Abstract
Objectives: To evaluate the effects of regular nasal irrigation on nasal microbiota and symptom improvement in allergic rhinitis patients. Methods: This retrospective study involved 202 allergic rhinitis patients. Participants were divided into a conventional treatment group (mometasone furoate spray and loratadine) and an observation group (nasal irrigation added to the conventional regimen). Nasal microbiota diversity, biofilm formation, nasal symptoms, and quality of life were assessed before and after a one month of treatment. A correlation analysis was performed to evaluate the relationships between nasal irrigation and clinical outcomes. Results: The nasal irrigation group exhibited significant reductions in nasal microbiota richness (P = 0.014), biofilm-forming Staphylococcus aureus and Staphylococcus epidermidis (P < 0.001, P = 0.001, respectively), with improvements in nasal pruritus, rhinorrhea, congestion, and sneezing (all P < 0.05), compared to the conventional treatment group. Post-treatment, the nasal irrigation group showed more notable improvement in quality of life scores (P = 0.007). Correlation analysis revealed significant associations between nasal irrigation, reduced microbial richness, and symptom severity. Conclusions: Regular nasal irrigation serves as an effective adjunctive treatment for allergic rhinitis by modulating nasal microbiota, alleviating symptoms, and enhancing quality of life.
Keywords: Allergic rhinitis, nasal irrigation, nasal microbiota, symptom improvement, quality of life, biofilm formation
Introduction
Allergic rhinitis is a common chronic condition characterized by inflammation of the nasal mucosa, resulting from an immunoglobulin E-mediated response to airborne allergens [1,2]. Clinically, it manifests as nasal congestion, sneezing, rhinorrhea, and nasal pruritus [3]. As the prevalence of allergic rhinitis continues to rise globally, its imposes a growing burden on individuals and healthcare systems, significantly affecting quality of life and daily functioning [4]. Despite advancements in pharmacotherapy, including antihistamines and intranasal corticosteroids, a subset of patients continues to experience persistent symptoms, highlighting the need for alternative or adjunctive therapeutic strategies [5,6].
Recent research has focused on the nasal microbiota and its potential role in the pathophysiology of allergic rhinitis [7]. The nasal cavity harbors a diverse microbial community essential for modulating the local immune environment and maintaining mucosal health [8]. Disruptions in this microbial community, known as dysbiosis, have been linked to various atopic and inflammatory diseases, including allergic rhinitis [9]. Specific bacterial species, through mechanisms such as biofilm formation, may exacerbate or sustain inflammation, contributing to the chronicity and severity of allergic symptoms [10]. Consequently, interventions targeting the nasal microbiota present a promising therapeutic approach.
Nasal irrigation, a technique that involves flushing the nasal cavity with a saline solution, has emerged as a potential adjunctive therapy for managing allergic rhinitis [11]. The rationale behind this approach is multifaceted, involving the mechanical removal of allergens, mucus, and pathogens, as well as potential modulation of the local microbiota [12]. Owing to its non-invasive nature and low risk of adverse effects, nasal irrigation is an attractive option for patients seeking complementary treatments. This study aims to analyze the correlation between regular nasal irrigation, nasal microbiota modulation, and symptom improvement in patients with allergic rhinitis.
Materials and methods
Case selection
A retrospective study was performed on 202 patients diagnosed with allergic rhinitis at Xishan People’s Hospital of Wuxi between January 2022 and May 2024. Patient data were retrieved from the hospital’s electronic medical record system and included demographic information, nasal microbiota profiles, symptom severity, and quality of life assessment. This study was approved by the Ethics Committee and Institutional Review Committee of Xishan People’s Hospital of Wuxi.
Inclusion criteria: (1) Diagnosis consistent with the “Chinese Guidelines for the Diagnosis and Treatment of Allergic Rhinitis (2022, Revised Edition)” [13]; (2) Age ≥ 7 years; (3) Positive skin prick tests (SPT) for allergens; (4) Presence of clinical symptoms such as sneezing, nasal congestion, and nasal pruritus [14]; (5) Complete clinical records; and (6) Completion of a one-month treatment course with regular follow-up.
Exclusion criteria: (1) Anatomical abnormalities of the nasal cavity, including nasal polyps or septal deviation [15]; (2) Allergic reactions to the medications; (3) History of nasal surgery; (4) Presence of severe comorbidities such as malignancies or organ failure; (5) Pregnancy or lactation; or (6) Diagnosed neurological diseases or cognitive impairments.
Intervening method
The conventional treatment group received mometasone furoate nasal spray (Schering-Plough Labo N.V., Belgium; import drug registration number H20140100). Each bottle contains 60 sprays, with each spray delivering 50 μg of mometasone furoate at a concentration of 0.05% (g/g). The prescribed dosage was one spray per nostril once daily. Additionally, patients were administered loratadine citrate tablets (Yangtze River Pharmaceutical Group, Guangzhou; registration number H20090138) at a dosage of one 8.8 mg tablet daily.
The observation group received the same conventional treatment combined with nasal irrigation. Nasal irrigation was performed using the PARI MONTESOL nasal irrigator (PARI, Germany; medical device registration number 2012-2661720) with 500 mL of saline warmed to approximately 40°C. Patients were instructed to sit with their heads tilted forward during the procedure. The olive-shaped tip of the irrigator was gently inserted into one nostril, keeping the mouth open without swallowing or speaking. The irrigation bulb was then pressed with consistent pressure to allow saline to flow through the nasal cavity and nasopharynx, exiting via the contralateral nostril and mouth. The procedure was repeated for the other nostril. After completing the irrigation, patients were guided to clear nasal secretions before applying the mometasone furoate nasal spray, following the same method as the routine group. The loratadine citrate tablet dosage remained consistent between the two groups. Both groups continued treatment for one month.
Data collection
Basic information
Patient baseline information encompassed age, body mass index (BMI), sex, smoking history, alcohol consumption history, presence of hypertension or diabetes, marital status, educational level, ethnicity, disease progression, total Immunoglobulin E (IgE) levels, eosinophil counts, and SPT results.
Prior to treatment initiation, two vials of fasting venous blood were drawn from each patient. One vial was anticoagulated and analyzed using an automated blood cell analyzer (Mindray BC6800, Mindray Bio-Medical Electronics Co., Ltd., China) to determine eosinophil counts and percentages. The other vial was centrifuged at 3,000 rpm for 10 minutes at low temperature (TLD 12A, Hunan Xiangxi Scientific Instrument Factory, China). The separated serum was then analyzed to determine total IgE levels using an automated enzyme scanner (InfinitiF50, Tecan, Switzerland).
Specific antigen detection was conducted using the SPT prior to treatment. The test area was the volar forearm or back. Allergen positions were marked using a non-irritating marker, with designated sites for positive and negative controls. Standardized allergen extracts were applied to the marked points, maintaining adequate distances to prevent cross-reactivity. A specialized lancet was used to gently puncture the epidermis, introducing a small quantity of allergen. Each pricking was performed with consistent and gentle pressure. Histamine solution and saline served as positive and negative controls, respectively. After 15-20 minutes, the diameters of wheals and surrounding erythema were measured. A wheal diameter ≥ 3 mm was considered a positive reaction, while < 3 mm was deemed negative [16].
Nasal microbiota
One day after completing the one-month treatment course, nasal mucosal secretions were collected under strict aseptic protocols. Hands and the endoscope lens were thoroughly disinfected before sampling. A sterile cotton swab were rotated two and a half times within the middle meatus, lateral to the middle turbinate, avoiding contact with the nasal sidewall and anterior vestibule. Two samples were obtained from each patient and stored in EP tubes for further analysis [17].
One sample was processed for total DNA extraction using a rapid DNA extraction kit (Tiangen Biotech, Beijing). Extracted DNA was subjected to 16S rRNA sequencing and bacterial identification at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). Raw sequencing reads were clustered into Operational Taxonomic Unit (OUT) using Uparse software based on 97% sequence similarity. Alpha diversity of the bacterial community was analyzed using Mothur software [18].
The second sample was enriched in tryptic soy broth until reaching a turbidity of 0.5 McFarland units, then inoculated into a 96-well plate and incubated at 37°C for 24 hours in a constant temperature incubator. Post-incubation, the wells were rinsed three times with Phosphate-Buffered Saline (PBS), fixed with methanol, and treated with ice-cold acetic acid. Biofilm formation was quantified by measuring absorbance at 590 nm using a microplate reader. Biofilm production ability was assessed by calculating the A value, defined as the mean absorbance of the test strain at 590 nm (A590) minus the mean A590 of the blank control. A threshold value, Ac, was established based on the standard strain (the same species without biofilm expression) A value and its three standard deviations. A test strain with an A value greater than Ac was considered biofilm-forming positive (BF positive). Each strain was tested in triplicate [19].
Nasal symptoms and quality of life
Following the completion of the one-month treatment, the severity of nasal symptoms and quality of life were assessed for each patient. Quality of life was evaluated using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) [20], both before and after treatment.
The RQLQ measures quality of life across seven domains: (1) Activity Limitations: Impact of nasal/eye symptoms on daily activities over the past seven days. (2) Sleep Problems: Difficulties in falling asleep, nighttime awakenings, and poor sleep quality. (3) Non-Nasal/Eye Symptoms: Indirect symptoms such as fatigue, thirst, and reduced work capacity. (4) Practical Problems: Inconveniences such as carrying tissues, frequent nose or eye rubbing, and nose blowing. (5) Nasal Symptoms: Severity of nasal congestion, runny nose, sneezing, and postnasal drip. (6) Eye Symptoms: Ocular discomfort, such as itchy eyes, tearing, and pain. (7) Emotional Impact: Emotional distress such as depression, restlessness, irritability, or embarrassment due to symptoms. Each dimension was scored from 0 (no impairment) to 6 (severe impairment), with total score ranging from 0 to 42. Higher scores indicate a poorer quality of life [20].
Statistical analysis
Data were analyzed using SPSS 29.0 statistical software (SPSS Inc., Chicago, IL, USA). Categorical data were presented as [n (%)], and continuous data with normal distribution were presented as mean ± standard deviation (X ± s). Comparisons between groups were performed using the independent samples t-test for continuous variables and the chi-square tests for categorical variables. A two-sided P-value < 0.05 was considered statistically significant.
Results
Basic information
Gender distribution was comparable between the two groups (P = 0.782) (Table 1). History of smoking and alcohol consumption, as well as comorbidities including hypertension and diabetes, were similarly distributed, with no significant differences noted (P > 0.05). Additionally, the average disease duration, total IgE levels, and eosinophil percentages were comparable between groups (P > 0.05). SPT results for allergens such as house dust mites, tree pollen, grass pollen, animal dander, and molds also showed no significant differences between the two groups (P > 0.05). These findings indicate that the baseline characteristics were well balanced, supporting the attribution of outcome differences to the interventions rather than pre-existing differences between groups.
Table 1.
Comparison of basic information between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 25.24 ± 6.65 | 25.19 ± 6.23 | 0.058 | 0.954 |
| Body Mass Index (kg/m2) | 23.23 ± 4.32 | 23.14 ± 3.65 | 0.150 | 0.881 |
| Female/Male | 51 (51%)/49 (49%) | 54 (52.94%)/48 (47.06%) | 0.076 | 0.782 |
| Smoking history (Yes/No) | 22 (22%)/78 (78%) | 24 (23.53%)/78 (76.47%) | 0.067 | 0.796 |
| Drinking history (Yes/No) | 28 (28%)/72 (72%) | 25 (24.51%)/77 (75.49%) | 0.318 | 0.573 |
| Hypertension (Yes/No) | 1 (1%)/99 (99%) | 0 (0%)/102 (100%) | None | 0.495 |
| Diabetes (Yes/No) | 2 (2%)/98 (98%) | 1 (0.98%)/101 (99.02%) | 0 | 0.986 |
| Marital Status (Married/Unmarried) | 32 (32%)/68 (68%) | 34 (33.33%)/68 (66.67%) | 0.041 | 0.840 |
| Educational Level (Junior college graduate or lower/College graduate or higher) | 43 (43%)/57 (57%) | 42 (41.18%)/60 (58.82%) | 0.069 | 0.793 |
| Ethnicity (Han/Other) | 86 (86%)/14 (14%) | 84 (82.35%)/18 (17.65%) | 0.504 | 0.478 |
| Course of illness | 0.79 ± 0.24 | 0.82 ± 0.37 | 0.534 | 0.594 |
| T IgE (IU/ML) | 113.30 ± 23.54 | 114.37 ± 23.54 | 0.324 | 0.747 |
| Eosinophils (%) | 2.40 ± 0.21 | 2.43 ± 0.34 | 0.763 | 0.446 |
| Skin Prick Test (+/-) | ||||
| House dust mites (Yes/No) | 36 (36%)/64 (64%) | 37 (36.27%)/65 (63.73%) | 0.002 | 0.968 |
| Tree pollen (Yes/No) | 29 (29%)/71 (71%) | 32 (31.37%)/70 (68.63%) | 0.135 | 0.713 |
| Grass pollen (Yes/No) | 27 (27%)/73 (73%) | 27 (26.47%)/75 (73.53%) | 0.007 | 0.932 |
| Animals (Yes/No) | 23 (23%)/77 (77%) | 19 (18.63%)/83 (81.37%) | 0.586 | 0.444 |
| Molds (Yes/No) | 14 (14%)/86 (86%) | 11 (10.78%)/91 (89.22%) | 0.481 | 0.488 |
T IgE: total immune globulin E.
Nasal microbiota diversity
The nasal irrigation group demonstrated a significantly lower Chao1 richness estimator compared to the conventional treatment group (t = 2.478, P = 0.014) (Figure 1). Similarly, the Ace index was significantly lower in the nasal irrigation group (t = 2.461, P = 0.015). The Simpson Diversity Index was slightly higher in the nasal irrigation group compared to the conventional treatment group (t = 2.046, P = 0.042). However, there was no significant difference in the Shannon-Wiener Diversity Index between the groups (t = 1.778, P = 0.078), indicating similar overall microbial diversity despite differences in richness and evenness.
Figure 1.
Comparison of nasal microbiota diversity between the two groups. A: Chao1 Richness Estimator; B: Ace; C: Simpson Diversity Index; D: Shannon-Wiener Diversity Index. Ace: Abundance-based Coverage Estimator. ns: no statistically significant difference; *: P < 0.05.
Quantitative analysis of biofilm formation
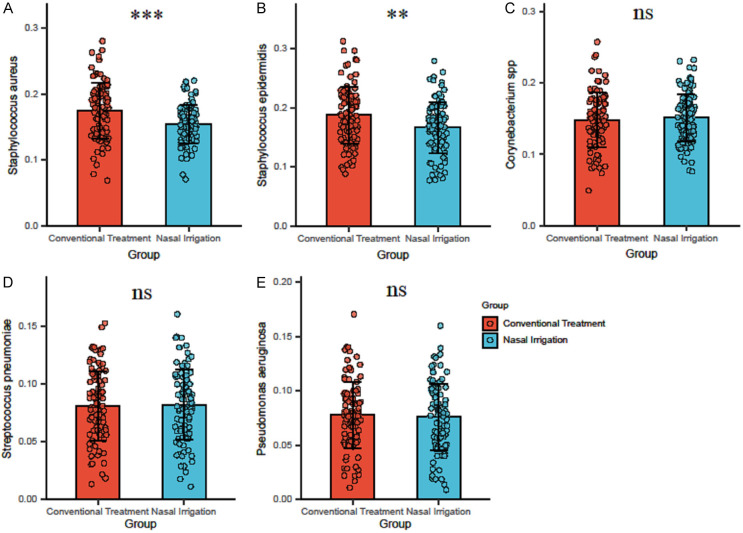
In the quantitative analysis of biofilm formation, Staphylococcus aureus levels were significantly lower in the nasal irrigation group (0.15 ± 0.03) compared to the conventional treatment group (0.17 ± 0.04) (t = 3.95, P < 0.001) (Figure 2). Similarly, Staphylococcus epidermidis biofilm levels were significantly reduced in the nasal irrigation group (0.17 ± 0.04) compared to the conventional treatment group (0.19 ± 0.05) (t = 3.268, P = 0.001). In contrast, there were no significant differences between the two groups for Corynebacterium spp, Streptococcus pneumoniae, and Pseudomonas aeruginosa (P > 0.05), indicating that nasal irrigation specifically reduced biofilm formation in certain microbial species.
Figure 2.
Comparison of biofilm formation between the two groups. A: Staphylococcus aureus; B: Staphylococcus epidermidis; C: Corynebacterium spp; D: Streptococcus pneumoniae; E: Pseudomonas aeruginosa. ns: no statistically significant difference; **: P < 0.01; ***: P < 0.001.
Nasal symptoms
The nasal irrigation group demonstrated a significantly higher rate of complete improvement in nasal pruritus compared to the conventional treatment group (χ2 = 11.366, P = 0.010) (Table 2). Furthermore, a higher proportion of patients in the nasal irrigation group experienced intermittent itching. Fewer patients reported tolerable ant-like sensations in the nasal irrigation group compared to the conventional treatment group, while the proportion of patients with intolerable ant-like sensations was similar between groups, indicating that nasal irrigation notably improved pruritus symptoms, particularly in achieving complete resolution.
Table 2.
Comparison of nasal pruritus between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | χ2 | P |
|---|---|---|---|---|
| Complete Improvement | 8 (8%) | 14 (13.73%) | 11.366 | 0.010 |
| Intermittent Itching | 31 (31%) | 49 (48.04%) | ||
| Sensation of Ants, Tolerable | 46 (46%) | 26 (25.49%) | ||
| Sensation of Ants, Intolerable | 15 (15%) | 13 (12.75%) |
For rhinorrhea, a significantly higher proportion of patients in the nasal irrigation group reported fewer than three episodes per day (χ2 = 12.193, P = 0.007) (Table 3). A higher percentage of patients in the nasal irrigation group also experienced rhinorrhea 3-5 times per day, while the proportion reporting 6-9 episodes per day was lower compared to the conventional treatment group. The proportion of patients experiencing rhinorrhea ≥ 10 times per day was similar between groups, suggesting that nasal irrigation was effective in reducing the overall frequency of rhinorrhea.
Table 3.
Comparison of rhinorrhea between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | χ2 | P |
|---|---|---|---|---|
| < 3 times/day | 8 (8%) | 21 (20.59%) | 12.193 | 0.007 |
| 3-5 times/day | 37 (37%) | 47 (46.08%) | ||
| 6-9 times/day | 43 (43%) | 25 (24.51%) | ||
| ≥ 10 times/day | 12 (12%) | 9 (8.82%) |
The nasal irrigation group showed a significantly higher rate of complete improvement in nasal congestion (χ2 = 13.111, P = 0.004) (Table 4). Additionally, more patients in the nasal irrigation group reported congestion only during conscious inhalation. Intermittent or alternating congestion was less prevalent in the nasal irrigation group compared to the conventional group. The frequency of oral breathing throughout the day was also lower in the nasal irrigation group compared to the conventional treatment group, indicating that nasal irrigation effectively alleviated nasal congestion symptoms.
Table 4.
Comparison of nasal congestion between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | χ2 | P |
|---|---|---|---|---|
| Complete Improvement | 12 (12%) | 28 (27.45%) | 13.111 | 0.004 |
| Congestion During Conscious Inhalation | 34 (34%) | 41 (40.20%) | ||
| Intermittent or Alternating Congestion | 37 (37%) | 19 (18.63%) | ||
| Oral Breathing Throughout the Day | 17 (17%) | 14 (13.73%) |
A significantly higher proportion of patients in the nasal irrigation group experienced sneezing fewer than three episodes per day (χ2 = 10.751, P = 0.013) (Table 5). Additionally, the proportion of patients sneezing 3-5 times per day was more prevalent in the nasal irrigation group, while sneezing frequency of 6-10 times per day was lower in the nasal irrigation group compared to in the conventional group. The proportion of patients experiencing sneezing ≥ 11 episodes per day was slightly lower in the nasal irrigation group, suggesting that regular nasal irrigation effectively reduced the frequency of sneezing episodes.
Table 5.
Comparison of sneezing between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | χ2 | P |
|---|---|---|---|---|
| < 3 times/day | 13 (13%) | 23 (22.55%) | 10.751 | 0.013 |
| 3-5 times/day | 31 (31%) | 45 (44.12%) | ||
| 6-10 times/day | 47 (47%) | 28 (27.45%) | ||
| ≥ 11 times/day | 9 (9%) | 6 (5.88%) |
Quality of life
Before treatment, Quality of life (QoL) scores were comparable between the two groups (t = 0.581, P = 0.562) (Table 6). However, after treatment, the nasal irrigation group showed a significantly greater improvement in QoL scores compared to the conventional treatment group (t = 2.74, P = 0.007), indicating that nasal irrigation substantially enhanced quality of life in patients with allergic rhinitis.
Table 6.
Comparison of QoL scores between the two groups
| Parameters | Conventional Treatment Group (n = 100) | Nasal Irrigation Group (n = 102) | t | P |
|---|---|---|---|---|
| Before Treatment | 33.02 ± 5.42 | 33.48 ± 5.87 | 0.581 | 0.562 |
| After Treatment | 27.38 ± 3.65 | 26.15 ± 2.65 | 2.740 | 0.007 |
QoL: Quality of life.
Correlation analysis
Correlation analysis revealed significant associations between regular nasal irrigation and multiple variables (Table 7). Nasal irrigation was negatively correlated with the Chao1 and Ace richness indices, indicating reduced microbial richness following the intervention. Similarly, notable negative correlations were found with Staphylococcus aureus and Staphylococcus epidermidis, suggesting decreased prevalence of these bacteria following nasal irrigation. Symptom improvements, including nasal pruritus, rhinorrhea, nasal congestion, and sneezing, were similarly negatively correlated with nasal irrigation. Additionally, QoL scores exhibited a negative correlation with nasal irrigation, reflecting overall quality of life improvement. A positive, though weaker, correlation was noted between the Simpson Diversity Index and nasal irrigation, suggesting a more even distribution of microbial species linked to nasal irrigation.
Table 7.
Correlation analysis of nasal microbiota, symptom improvement with nasal irrigation
| Rho | P | |
|---|---|---|
| Chao1 Index | -0.179 | 0.011 |
| Ace Index | -0.199 | 0.004 |
| Simpson Index | 0.141 | 0.045 |
| Staphylococcus aureus | -0.256 | P < 0.001 |
| Staphylococcus epidermidis | -0.219 | 0.002 |
| Nasal Pruritus | -0.194 | 0.006 |
| Rhinorrhea | -0.228 | 0.001 |
| Nasal Congestion | -0.219 | 0.002 |
| Sneezing | -0.217 | 0.002 |
| QoL scores | -0.195 | 0.005 |
QoL: Quality of life.
Discussion
This study highlights the significant adjunctive effects of regular nasal irrigation in the management of allergic rhinitis, demonstrating improvements in nasal symptoms, microbial composition, and patient quality of life. The most pertinent finding was the reduction in microbial richness and diversity, as evidenced by decreased Chao1 and Ace indices. Regular nasal irrigation appeared to modulate the nasal microbiota by reducing the abundance of specific bacterial species, including Staphylococcus aureus and Staphylococcus epidermidis. This alteration in nasal flora may stem from the mechanical removal of superficial microbes and changes in the local nasal environment that inhibit bacterial growth and biofilm formation [21,22]. The reduction in biofilm-producing bacteria is particularly significant, as biofilms protect bacteria from antibiotics and immune responses, potentially sustaining inflammation and allergic symptoms [23].
By mechanically flushing out mucus and allergens, nasal irrigation potentially reduces the allergenic burden in the nasal passages [24], leading to reduced immune cell activation and attenuated allergic responses [25]. In this study, the observed reduction in microbial load, specifically of certain biofilm-forming species, may have contributed to a less inflammatory milieu of nasal epithelium [26]. This is consistent with the clinical improvements observed in nasal congestion, sneezing, and pruritus, which are hallmark symptoms of allergic rhinitis driven by underlying inflammatory processes.
Simultaneously, improvements in the Simpson Diversity Index suggest that, although microbial richness decreases, species evenness within the nasal microbiota was enhanced [27]. A more balanced microbiota community may contribute to symptomatic improvements [28], as balanced microbial ecosystems are generally more resilient and less susceptible to domination by pathogenic or opportunistic bacteria, fostering a local environment less conducive to sustained inflammation and symptom persistence in individuals with allergic rhinitis [29,30].
Another potential mechanism underlying symptom improvement following nasal irrigation is the modulation of immune responses [31]. The nasal mucosa functions not only as a barrier but also an active immune interface [32]. Changes in the microbiota can influence both the innate and adaptive immune systems, potentially leading to modified local immune responses [33]. A reduction in specific bacterial populations may decrease the production of inflammatory cytokines or chemokines, thus mitigating the inflammatory cascade that typically characterizes allergic reactions [34]. These findings highlight the complex interactions between the host immune system and the nasal microbiota, warranting further investigation in future studies.
The enhanced quality of life observed in the nasal irrigation group also deserves attention. Quality of life in patients with allergic rhinitis is often compromised by symptoms such as nasal congestion, impaired sleep, and general discomfort [35]. Our results indicate that regular nasal irrigation, by ameliorating these specific symptoms, leads to tangible improvements in daily life activities and overall well-being. Beyond direct symptom relief, the improvement may also be partly attributed to psychological benefits, such as an enhanced sense of control over the disease, which can improve overall perceived health [16].
At the molecular level, nasal irrigation may affect the expression of tight junction proteins and other components of the nasal epithelium barrier [36]. Mechanical stimulation from irrigation may enhance mucosal barrier integrity, reducing allergen penetration and thus alleviating symptom severity [26]. Enhanced barrier integrity could limit the interaction between allergens and immune cells, diminishing the inflammatory cascades responsible for allergic rhinitis symptoms [37].
The clinical implications of these findings are substantial, suggesting that nasal irrigation could potentially redefine standard management protocols for allergic rhinitis, particularly for patients with persistent symptoms despite pharmacotherapy. Future studies should focus on comparing different irrigation solutions and devices to refine and optimize treatment strategies, assessing factors such as volume, frequency, and temperature. A personalized approach may optimize therapeutic outcomes in patients with allergic rhinitis.
While this study provides valuable insights into the effects of nasal irrigation on nasal microbiota and symptom improvement in patients with allergic rhinitis, several limitations must be acknowledged. First, the short study duration limits our understanding of the long-term efficacy and sustainability of the intervention. Additionally, the sample size may not fully represent the broader allergic rhinitis population, thereby affecting the generalizability of the findings. Furthermore, variations in irrigation techniques and patient compliance could introduce inconsistencies in treatment outcomes. Future studies should incorporate larger, more diverse populations and incorporate long-term follow-up to better evaluate the durability of treatment effects and optimize treatment protocols.
Conclusion
In conclusion, this study highlights nasal irrigation as an effective complementary intervention in the management of allergic rhinitis, leading to modulation of the nasal microbiota, symptom relief, and enhanced quality of life. Through mechanical removal of allergens and alterations in microbiota composition, nasal irrigation reduces inflammation and modulates immune responses, both of which are critical in the pathophysiology of allergic rhinitis. These findings pave the way for further research into the interplay between nasal microbiota and host immunity, potentially unveiling novel therapeutic targets for allergic diseases.
Disclosure of conflict of interest
None.
References
- 1.Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, Luong A, Rodriguez K, Sedaghat AR, Toskala E, Villwock J, Abdullah B, Akdis C, Alt JA, Ansotegui IJ, Azar A, Baroody F, Benninger MS, Bernstein J, Brook C, Campbell R, Casale T, Chaaban MR, Chew FT, Chambliss J, Cianferoni A, Custovic A, Davis EM, DelGaudio JM, Ellis AK, Flanagan C, Fokkens WJ, Franzese C, Greenhawt M, Gill A, Halderman A, Hohlfeld JM, Incorvaia C, Joe SA, Joshi S, Kuruvilla ME, Kim J, Klein AM, Krouse HJ, Kuan EC, Lang D, Larenas-Linnemann D, Laury AM, Lechner M, Lee SE, Lee VS, Loftus P, Marcus S, Marzouk H, Mattos J, McCoul E, Melen E, Mims JW, Mullol J, Nayak JV, Oppenheimer J, Orlandi RR, Phillips K, Platt M, Ramanathan M Jr, Raymond M, Rhee CS, Reitsma S, Ryan M, Sastre J, Schlosser RJ, Schuman TA, Shaker MS, Sheikh A, Smith KA, Soyka MB, Takashima M, Tang M, Tantilipikorn P, Taw MB, Tversky J, Tyler MA, Veling MC, Wallace D, Wang Y, White A, Zhang L. International consensus statement on allergy and rhinology: allergic rhinitis - 2023. Int Forum Allergy Rhinol. 2023;13:293–859. doi: 10.1002/alr.23090. [DOI] [PubMed] [Google Scholar]
- 2.Vardouniotis A, Doulaptsi M, Aoi N, Karatzanis A, Kawauchi H, Prokopakis E. Local allergic rhinitis revisited. Curr Allergy Asthma Rep. 2020;20:22. doi: 10.1007/s11882-020-00925-5. [DOI] [PubMed] [Google Scholar]
- 3.Okano M, Fujieda S, Gotoh M, Kurono Y, Matsubara A, Ohta N, Kamijo A, Yamada T, Nakamaru Y, Asako M, Sakurai D, Terada T, Yonekura S, Sakashita M, Okubo K. Executive summary: Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2023;72:41–53. doi: 10.1016/j.alit.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, Suzaki H, Fujieda S, Masuyama K. Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69:331–345. doi: 10.1016/j.alit.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Jung MA, Hwang YH, Pyun BJ, Lee JY, Jung DH, Ji KY, Kim T. Anti-allergic effects of Asarum heterotropoides on an ovalbumin-induced allergic rhinitis murine model. Biomed Pharmacother. 2021;141:111944. doi: 10.1016/j.biopha.2021.111944. [DOI] [PubMed] [Google Scholar]
- 6.Campo P, Canonica GW. Local allergic rhinitis. J Allergy Clin Immunol Pract. 2024;12:1430–1433. doi: 10.1016/j.jaip.2024.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Wise SK, Damask C, Greenhawt M, Oppenheimer J, Roland LT, Shaker MS, Wallace DV, Lang DM. A synopsis of guidance for allergic rhinitis diagnosis and management from ICAR 2023. J Allergy Clin Immunol Pract. 2023;11:773–796. doi: 10.1016/j.jaip.2023.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Ponda P, Carr T, Rank MA, Bousquet J. Nonallergic rhinitis, allergic rhinitis, and immunotherapy: advances in the last decade. J Allergy Clin Immunol Pract. 2023;11:35–42. doi: 10.1016/j.jaip.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Immunol Allergy Clin North Am. 2021;41:613–625. doi: 10.1016/j.iac.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, Melén E, Palomares O, Scadding GK, Togias A, Toppila-Salmi S. Allergic rhinitis. Nat Rev Dis Primers. 2020;6:95. doi: 10.1038/s41572-020-00227-0. [DOI] [PubMed] [Google Scholar]
- 11.Gnanasekaran S, Jayaraj V, V BY, Selvam MP, Rajendran V. Evaluating the efficacy of nasal irrigation in postoperative functional endoscopic sinus surgery patients: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2024;281:3903–3913. doi: 10.1007/s00405-024-08535-x. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein JA, White AA, Han JK, Lang DM, Elkayam D, Baroody FM. Review of evidence supporting the use of nasal corticosteroid irrigation for chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2023;130:46–57. doi: 10.1016/j.anai.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:367–378. doi: 10.1016/S2468-1253(21)00261-2. [DOI] [PubMed] [Google Scholar]
- 14.Gupta KK, Anari S. Medical management of rhinitis in pregnancy. Auris Nasus Larynx. 2022;49:905–911. doi: 10.1016/j.anl.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Head K, Snidvongs K, Glew S, Scadding G, Schilder AG, Philpott C, Hopkins C. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. 2018;6:Cd012597. doi: 10.1002/14651858.CD012597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosca MA, Trincianti C, Naso M, Nosratian V, Ciprandi G. Treatment of allergic rhinitis in clinical practice. Curr Pediatr Rev. 2024;20:271–277. doi: 10.2174/1573396320666230912103108. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, He S, Wei Y, Liu Y, Xu Q, Lin X, Chen C, Lin W, Wang Y, Li L, Xu Y. Fecal and serum metabolomic signatures and gut microbiota characteristics of allergic rhinitis mice model. Front Cell Infect Microbiol. 2023;13:1150043. doi: 10.3389/fcimb.2023.1150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Zhang R, Li J, Wang H, Wang M, Ren Q, Fang Y, Tian L. Association between gut and nasal microbiota and allergic rhinitis: a systematic review. J Asthma Allergy. 2024;17:633–651. doi: 10.2147/JAA.S472632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P. The role of diet and nutrition in allergic diseases. Nutrients. 2023;15:3683. doi: 10.3390/nu15173683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soler R, de la Hoz B, Badia X, Mercadal J, Lozano R, Benavides A, Roset M. Validation of the spanish version of the rhinoconjunctivitis quality of life questionnaire (RQLQ) Rev Clin Esp. 2004;204:131–138. doi: 10.1157/13058825. [DOI] [PubMed] [Google Scholar]
- 21.Eschenbacher WH. Nasal saline irrigation with steroids: reviewing a common practice carefully. Ann Allergy Asthma Immunol. 2023;130:2–3. doi: 10.1016/j.anai.2022.09.030. [DOI] [PubMed] [Google Scholar]
- 22.de Gabory L, Kérimian M, Sagardoy T, Verdaguer A, Gauchez H. Paediatric nasal irrigation: the “fencing” method. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138:107–113. doi: 10.1016/j.anorl.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Luz-Matsumoto GR, Cabernite-Marchetti E, Sasaki LSK, Marquez GJ, Lacerda LS, Almeida TR, Kosugi EM. Nasal irrigation with corticosteroids in Brazil: the clinical response of 1% compounded budesonide drops and betamethasone cream. Braz J Otorhinolaryngol. 2022;88(Suppl 5):S32–S41. doi: 10.1016/j.bjorl.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panta P, Chatti K, Andhavarapu A. Do saline water gargling and nasal irrigation confer protection against COVID-19? Explore (NY) 2021;17:127–129. doi: 10.1016/j.explore.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt Y, Yildirim YS. Effectiveness of pediatric nasal irrigation solution with or without xylitol. Int J Pediatr Otorhinolaryngol. 2022;158:111183. doi: 10.1016/j.ijporl.2022.111183. [DOI] [PubMed] [Google Scholar]
- 26.Salati H, Bartley J, White DE. Nasal saline irrigation - a review of current anatomical, clinical and computational modelling approaches. Respir Physiol Neurobiol. 2020;273:103320. doi: 10.1016/j.resp.2019.103320. [DOI] [PubMed] [Google Scholar]
- 27.Czech EJ, Overholser A, Schultz P. Allergic rhinitis. Prim Care. 2023;50:159–178. doi: 10.1016/j.pop.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Alenezi A, Qureshi H, Ahmed OG, Ramanathan M Jr. Air quality, allergic rhinitis, and asthma. Otolaryngol Clin North Am. 2024;57:293–307. doi: 10.1016/j.otc.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Pagel JML, Mattos JL. Allergic rhinitis and its effect on sleep. Otolaryngol Clin North Am. 2024;57:319–328. doi: 10.1016/j.otc.2023.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy. 2022;77:3309–3319. doi: 10.1111/all.15454. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. 2021;76:3383–3389. doi: 10.1111/all.15044. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui ZA, Walker A, Pirwani MM, Tahiri M, Syed I. Allergic rhinitis: diagnosis and management. Br J Hosp Med (Lond) 2022;83:1–9. doi: 10.12968/hmed.2021.0570. [DOI] [PubMed] [Google Scholar]
- 33.Bayar Muluk N, Cingi C. Biologics in allergic rhinitis. Eur Rev Med Pharmacol Sci. 2023;27:43–52. doi: 10.26355/eurrev_202310_34069. [DOI] [PubMed] [Google Scholar]
- 34.Gani F, Cottini M, Landi M, Berti A, Comberiati P, Peroni D, Senna G, Lombardi C. Allergic rhinitis and COVID-19: friends or foes? Eur Ann Allergy Clin Immunol. 2022;54:53–59. doi: 10.23822/EurAnnACI.1764-1489.234. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Y, Wang C, Wang G, Guo X, Jiang S, Zuo X, Wang X, Hsu AC, Qi M, Wang F. Airway microbiome and serum metabolomics analysis identify differential candidate biomarkers in allergic rhinitis. Front Immunol. 2021;12:771136. doi: 10.3389/fimmu.2021.771136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapiala J, Hyvärinen A, Toppila-Salmi S, Suihko E, Penttilä E. Nasal saline irrigation: prescribing habits and attitudes of physicians and pharmacists. Scand J Prim Health Care. 2021;39:35–43. doi: 10.1080/02813432.2021.1880123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audag N, Cnockaert P, Reychler G, Poncin W. Consensus on nasal irrigation in infants: a delphi study. Ann Otol Rhinol Laryngol. 2023;132:674–683. doi: 10.1177/00034894221112514. [DOI] [PubMed] [Google Scholar]