Abstract
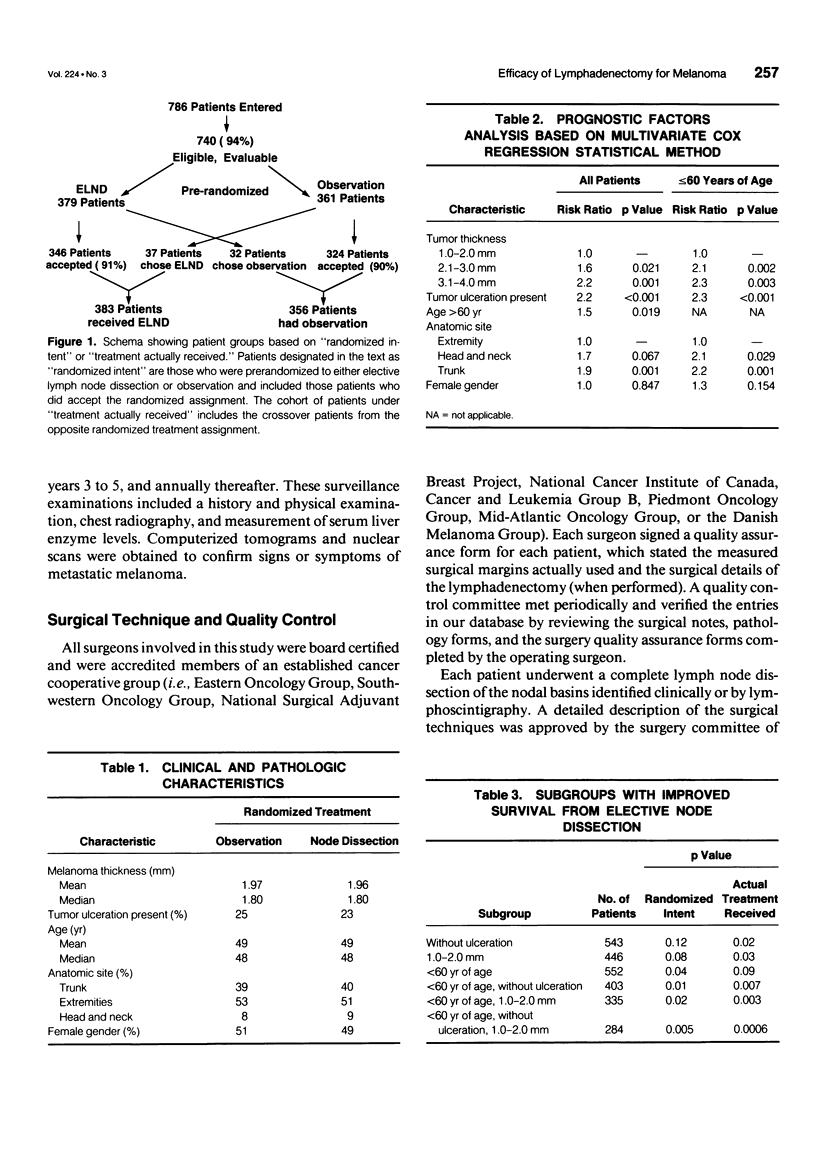
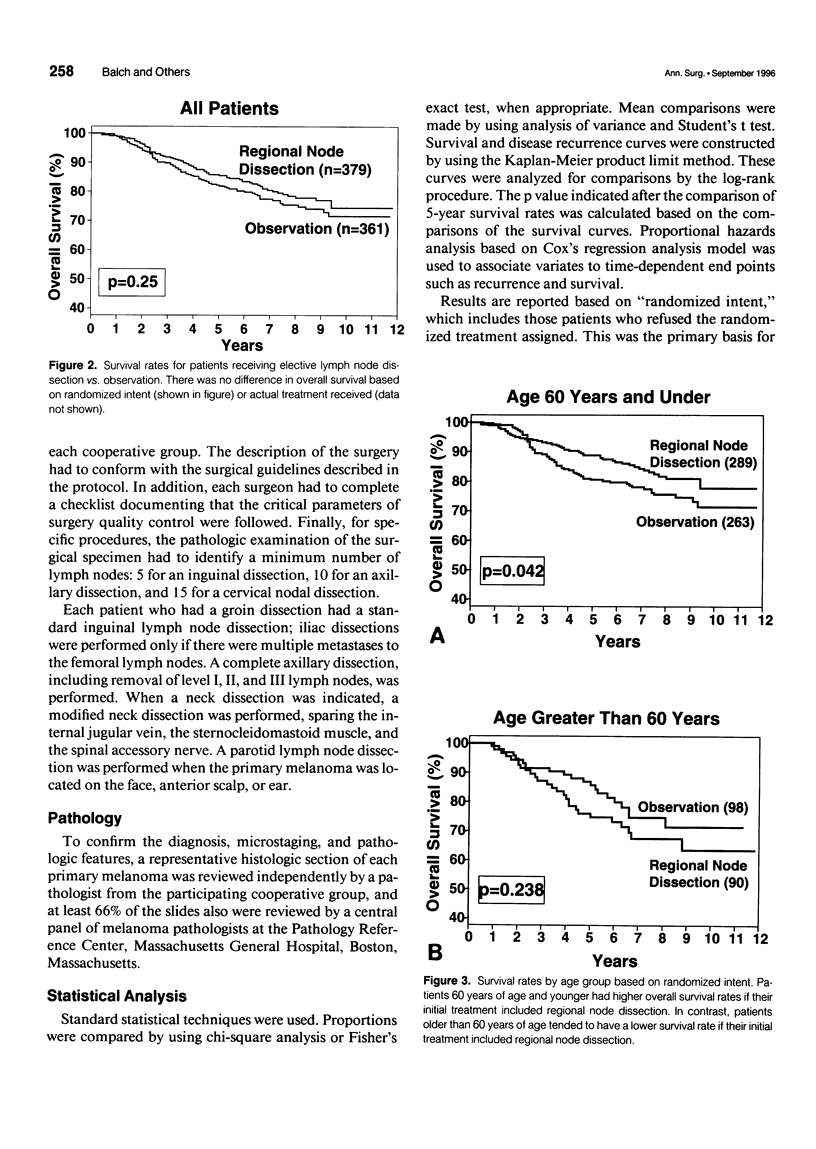
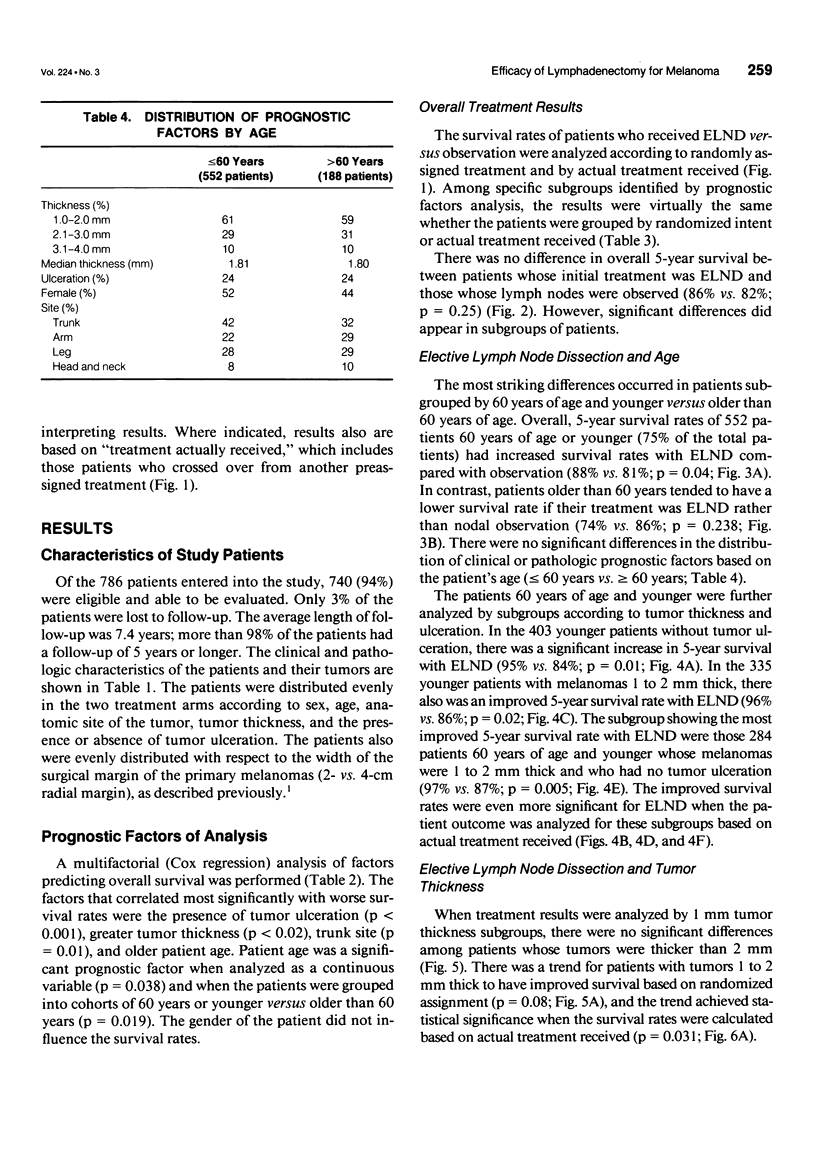
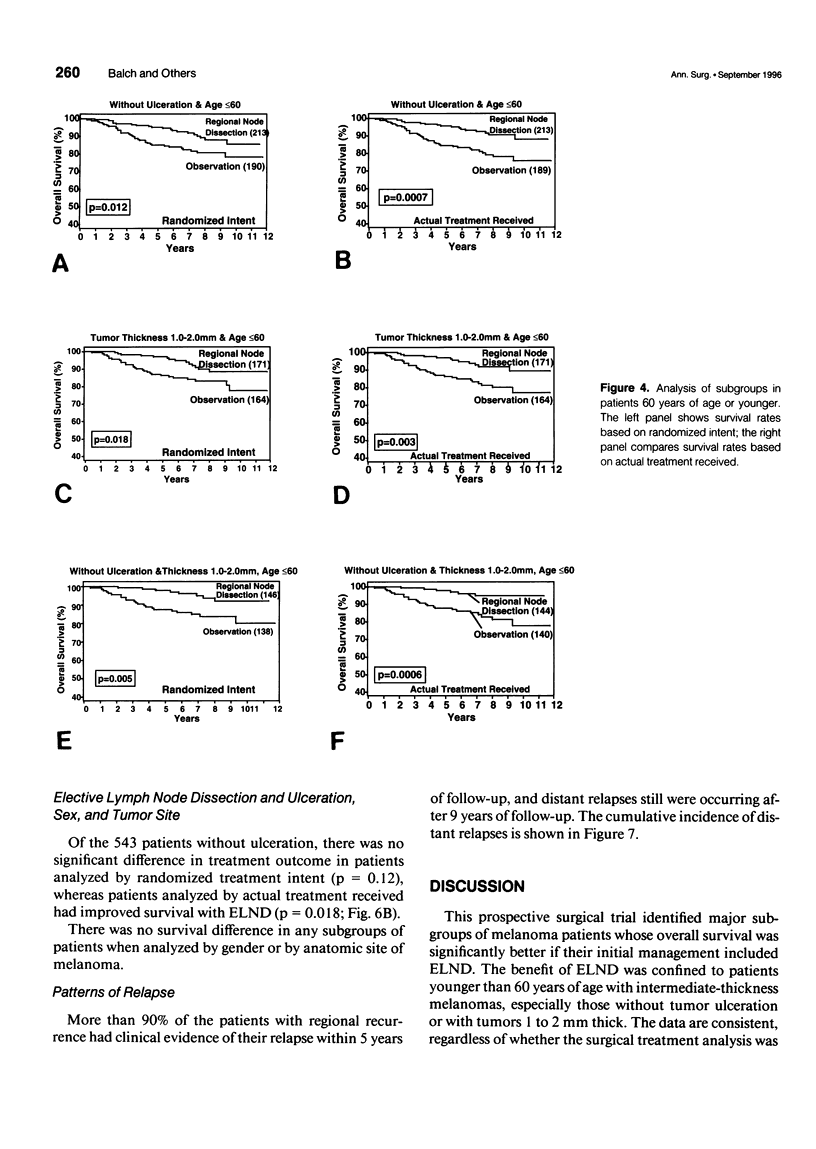
OBJECTIVE: A prospective multi-institutional randomized surgical trial involving 740 stage I and II melanoma patients was conducted by the Intergroup Melanoma Surgical Program to determine whether elective (immediate) lymph node dissection (ELND) for intermediate-thickness melanoma (1-4 mm) improves survival rates compared with clinical observation of the lymph nodes. A second objective was to define subgroups of melanoma patients who would have a higher survival with ELND. METHODS: The eligible patients were stratified according to tumor thickness, anatomic site, and ulceration, and then were prerandomized to either ELND or nodal observation. Femoral, axillary, or modified neck dissections were performed using standardized surgical guidelines. RESULTS: The median follow-up was 7.4 years. A multifactorial (Cox regression) analysis showed that the following factors independently influenced survival: tumor ulceration, trunk site, tumor thickness, and patient age. Surgical treatment results were first compared based on randomized intent. Overall 5-year survival was not significantly different for patients who received ELND or nodal observation. However, the 552 patients 60 years of age or younger (75% of total group) with ELND has a significantly better 5-year survival. Among these patients, 5-year survival was better with ELND versus nodal observation for the 335 patients with tumors 1 to 2 mm thick, the 403 patients without tumor ulceration, and the 284 patients with tumors 1 to 2 mm thick and no ulceration. In contrast, patients older than 60 years of age who had ELND actually had a lower survival trend than those who had nodal observation. When survival rates were compared based on treatment actually received (i.e., including crossover patients), the patients with significantly improved 5-year survival rates after ELND included those with tumors 1 to 2 mm thick, those without tumor ulceration, and those 60 years of age or younger with tumors 1 to 2 mm thick or without ulceration. CONCLUSION: This is the first randomized study to prove the value of surgical treatment for clinically occult regional metastases. Patients 60 years or age or younger with intermediate-thickness melanomas, especially with nonulcerative melanoma and those with tumors 1 to 2 mm thick, may benefit from ELND. However, because some patients still are developing distant disease, these results should be considered an interim analysis.
Full text
PDF

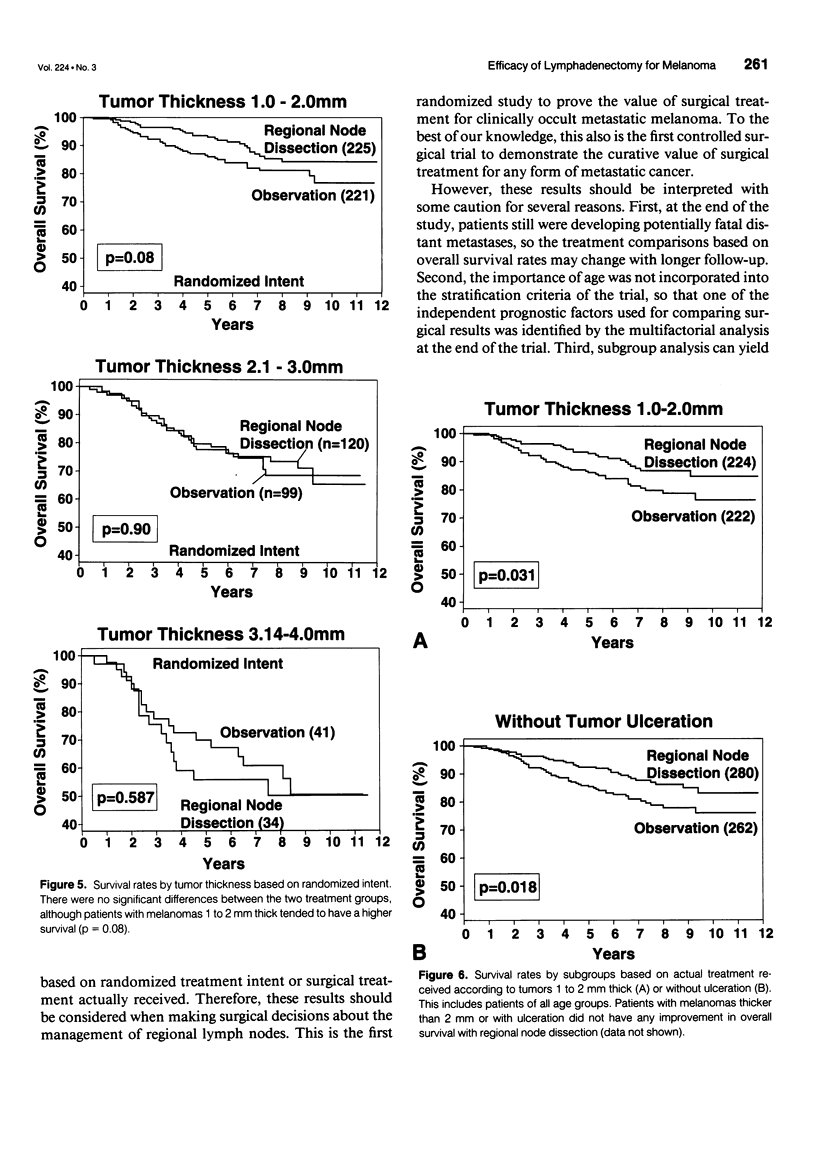
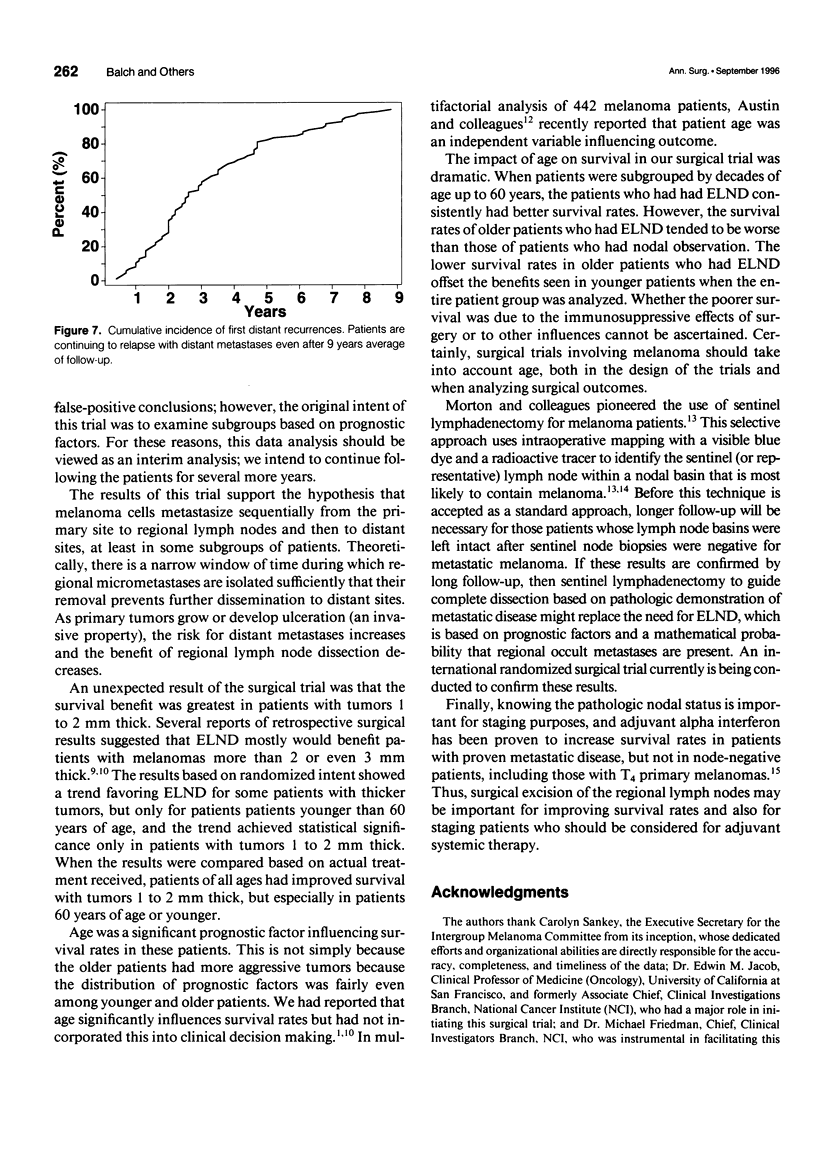

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin P. F., Cruse C. W., Lyman G., Schroer K., Glass F., Reintgen D. S. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994 Nov;1(6):487–494. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Halpern N. B., Maddox W. A. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978 Dec;188(6):732–742. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Richards P. C., Maddox W. A. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979 Mar;43(3):883–888. doi: 10.1002/1097-0142(197903)43:3<883::aid-cncr2820430316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979 Aug;86(2):343–351. [PubMed] [Google Scholar]
- Coates A. S., Ingvar C. I., Petersen-Schaefer K., Shaw H. M., Milton G. W., O'Brien C. J., Thompson J. F., McCarthy W. H. Elective lymph node dissection in patients with primary melanoma of the trunk and limbs treated at the Sydney Melanoma unit from 1960 to 1991. J Am Coll Surg. 1995 Apr;180(4):402–409. [PubMed] [Google Scholar]
- Kirkwood J. M., Strawderman M. H., Ernstoff M. S., Smith T. J., Borden E. C., Blum R. H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996 Jan;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., McCarthy W. H., Pearson L., Balch C. M., Soong S. J. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: results of surgical treatment in 1319 patients. Br J Surg. 1982 Feb;69(2):108–111. doi: 10.1002/bjs.1800690217. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Wen D. R., Wong J. H., Economou J. S., Cagle L. A., Storm F. K., Foshag L. J., Cochran A. J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- Reintgen D., Cruse C. W., Wells K., Berman C., Fenske N., Glass F., Schroer K., Heller R., Ross M., Lyman G. The orderly progression of melanoma nodal metastases. Ann Surg. 1994 Dec;220(6):759–767. doi: 10.1097/00000658-199412000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Ivins J. C., Pritchard D. J., Soule E. H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978 Mar;41(3):948–956. doi: 10.1002/1097-0142(197803)41:3<948::aid-cncr2820410324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorski V. V., Kirov S. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982 Jun 1;49(11):2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979 May 31;300(22):1242–1245. doi: 10.1056/NEJM197905313002203. [DOI] [PubMed] [Google Scholar]


