated); parallel lines represent identical mutations. Color coding is as follows: red, highland PNG; blue, coastal PNG/New Britain; green, Trobriand Islands; yellow, eastern Indonesia.
Table 5.
Demographic Inferences for Y-Chromosome Mutations, Based on Associated Y-Chromosome STR Haplotype Variation
|
Median (95% Equal-Tailed Interval) |
||||
| Y-Chromosome Mutation(Y-Chromosome Background) | Initial Effective Population Size/1,000 Individuals | Population Growth Rate/Generation(×10−3) | Start of Population Expansion(×103 years) | TMRCA(×103 years) |
| Prior probabilitiesa | .40 (.06–2.86) | 6.9 (.3–36.9) | 4.9 (.1–64.6) | 17.1 (1.6–100.6) |
| Posterior probabilities: | ||||
| DYS390.1 deletion (RPS4Y711T) | .15 (.05–.46) | 24.7 (9.3–56.9) | 3.7 (1.7–9.0) | 6.6 (3.0–17.5) |
| M4G/M5T (M9G) | .24 (.08–.72) | 13.7 (4.8–35.3) | 5.1 (2.2–13.0) | 9.7 (4.2–25.6) |
| M119C (M9G) | .25 (.08–.74) | 18.3 (7.1–39.9) | 3.9 (1.8–9.5) | 12.4 (5.0–34.0) |
| RPS4Y711Tb | .29 (.08–.85) | 13.7 (5.0–32.3) | 5.1 (2.2–13.7) | 11.8 (5.1–32.8) |
Used for calculations (for details, see the Statistical Analyses subsection).
Including RPS4Y711T chromosomes with the DYS390.1 and DYS390.3 deletions (n=132).
Figure 5.
Bayesian-based demographic inference of associated Y-chromosome STR variation concerning the DYS390.1del/RPS4Y711T haplotype (a and b), the M119C/M9G haplotype (c and d), and the M4G/M5T/M9G haplotype (e and f). Prior probabilities (dashed lines) and posterior-probability distributions (continuous lines) are shown for the time when population expansion began (a, c, and e) and for the TMRCA (b, d, and f).
M119C/M9G Haplotype
The M119C/M9G haplotype was found in all eastern/southeastern Asian populations, at frequencies ranging from 4%, in Korea, to 79%, in Taiwan. In Melanesia, it was absent from coastal PNG and New Britain and was nearly absent (1 of 31) from highland PNG; however, it was observed at a much higher frequency (28%) in the Trobriand Islanders. This haplotype was also absent from Australia and Polynesia. The M119C/M9G haplotype was observed in a total of 111 individuals, in whom 59 Y-chromosome STR haplotypes (h=.98) were found. The TMRCA of these individuals was estimated to be 12,400 years, and a signal of moderate population growth (growth rate .018/generation) was detected, dating to the start of a population expansion ∼3,900 years ago (table 4 and fig. 5).
RPS4Y711T Haplotype
Individuals carrying the RPS4Y711T chromosome were found at low frequencies in all eastern/southeastern Asian populations, including that of eastern Indonesia, but not in Taiwan Aborigines. In Australia, RPS4Y711T was found only in Arnhem Land (6 of 60) and not in the sample from the Great Sandy Desert. RPS4Y711T was absent also from Melanesia and Polynesia (table 4 and fig. 1). The total number of individuals carrying the RPS4Y711T haplotype (n=26) was too small to permit accurate demographic inferences. However, in Australia and Melanesia/Polynesia, RPS4Y711T chromosomes do exist at high frequency, in association with the Y-chromosome STR DYS390.1 and DYS390.3 deletions, respectively, and inclusion of RPS4Y711T chromosomes with the DYS390.1 and DYS390.3 deletions raises the sample size to 132. For this demographic analysis, we excluded the DYS390.1 and DYS390.3 subloci from the Y-chromosome STR haplotype coding, since these deletions violate the single-step–mutation model, and considered variation only at the DYS390.2 sublocus. The resulting TMRCA is 11,800 years, with the start of population expansion having occurred ∼5,100 years ago (table 5).
DYS390.1del/RPS4Y711T Haplotype
The DYS390.1del/RPS4Y711T haplotype was found at high frequency in both population samples from Australia (53% in Arnhem Land and 69% in the Great Sandy Desert). This haplotype was not observed anywhere else, including Melanesia (table 4 and fig. 1). In the 56 individuals with this haplotype, 31 Y-chromosome STR haplotypes were observed. The TMRCA of those individuals was estimated to be 6,600 years. A strong signal of population growth (growth rate .025/generation), the strongest that has been reported for any Y-chromosome mutation analyzed with this model (Kayser et al. 2000a), was observed, indicating that the beginning of population expansion occurred ∼3,700 years ago (table 5 and fig. 5).
DYS390.3del/M9G Haplotype
Previously, the DYS390.3 deletion had been found, in all cases, to be associated with the RPS4Y711T (M9C) chromosome (Kayser et al. 2000a). However, in three individuals from Alor Island in the Nusa Tenggara region and in one individual from coastal PNG, this deletion was observed on M9G (RPS4Y711C) chromosomes (table 4 and fig. 1). One possible explanation is recurrent mutation involving the Y-chromosome SNP loci, but, since this would require the presence of mutations at both RPS4Y711 and M9, a more likely explanation is recurrence of the DYS390.3 deletion. In their seven-locus Y-chromosome STR haplotypes, the three individuals from Alor Island who have the DYS390.3del/M9G haplotype differ from each other by one-step mutations at just two loci, which makes a recent common origin of the DYS390.3 deletion on M9G very likely. The single coastal PNG individual, however, differs from the Alor Island consensus haplotype by one-step mutations at four of the seven loci analyzed. Although this may represent an origin shared by the Alor Island individuals, a separate origin of the deletion in coastal PNG is also possible.
M9G and the Ancestral Haplotype
There were 196 individuals carrying M9G chromosomes, who were distributed across all populations (table 4 and fig. 1). M9G chromosomes most likely represent the common ancestor of the majority of non-African Y chromosomes, and many markers are known to occur on the M9G background (Underhill et al. 1997, 2000). Since the M9G chromosomes observed in the present study most likely represent a rather heterogeneous group of not-yet-identified haplotypes, they are less useful for investigation of population history. The same is true for the ancestral haplotype, which was found in just nine individuals (table 4 and fig. 1).
In an attempt to further differentiate the M9G chromosomes, we analyzed a newly available marker, M175, in which a 5-bp deletion occurs on M9G chromosomes in eastern Asian populations (Underhill et al. 2000). All Melanesian, eastern Indonesian, Polynesian, and Australian M9G chromosomes (n=105) were analyzed with M175, but none proved to have the M175 deletion.
Population-Relationship Analyses
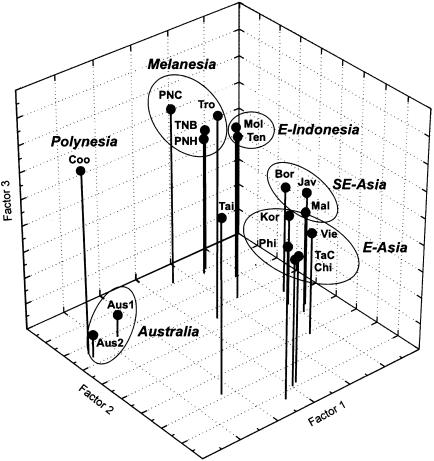
To investigate population relationships, a three-dimensional PC analysis of the nine Y-chromosome SNP/STRdel haplotypes was performed (fig. 6). The first three coordinates account for 81% of the total variance. The PC analysis indicates a close correspondence between the clustering based on the Y-chromosome haplotypes and geography. The eastern/southeastern Asian populations (except for eastern Indonesia and Taiwan) are clustered into two groups, whereas the Melanesian populations group together, close to the two eastern Indonesian populations. The two Australian populations cluster closely together, separated from all Melanesian and Asian populations, whereas the Polynesians also appear to be separated from Melanesia and Asia and are differentiated from the Australians by the third PC.
Figure 6.
PC plot of Y-chromosome haplotype frequencies for 18 populations from eastern/southeastern Asia, Melanesia, Polynesia, and Australia (for abbreviations, see the legend to figure 1). Geographic regions are highlighted.
These results do not indicate any close relationship between Melanesian and Australian populations. Moreover, pairwise FST values between Melanesian and Australian populations, based on the Y-chromosome SNP/STRdel haplotypes, are .241–.397, and pairwise RST values based on the Y-chromosome STR haplotypes are .273–.482; all values are statistically significant (P<.0003) when corrected for multiple comparisons, indicating significant genetic differences between Melanesian and Australian populations.
The highland and coastal PNG/Melanesian populations all group together within the PC plot (fig. 6). Moreover, pairwise FST and RST analyses also revealed very small genetic differences (FST=.065 and RST=.002) between coastal and highland PNG, which were not statistically significant when corrected for multiple comparisons. The same result was obtained when PNG Highlanders were compared with island Melanesians from New Britain (FST=-.030; P=.694 vs. RST=.052; P=.057), whereas comparison of PNG Highlanders with Trobriand Islanders results in a higher FST, .108 (P=.002), but in a comparatively low RST, .05 (P=.012). Thus, the Y-chromosome results indicate an overall close genetic relationship among highland PNG, coastal PNG, and Island Melanesian populations.
Genetic Distance, Based on the Y Chromosome and mtDNA, versus Geographic Distance
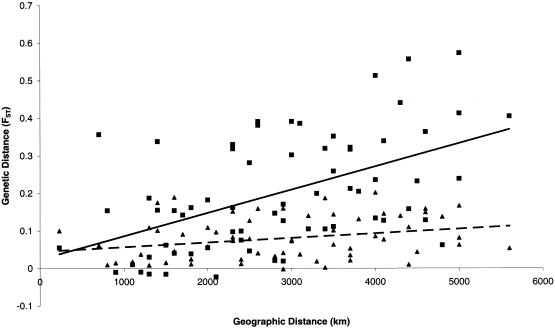
FST values based on Y-chromosome SNP haplotypes for each pair of the 18 populations in this study were significantly correlated (r=.684; P<.001) with RST values based on Y-chromosome STR haplotypes. Comparing the Y-chromosome genetic distance (based on pairwise FST values for the Y-chromosome SNP/STRdel haplotypes) with the geographic distance between each pair of populations reveals a significantly positive correlation (r=.535; P=.002); the same result was found when genetic distances were based on pairwise RST values for the Y-chromosome STR haplotypes (r=.409; P=.037).
Of the 18 populations analyzed in this present study, 10 had been previously typed for SNPs in the two hypervariable segments of the mtDNA control region (sample sizes are in parentheses): Moluccans (49), Malays (81), Taiwan Aborigines (82), Filipinos (176), and the populations from coastal PNG (48), highland PNG (69), Nusa Tenggara (94), Borneo (95), Australia Arnhem Land (95), and Australia Sandy Desert (105) (Melton et al. 1995; Redd and Stoneking 1999); mtDNA data for populations from the same geographic regions (but not identical to the samples used for Y chromosome analysis) were also available for the Javanese (98) and Chinese (103) populations (Melton et al. 1995). For these 12 populations, various Y-chromosome/mtDNA comparisons were performed that were based on pairwise FST/RST values; no significant correlations were found. However, when pairwise FST values based on haplotypes from mtDNA-SNPs and Y-chromosome SNPs were compared with geographic distance, a nonsignificant positive correlation for mtDNA (r=.28; P=.081) and a highly significant positive correlation for Y-chromosome DNA (r=.52; P<.001) were found (fig. 7).
Figure 7.
Correlation analysis of genetic distance (FST based on SNP markers) and geographic distance (km), for 12 populations from eastern/southeastern Asia, Melanesia, and Australia (for particular populations, see the text), for mtDNA (triangles, dashed trendline) and Y-chromosome DNA (squares, continuous trendline).
Discussion
Y-chromosome haplotypes differ greatly between Melanesia and Australia. The two most prevalent haplotypes in Melanesia (M4G/M5T/M9G and DYS390.3del/RPS4Y711T, which account for 39%–55% of Melanesian Y-chromosome haplotypes) are not found in Australia. Other low-frequency haplotypes in Melanesia, such as M119C/M9G, M122C/M9G, and DYS390.3del/M9G, are also absent from Australia (except for M122C/M9G, which was found in a single Australian). Conversely, the most prevalent haplotype in Australia, DYS390.1del/RPS4Y711T (frequency 53%–69%), is not found in Melanesia, nor are the low-frequency Australian haplotypes (RPS4Y711T and completely ancestral) found in Melanesia. The only haplotype that occurs at an appreciable frequency in both Melanesia and Australia is M9G, but M9G exists nearly everywhere in the world except Africa (Underhill et al. 1997, 2000) and, hence, represents a rather heterogeneous group of Y chromosomes, which are not informative for discernment of population relationships. Additional markers on the M9G background (such as M175, which, unfortunately, was not polymorphic in our sample of Melanesian/Australian M9G chromosomes) are needed in order to allow better characterization of the M9G chromosomes in Australia and Melanesia.
Even with the inclusion of M9G, the strong differences between Melanesia and Australia Y-chromosome–haplotype distribution are still demonstrated by the clear separation of populations belonging to the two groups in the PC analysis and by statistically significant differences in FST and RST values involving all pairwise comparisons between Melanesian and Australian populations. We thus conclude that the Y-chromosome histories in Melanesia and Australia have been quite distinct. The male and female histories of the Melanesian and Australian populations therefore appear to have been concordant, since studies of mtDNA diversity have found similar differences between the mtDNA gene pools of Melanesia (represented by PNG) and Australia (Stoneking et al. 1990; Redd and Stoneking 1999).
The Y-chromosome (and mtDNA) results do appear to conflict with studies of classical markers (Keats 1977; Nei and Roychoudhury 1993), RFLP loci (Roychoudhury 1984; Mountain and Cavalli-Sforza 1997), α-globin haplotypes (Roberts-Thomson et al. 1996), and HLA loci (Gao and Serjeantson 1991a; Mack et al. 2000), which show a common origin of Australians and PNG Highlanders. However, as discussed in detail elsewhere (Redd and Stoneking 1999), these studies often do not include all relevant populations and/or do not analyze the data fully. For example, when Alu-insertion polymorphisms were analyzed via the building of a tree of population relationships, PNG Highlanders were shown clustered with Australians (Batzer et al. 1994; Stoneking et al. 1997); PC-analyses (which incorporate more of the information present in the data) of the same data (Harpending et al. 1996; Stoneking et al. 1997) revealed closer affinities between highland PNG and Africa and between Australia and southern India. Thus, it is not clear whether these autosomal loci really do conflict with the Y-chromosome/mtDNA results; additional population samples, loci, and methods of analysis are needed to resolve this issue.
One possible way to reconcile the Y-chromosome results with those of the autosomal markers listed above comes from consideration of the timescale of population-history events in Melanesia and Australia. Humans first colonized Sahul (the combined Australia–New Guinea landmass) some 50,000 years ago (Roberts et al. 1990; Thorne et al. 1999), and Australia and New Guinea were not separated by the rise in sea levels until ∼8,000 years ago (White and O'Connell 1982; Jones 1995). We therefore hypothesize that the Y-chromosome (and mtDNA) differences between PNG and Australia arose after the separation and isolation of Australia and New Guinea, whereas the autosomal markers reflect their preceding shared history. The date for the Australia-specific DYS390.1 deletion supports this hypothesis: it arose in Australia ∼6,600 years ago, after the separation of Australia and New Guinea, Moreover, the RPS4Y711T mutation, on which the DYS390.1 deletion occurred, is widespread in Asia, Oceania, and the New World (Bergen et al. 1999; Karafet et al. 1999) and arose before the separation of Australia and New Guinea (the TMRCA for our sample is 11,800 years). The RPS4Y711T mutation therefore could be an ancient link between Australian and Melanesian Y chromosomes (as could the M9G chromosomes, although more markers are needed to confirm this speculation).
The Melanesian-specific M4G/M5T mutations and the DYS390.3 deletion (RPS4Y711T) are dated to ∼9,700 and ∼10,900 (Kayser et al., in press) years ago, respectively, which is slightly earlier than the separation of Australia and New Guinea. However, the 95% equal-tailed intervals for the TMRCAs are 4,200–25,600 and 4,700–30,900 (Kayser et al., in press) years, respectively, which means that it is possible that these mutations arose in New Guinea after the latter's separation from Australia. Alternatively, the populations in Sahul may have been structured such as these older mutations that arose in the New Guinea part of Sahul but that did not spread to the Australian part of Sahul.
The mtDNA data appear to contradict this hypothesis, since several of the mtDNA mutations specific to the highlands of PNG arose ⩾40,000 years ago (Redd and Stoneking 1999). However, there is uncertainty regarding the mutation rate for mtDNA (Jazin et al. 1998; Sigurðardóttir et al. 2000), and, for these mtDNA mutations, a faster rate could lead to dates that are compatible with this hypothesis. For most autosomal loci that appear to link Australia and Melanesia, such as α-globin haplotypes (Roberts-Thomson et al. 1996) and HLA (Gao and Serjeantson 1991a; Mack et al. 2000), the ages of specific mutations have not been determined. Thus, it remains to be seen whether the apparent discrepancies among the various genetic loci can be explained by this hypothesis.
The Y chromosome shows an unusual pattern of variation within Australia, in that Australia is characterized by high frequencies of a unique haplotype, DYS390.1del/RPS4Y711T, that has not been found anywhere else in the world. This haplotype was found at a frequency of 53.3% in the sample from Arnhem Land and at a frequency of 68.6% in the sample from the Great Sandy Desert. In an earlier study (Forster et al. 1998), the DYS390.1 deletion had also been identified (by sequence analysis) in Australians from the Northern Territory and from Kimberley, at frequencies of 23.7% and 100% (the latter sample comprised just eight individuals), respectively, but the RPS4Y711 marker was not analyzed in that study. Another study of a sample from the Northern Territory found short DYS390 alleles (18–20 repeats), at a frequency of 46.8% (Vandenberg et al. 1999). These short DYS390 alleles probably represent the DYS390.1/RPS4Y711T haplotype, although DYS390 sequence analysis and typing of the RPS4Y711 marker are needed to clearly identify the haplotype. However, if one assumes that all Australian Y chromosomes with short DYS390 alleles belong to the DYS390.1/RPS4Y711T haplotype, then all studies to date concur in finding this unique haplotype at high frequency throughout Australia.
On the basis of our data, the age of the DYS390.1 deletion on the RPS4Y711T chromosome background was estimated to be 6,600 years, which seems surprisingly recent, given the high frequency of this haplotype in all Australian populations studied so far. Can the high frequency of this Y-chromosome haplotype be explained by population history (i.e., genetic drift), or is another mechanism, such as selection, involved? For the DYS390.1del/RPS4Y711T haplotype, demographic analysis indicated a population expansion beginning ∼3,700 years ago (table 5). To see whether this expansion is specific to this haplotype (which would indicate selection) or is also characteristic of other Australian Y chromosomes (which would indicate population history), we performed the same analysis for the Y-chromosome STR haplotypes for all Australian M9G chromosomes (n=30). Although the problem of founder effects is ignored in this analysis, since M9G did not originate in Australia, the Australian M9G chromosomes indicate a population expansion beginning ∼4,700 years ago (95% equal-tailed interval 1,400–15,800 years ago), which is not significantly different from that for DYS390.1del/RPS4Y711T. Thus, it appears that the high frequency of the DYS390.1del/RPS4Y711T haplotype in Australians can be explained by a population expansion in Australia that started ∼3,700 years ago, in a few hundred individuals. This is in remarkably good agreement with archaeological evidence for a mid-Holocene “intensification” in Australian prehistory; beginning ∼4,000 years ago, new tool types such as microliths occur, many sites were occupied for the first time, other sites show a higher density of material being discarded (indicating more intense use of these sites), new resources were exploited, and the dingo was introduced into Australia (Bowdler 1997). This has been interpreted as cultural evidence for population growth combined with a change in social structure (Lourandos 1985; Lourandos and Ross 1994; Bowdler 1997), and the genetic evidence presented here similarly indicates population growth during this time. To what extent this cultural change was motivated by external versus internal factors is still an open question; since the Australian-specific DYS390.1 deletion has not been found in any other population, it is tempting to argue that this mutation arose in Australia and, hence, that there is no evidence for any significant external genetic contribution to Australia ∼4,000 years ago; but more populations need to be surveyed to verify that this mutation did indeed arise in Australia. Redd and Stoneking (1999) have recently reported evidence for a population expansion in Australia, on the basis of mtDNA data. This expansion was dated to ∼68,000 years ago, which clearly indicates an expansion different than what has been suggested by the Y-chromosome data in the present study. Since mtDNA SNPs have a mutation rate ∼10,000–100,000 times lower than that for Y-chromosome STRs (Bonatto and Salzano 1997; Kayser et al. 2000b; Sigurðardóttir et al. 2000), population expansions as recent as a few thousand years ago, as suggested by the Y-chromosome STR data, would not be detectable by mtDNA analysis, unless the population had undergone a very dramatic bottleneck.
The Y-chromosome haplotypes also provide information concerning Melanesian population relationships. The M4G/M5T/M9G haplotype is found at a frequency of 29%–36% in highland and coastal PNG and Island Melanesia, but the only other location where it is found is eastern Indonesia. Since highland PNG has both the highest frequency of this haplotype and the highest associated Y-chromosome STR diversity, we suggest that the mutations defining this haplotype (M4G and M5T) arose in highland New Guinea ∼9,700 years ago (table 5). It is also possible that this haplotype arose instead in coastal New Guinea; in either case, it must have occurred initially in a Papuan-speaking population, but it is now also present in Austronesian-speaking populations in Melanesia, including those of coastal PNG, New Britain, and the Trobriand Islands, as well as those of eastern Indonesia. Since Austronesian languages arrived in Melanesia ∼3,500 years ago (Bellwood 1978, 1989), the presence of this Papuan Y-chromosome haplotype in Austronesian populations indicates substantial male admixture between Papuan speakers and Austronesian speakers. Alternatively, it could be that the present-day Austronesian-speaking populations in coastal PNG and Island Melanesia used to be Papuan-speaking populations that adopted Austronesian languages. However, such language replacements would have been accompanied by significant genetic contributions from the Austronesian speakers, as indicated by other genetic evidence discussed below. Moreover, in an earlier report, we had suggested that the DYS390.3del/RPS4Y711T haplotype, which is the predominant haplotype in Polynesians, arose in Melanesia prior to the arrival of Austronesian speakers and, hence, that there had been male admixture between the Papuan speakers and the Austronesian speakers (Kayser et al. 2000a).
The association of the M4G/M5T/M9G haplotype with Papuan speakers is further borne out by the distribution of this haplotype in eastern Indonesia; it is found at a frequency of 21% in the Papuan-speaking populations from Hiri and Ternate in the Moluccas but is found at a much lower frequency (6%) in the Austronesian-speaking populations from the Nusa Tenggaras. Moreover, in the Nusa Tenggara sample, this haplotype is restricted to Alor, the only island, in the sample, that also has Papuan speakers today (although all sampled individuals from Alor were Austronesian speakers).
Recently, Su et al. (2000) proposed that the lack of the M4G/M5T/M9G haplotype (which they term “haplotype H17”) in Polynesians argues against any substantial contribution of Melanesian Y chromosomes to Polynesia. Although we concur with their results, in that we do not find this widespread Melanesian haplotype in Polynesia, we propose that the absence of this haplotype in Polynesia reflects a bottleneck during the colonization of Polynesia. Our results show that Polynesian Y-chromosome SNP diversity and Y-chromosome STR diversity are both greatly reduced compared with that in Asian, Melanesian, and Australian populations (table 1), indicating that there was indeed a profound bottleneck during the colonization of Polynesia, as supported by mtDNA (Redd et al. 1995; Sykes et al. 1995; Lum et al. 1998) and nuclear genetic evidence (Flint et al. 1989; Lum et al. 1998). We also have argued elsewhere that the DYS390.3del/RPS4Y711T haplotype, which is predominant in Polynesians, arose in Melanesia and, hence, indicates that most (if not all) Polynesian Y chromosomes are in fact of Melanesian origin (Kayser et al. 2000a).
Two other Y-chromosome haplotypes, M119C/M9G and M122C/M9G, are present in coastal PNG and Island Melanesia, are rare or absent in highland PNG, and are widely distributed across Asia (table 4). They are undoubtedly of Asian origin, with coalescence times of 12,400 years ago for M119C (table 5) and 11,100 years ago for M122C (Kayser et al. 2000a), and were probably brought to Melanesia by the Austronesian expansion, since both haplotypes show signals of a population expansion beginning ∼3,900–6,000 years ago (table 5), (Kayser et al. 2000a). The M119C/M9G haplotype is of particular interest in that it reaches highest frequency in the aboriginal Taiwanese population and, hence, may have arisen there. If this haplotype did arise in Taiwan, then the Y chromosome indicates that the genetic trail for the Austronesian expansion began in Taiwan, in agreement with both mtDNA evidence (Melton et al. 1995; Redd et al. 1995; Sykes et al. 1995) and linguistic evidence (Blust 1984/85, 1999). However, other haplotypes, such as M122C/M9G, do not show any indication of a Taiwanese origin, and, moreover, the aboriginal Taiwanese sample shows reduced Y- chromosome SNP (but not Y-chromosome STR) diversity (table 1), so bottleneck effects may have influenced haplotype frequencies in Taiwan. Moreover, M119C/M9G does not occur in Polynesia (Kayser et al. 2000a; Su et al. 2000), so the presence of this haplotype in some parts of Island Melanesia may not be associated with the Austronesian expansion. It thus remains to be seen whether, for the Austronesian expansion, the Y-chromosome data indicate a genetic trail from Taiwan or, instead, a southeastern-Asian origin, as has been suggested by Su et al. (2000).
Among the coastal and Island Melanesian populations sampled here, the M119C/M9G haplotype is present only in the Trobriand Islands, at a frequency of 28%. The Trobriand Islanders also show an elevated frequency of two other markers associated with the Austronesian expansion: the mtDNA 9-bp deletion is found at a frequency of 75% in the Trobriand Islands (Hagelberg et al. 1999), compared with a frequency of 15%–42% in coastal PNG and New Britain (Hertzberg et al. 1989; Stoneking and Wilson 1989; Merriwether et al. 1999), whereas the HLA-DPB1 0501 allele, which is widespread throughout Asia and has a frequency of 62%–72% in Polynesia and 27%–72% in coastal PNG/New Britain (Zimdahl et al. 1999; Mack et al. 2000), is nearly fixed (frequency 97.6%) in Trobriand Islanders (Zimdahl et al. 1999). The Y-chromosome, mtDNA, and HLA data thus all seem to indicate that the genetic influence that the incoming Austronesians had on the Trobriand Islanders was greater than their genetic influence elsewhere in Melanesia. Other more-detailed studies of specific populations in the Pacific have similarly revealed variation in the genetic contribution of Austronesians to existing populations (Merriwether et al. 1999), indicating the importance of local effects during the colonization of the Pacific.
Although the Y-chromosome and mtDNA results are, in general, concordant, in that both indicate independent histories for Australian and Melanesian populations, they disagree with respect to relationships between highland and coastal PNG populations. The coastal and highland PNG populations differ significantly with respect to mtDNA (Stoneking et al. 1990; Redd and Stoneking 1999), but, for the Y chromosome, neither FST values (which are based on Y-chromosome SNP loci) nor RST values (which are based on Y-chromosome STR loci) differ significantly between the two populations. Various HLA alleles also have significant frequency differences between the highland and coastal PNG populations (Gao and Serjeantson 1991a, 1991b, 1992; Zimdahl et al. 1999; Mack et al. 2000), although the potential influence that selection has on HLA loci complicates population-relationship interpretations based on these loci. The similarity between the coastal and highland PNG populations with respect to the Y chromosome is due primarily to the shared presence, at relatively high frequency, of the M9G and M4G/M5T/M9G haplotypes. As discussed above, the M9G haplotype is an ancestral haplotype for non-African populations and, hence, is not informative for population relationships. However, the M4G/M5T/M9G haplotype probably arose in highland PNG (as discussed above) and thus must have spread via migration from highland to coastal PNG. Since this Y-chromosome–haplotype similarity is not reflected in the patterns of mtDNA variation, presumably this migration involved primarily males.
This evidence for greater similarity/mobility of the Y chromosome than of mtDNA in PNG runs counter to what has been reported for other human populations (Salem et al. 1996; Perez-Lezaun et al. 1999). In particular, among European populations a comparison of mtDNA and Y-chromosome genetic distances versus geographic distances found much less similarity for the Y chromosome than for mtDNA, a result that was interpreted as reflecting patrilocality of these societies (Seielstad et al. 1998)—on marrying, women tend to move to the residence of the male. However, this analysis was criticized for using different populations and different types of markers for the mtDNA and Y-chromosome data (Stoneking 1998). We therefore analyzed, in 12 populations from Asia, Melanesia, and Australia, the correlation between genetic distances (FST values based on SNPs) for mtDNA and Y-chromosome haplotypes and geographic distance, and we found the same striking pattern: the correlation between Y-chromosome genetic distance and geographic distance was high and statistically significant, whereas the correlation between mtDNA genetic distance and geographic distance was lower and not significant (fig. 7). Overall, then, despite the local exception involving coastal and highland PNG, our results agree with those of other studies, in that differences among populations are greater with respect to the Y chromosome than they are for mtDNA. In Asia/Melanesia/Australia, as elsewhere in the world, women are apparently moving around more frequently than men.
Acknowledgments
We thank the original donors of samples, and we thank N. Saha, A. G. Soemantri, A. S. M. Sofro, K. Bhatia, J. Kuhl, N. Kretchmer, D. Bugawan, E. Hagelberg, S. Ulijaszek, K. Katayama, L. Roewer, J. Martinson, B. Budowle, and C. Tyler-Smith for providing DNA and/or blood samples. C. Tyler-Smith is additionally acknowledged for sharing data prior to publication. This work was funded by a National Science Foundation grant (to M.S.) and by the Max Planck Society.
Electronic-Database Information
URLs for data in this article are as follows:
- ARLEQUIN, http://lgb.unige.ch/arlequin/ (for ARLEQUIN version 2.000)
- Phylogenetic Network Analysis Shareware Software, http://www.fluxus-engineering.com/sharenet.htm (for Network 2.0b software)
- University of Aberdeen Department of Mathematical Sciences, http://www.maths.abdn.ac.uk/ (for BATWING)
References
- Allen B (1989) When did humans first colonize Australia? Search 20:149–154 [Google Scholar]
- Allen J, Flannery T, Gosden C, Jones R, White JP (1988) Pleistocene dates for the human occupation of New Ireland, Northern Melanesia. Nature 331:707–709 [DOI] [PubMed] [Google Scholar]
- Bandelt H-J, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48 [DOI] [PubMed] [Google Scholar]
- Batzer MA, Stoneking M, Alegria-Hartman M, Bazan H, Kass DH, Shaikh TH, Novick GE, Ioannou PA, Scheer WD, Herrera RJ, Deininger PL (1994) African origin of human-specific polymorphic Alu insertions. Proc Natl Acad Sci USA 91:12288–12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwood PS (1978) Man's conquest of the Pacific: the prehistory of Southeast Asia and Oceania. Oxford University Press, Oxford [Google Scholar]
- ——— (1987) The Polynesians. Thames & Hudson, London [Google Scholar]
- ——— (1989) The colonization of the Pacific: some current hypotheses. In: Hill AVS, Serjeantson SW (eds) The colonization of the Pacific: a genetic trail. Oxford University Press, Oxford, pp 1–59 [Google Scholar]
- Bergen AW, Wang C-Y, Tsai J, Jefferson K, Dey C, Smith KD, Park S-C, Tsai S-J, Goldman D (1999) An Asian-Native American paternal lineage identified by RPS4Y resequencing and by microsatellite haplotyping. Ann Hum Genet 63:63–80 [DOI] [PubMed] [Google Scholar]
- Blust R (1984/85) The Austronesian homeland: a linguistic perspective. Asian Perspect 26:45–67 [Google Scholar]
- ——— (1999) Subgrouping, circularity and extinction: some issues in Austronesian comparative linguistics. Symp Ser Inst Linguist Acad Sin 1:31–94 [Google Scholar]
- Bonatto SL, Salzano FM (1997) Diversity and age of the four major mtDNA haplogroups, and their implications for the peopling of the New World. Am J Hum Genet 61:1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdler S (1997) Building on each other's myths: archaeology and linguistics in Australia. In: McConvell P, Evans N (eds) Archaeology and linguistics: Aboriginal Australia in global perspective. Oxford University Press, Oxford, pp 17–26 [Google Scholar]
- Flint J, Boyce AJ, Martinson JJ, Clegg JB (1989) Population bottlenecks in Polynesia revealed by minisatellites. Hum Genet 83:257–263 [DOI] [PubMed] [Google Scholar]
- Forster P, Kayser M, Meyer E, Roewer L, Pfeiffer H, Benkmann H, Brinkmann B (1998) Phylogenetic resolution of complex mutational features at Y-STR DYS390 in Aboriginal Australians and Papuans. Mol Biol Evol 15:1108–1114 [DOI] [PubMed] [Google Scholar]
- Gao X, Serjeantson SW (1991a) Diversity in HLA-DR4-related DR, DQ haplotypes in Australia, Oceania, and China. Hum Immunol 32:269–276 [DOI] [PubMed] [Google Scholar]
- ——— (1991b) Heterogeneity in HLA-DR2-related DR, DQ haplotypes in eight populations of Asia-Oceania. Immunogenetics 34:401–408 [DOI] [PubMed] [Google Scholar]
- ——— (1992) Analysis of 2600 HLA-DR, DQ haplotypes in Asia-Oceania. In: Tsuji K, Aizawan M, Sasazuki T (eds) HLA 1991: Proceedings of the 11th International Histocompatibility Workshop and Conference. Oxford University Press, Oxford, pp 229–232 [Google Scholar]
- Groube LM, Chappell J, Muke J, Price D (1986) A 40,000 year-old human occupation site at Huon Peninsula, Papua New Guinea. Nature 324:453–455 [DOI] [PubMed] [Google Scholar]
- Hagelberg E, Kayser M, Nagy M, Roewer L, Zimdahl H, Krawczak M, Lio P, Schiefenhövel W (1999) Molecular genetic evidence for the human settlement of the Pacific: analysis of mitochondrial DNA, Y chromosome and HLA markers. Philos Trans R Soc Lond B Biol Sci 354:141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpending H, Relethford J, Sherry ST (1996) Methods and models for understanding human diversity. In: Boyce AJ, Mascie-Taylor CGN (eds) Molecular biology and human diversity. Cambridge University Press, Cambridge, pp 283–299 [Google Scholar]
- Hertzberg M, Mickleson KNP, Serjeantson SW, Prior JF, Trent RJ (1989) An Asian-specific 9-bp deletion of mitochondrial DNA is frequently found in Polynesians. Am J Hum Genet 44:504–510 [PMC free article] [PubMed] [Google Scholar]
- Hill AVS, O'Shaughnessy DFO, Clegg JB (1989) Haemoglobin and globin gene variants in the Pacific. In: Hill AVS, Serjeantson SW (eds) The colonization of the Pacific: a genetic trail. Oxford University Press, Oxford, pp 246–285 [Google Scholar]
- Jazin E, Soodyall H, Jalonen P, Lindholm E, Stoneking M, Gyllensten U (1998) Mitochondrial mutation rate revisited: hot spots and polymorphism. Nat Genet 18:109–110 [DOI] [PubMed] [Google Scholar]
- Jones R (1995) Tasmanian archaeology: establishing the sequences. Annu Rev Anthropol 24:423–446 [Google Scholar]
- Karafet TM, Zegura SL, Posukh O, Osipova L, Bergen A, Long J, Goldman D, Klitz W, Harihara S, de Knijff P, Wiebe V, Griffiths RC, Templeton AR, Hammer MF (1999) Ancestral Asian source(s) of New World Y-chromosome founder haplotypes. Am J Hum Genet 64:817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M, Brauer S, Weiss G, Underhill PA, Roewer L, Schiefenhövel W, Stoneking M (2000a) Melanesian origin of Polynesian Y chromosomes. Curr Biol 10:1237–1246 [DOI] [PubMed] [Google Scholar]
- ———. Melanesian origin of Polynesian Y chromosomes: correction. Curr Biol (in press) [DOI] [PubMed] [Google Scholar]
- Kayser M, Caglià A, Corach D, Fretwell N, Gehrig C, Graziosi G, Heidorn F, et al (1997) Evaluation of Y chromosomal STRs: a multicenter study. Int J Legal Med 110:125–133, 141–149 [DOI] [PubMed] [Google Scholar]
- Kayser M, Roewer L, Hedman M, Henke L, Henke J, Brauer S, Krüger C, Krawczak M, Nagy M, Dobosz T, Szibor R, de Knijff P, Stoneking M, Sajantila A (2000b) Characteristics and frequency of germline mutations at microsatellite loci from the human Y chromosome, as revealed by direct observation in father/son pairs. Am J Hum Genet 66:1580–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats B (1977) Genetic structure of the indigenous populations in Australia and New Guinea. J Hum Evol 6:319–339 [Google Scholar]
- Kirch PV (1995) The Lapita peoples. Blackwell, Cambridge, MA [Google Scholar]
- Legendre P, Lapointe F-J, Casgrain P (1994) Modeling brain evolution from behaviour: a permutational regression approach. Evolution 48:1487–1499 [DOI] [PubMed] [Google Scholar]
- Lourandos H (1985) Intensification and Australian prehistory. In: Price TD, Brown JA (eds) Prehistoric hunter-gatherers: the emergence of cultural complexity. Academic Press, Orlando, pp 384–423 [Google Scholar]
- Lourandos H, Ross A (1994) The great `intensification debate’: its history and place in Australian archaeology. Aust Archaeol 39:54–63 [Google Scholar]
- Lum JK, Cann RL, Martinson JJ, Jorde LB (1998) Mitochondrial and nuclear genetic relationships among Pacific Island and Asian populations. Am J Hum Genet 63:613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JK, Rickards O, Ching C, Cann RL (1994) Polynesian mitochondrial DNAs reveal three deep maternal lineage clusters. Hum Biol 66:567–590 [PubMed] [Google Scholar]
- Mack SJ, Bugawan TL, Moonsamy PV, Erlich JA, Trachtenberg EA, Paik YK, Begovich AB, Saha N, Beck HP, Stoneking M, Erlich HA (2000) Evolution of Pacific/Asian populations inferred from HLA class II allele frequency distributions. Tissue Antigens 55:383–400 [DOI] [PubMed] [Google Scholar]
- Melton T, Peterson R, Redd AJ, Saha N, Sofro ASM, Martinson J, Stoneking M (1995) Polynesian genetic affinities with Southeast Asian populations as identified by mtDNA analysis. Am J Hum Genet 57:403–414 [PMC free article] [PubMed] [Google Scholar]
- Merriwether DA, Friedlaender JS, Mediavilla J, Mgone C, Gentz F, Ferrell RE (1999) Mitochondrial DNA variation is an indicator of Austronesian influence in Island Melanesia. Am J Phys Anthropol 110:243–270 [DOI] [PubMed] [Google Scholar]
- Mountain JL, Cavalli-Sforza LL (1997) Multilocus genotypes, a tree of individuals, and human evolutionary history. Am J Hum Genet 61:705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Roychoudhury AK (1993) Evolutionary relationships of human populations on a global scale. Mol Biol Evol 10:927–943 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy DR, Hill AVS, Bowden DK, Weatherall DJ, Clegg JB (1990) Globin gene in Micronesia: origin and affinities of Pacific Island peoples. Am J Hum Genet 46:144–155 [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Gosden C (1994) 35,000-year-old sites in the rainforests of West New Britain, Papua New Guinea. Antiquity 68:604–610 [Google Scholar]
- Perez-Lezaun A, Calafell F, Comas D, Mateu E, Bosch E, Martinez-Arias R, Clarimon J, Fiori G, Luiselli D, Facchini F, Pettener D, Bertranpetit J (1999) Sex-specific migration patterns in Central Asian populations, revealed by analysis of Y-chromosome short tandem repeats and mtDNA. Am J Hum Genet 65:208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd AJ, Clifford SL, Stoneking M (1997) Multiplex DNA typing of short-tandem-repeat loci on the Y chromosome. Biol Chem 378:923–927 [DOI] [PubMed] [Google Scholar]
- Redd AJ, Stoneking M (1999) Peopling of Sahul: mtDNA variation in aboriginal Australian and Papua New Guinea populations. Am J Hum Genet 65:808–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd AJ, Takezaki N, Sherry ST, McGarvey ST, Sofro AS, Stoneking M (1995) Evolutionary history of the COII/tRNALys intergenic 9 base pair deletion in human mitochondrial DNAs from the Pacific. Mol Biol Evol 12:604–615 [DOI] [PubMed] [Google Scholar]
- Roberts RG, Jones R, Smith MA (1990) Thermoluminescence dating of a 50,000-year-old human occupation site in northern Australia. Nature 345:153–156 [Google Scholar]
- Roberts-Thomson JM, Martinson JJ, Norwich JT, Harding RM, Clegg JB, Boettcher B (1996) An ancient common origin of Aboriginal Australians and New Guinean highlanders is supported by α-globin haplotype analysis. Am J Hum Genet 58:1017–1024 [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury AK (1984) Genetic relationship between Indian tribes and Australian Aborigines. Hum Hered 34:314–320 [DOI] [PubMed] [Google Scholar]
- Salem A-H, Badr FM, Gaballah MF, Pääbo S (1996) The genetics of traditional living: Y-chromosomal and mitochondrial lineages in the Sinai Peninsula. Am J Hum Genet 59:741–743 [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excoffier L (2000) ARLEQUIN, version 2.000: a software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Geneva [Google Scholar]
- Seielstad M, Minch E, Cavalli-Sforza LL (1998) Genetic evidence for a higher female migration rate in humans. Nat Genet 20:278–280 [DOI] [PubMed] [Google Scholar]
- Sigurðardóttir S, Helgason A, Gulcher JR, Stefansson K, Donnelly P (2000) The mutation rate in the human mtDNA control region. Am J Hum Genet 66:1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M (1985) The Lapita cultural complex: origins, distribution, contemporaries and successors. In: Kirk R, Szathmary E (eds) Out of Asia. Journal of Pacific History, Canberra, pp 185–206 [Google Scholar]
- Stoneking M (1998) Women on the move. Nat Genet 20:219–220 [DOI] [PubMed] [Google Scholar]
- Stoneking M, Fontius JJ, Clifford SL, Soodyall H, Arcot SS, Saha N, Jenkins T, Tahir MA, Deininger PL, Batzer MA (1997) Alu insertion polymorphisms and human evolution: evidence for a larger population size in Africa. Genome Res 7:1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneking M, Jorde LB, Bhatia K, Wilson AC (1990) Geographic variation in human mitochondrial DNA from Papua New Guinea. Genetics 124:717–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneking M, Wilson AC (1989) Mitochondrial DNA. In: Hill AVS, Serjeantson S (eds) The colonization of the Pacific: a genetic trail. Oxford University Press, Oxford, pp 215–245 [Google Scholar]
- Su B, Jin L, Underhill P, Martinson J, Saha N, McGarvey ST, Shriver MD, Chu J, Oefner P, Chakraborty R, Deka R (2000) Polynesian origins: insights from the Y chromosome. Proc Natl Acad Sci USA 97:8225–8228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Xiao J, Underhill P, Deka R, Zhang W, Akey J, Huang W, Shen D, Lu D, Luo J, Chu J, Tan J, Shen P, Davis R, Cavalli-Sforza L, Chakraborty R, Xiong M, Du R, Oefner P, Chen Z, Jin L (1999) Y-chromosome evidence for a northward migration of modern humans into eastern Asia during the last Ice Age. Am J Hum Genet 65:1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B, Leiboff A, Low-Beer J, Tetzner S, Richards M (1995) The origins of the Polynesians: an interpretation from mitochondrial lineage analysis. Am J Hum Genet 57:1463–1475 [PMC free article] [PubMed] [Google Scholar]
- Thorne A, Grun R, Mortimer G, Spooner N, Simpson J, McCulloch M, Taylor L, Curnoe D (1999) Australia's oldest human remains: age of the Lake Mungo 3 skeleton. J Hum Evol 36:591–612 [DOI] [PubMed] [Google Scholar]
- Tsintsof AS, Hertzberg MS, Prior JF, Mickleson KNP, Trent RJ (1990) α-Globin gene markers identify genetic differences between Australian aborigines and Melanesians. Am J Hum Genet 46:138–143 [PMC free article] [PubMed] [Google Scholar]
- Underhill PA, Jin L, Lin AA, Mehdi SQ, Jenkins T, Vollrath D, Davis RW, Cavalli-Sforza LL, Oefner PJ (1997) Detection of numerous Y chromosome biallelic polymorphisms by denaturing high-performance liquid chromatography. Genome Res 7:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill PA, Shen P, Lin AA, Jin L, Passarino G, Yang WH, Kauffman E, Bonne-Tamir B, Bertranpetit J, Francalacci P, Ibrahim M, Jenkins T, Kidd JR, Mehdi SQ, Seielstad MT, Wells RS, Piazza A, Davis RW, Feldman MW, Cavalli-Sforza LL, Oefner PJ (2000) Y chromosome sequence variation and the history of human populations. Nat Genet 26:358–361 [DOI] [PubMed] [Google Scholar]
- Vandenberg N, Van Oorschot RAH, Tyler-Smith C, Mitchell RJ (1999) Y-chromosome-specific microsatellite variation in Australian Aborigines. Hum Biol 71:915–931 [PubMed] [Google Scholar]
- van Holst Pellekaan S, Frommer M, Sved J, Boetcher B (1997) Mitochondrial D-loop diversity in Australian riverine and Australian desert Aborigines. Electrophoresis 18:1538–1543 [DOI] [PubMed] [Google Scholar]
- ——— (1998) Mitochondrial control-region sequence variation in Aboriginal Australians. Am J Hum Genet 62:435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, O'Connell JF (1982) A prehistory of Australia, New Guinea, and Sahul. Academic Press, New York [Google Scholar]
- Wickler S, Spriggs M (1988) Pleistocene human occupation of the Solomon Islands, Melanesia. Antiquity 62:703–706 [Google Scholar]
- Wilson IJ, Balding DJ (1998) Genealogical inference from microsatellite data. Genetics 150:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenchitsomanus PT, Summers KM, Bhatia KK, Board PG (1986) A single α-globin gene deletion in Australian Aborigines. Aust J Exp Biol Med Sci 64:297–306 [DOI] [PubMed] [Google Scholar]
- Zimdahl H, Schiefenhövel W, Kayser M, Roewer L, Nagy M (1999) Towards understanding the origin and dispersal of Austronesians in the Solomon Sea: HLA class II polymorphism in eight distinct populations of Asia-Oceania. Eur J Immunogenet 26:405–416 [DOI] [PubMed] [Google Scholar]