Abstract
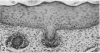
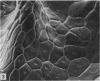
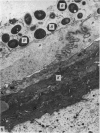
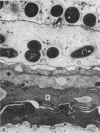
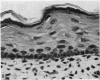
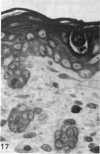
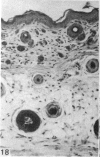
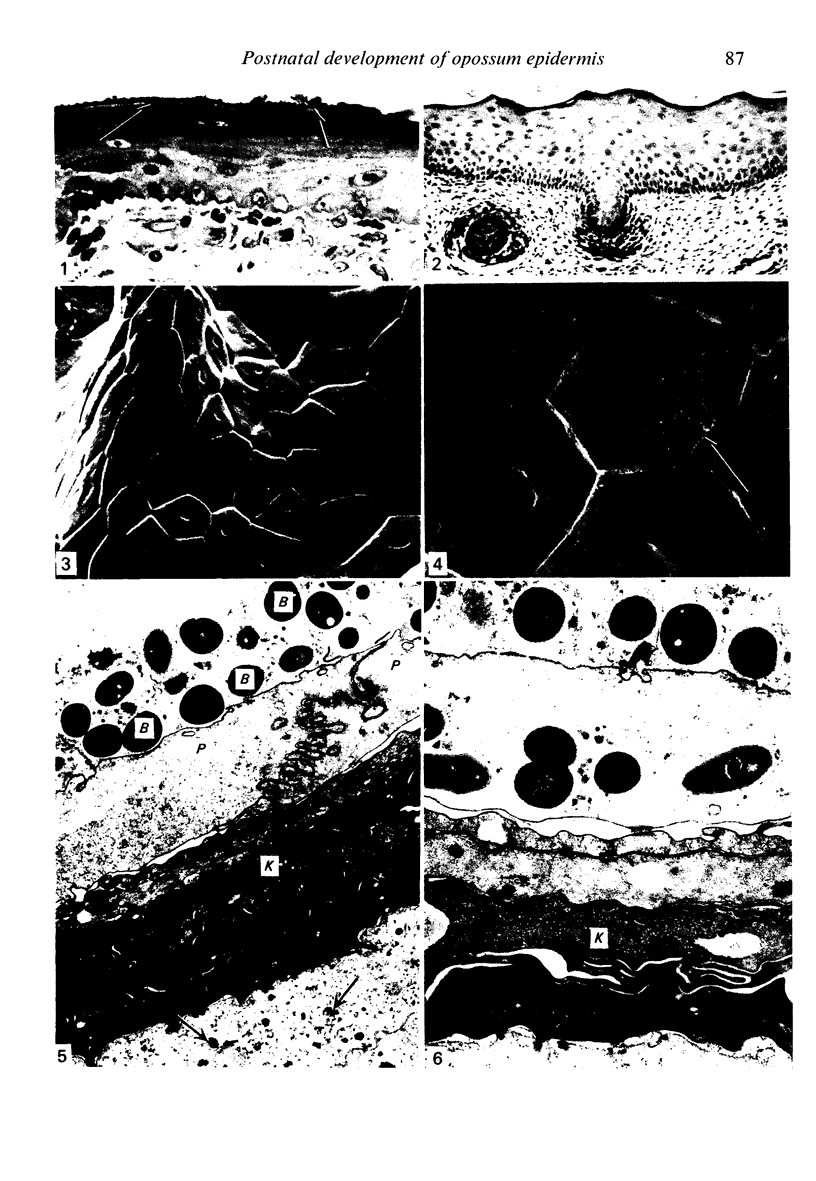
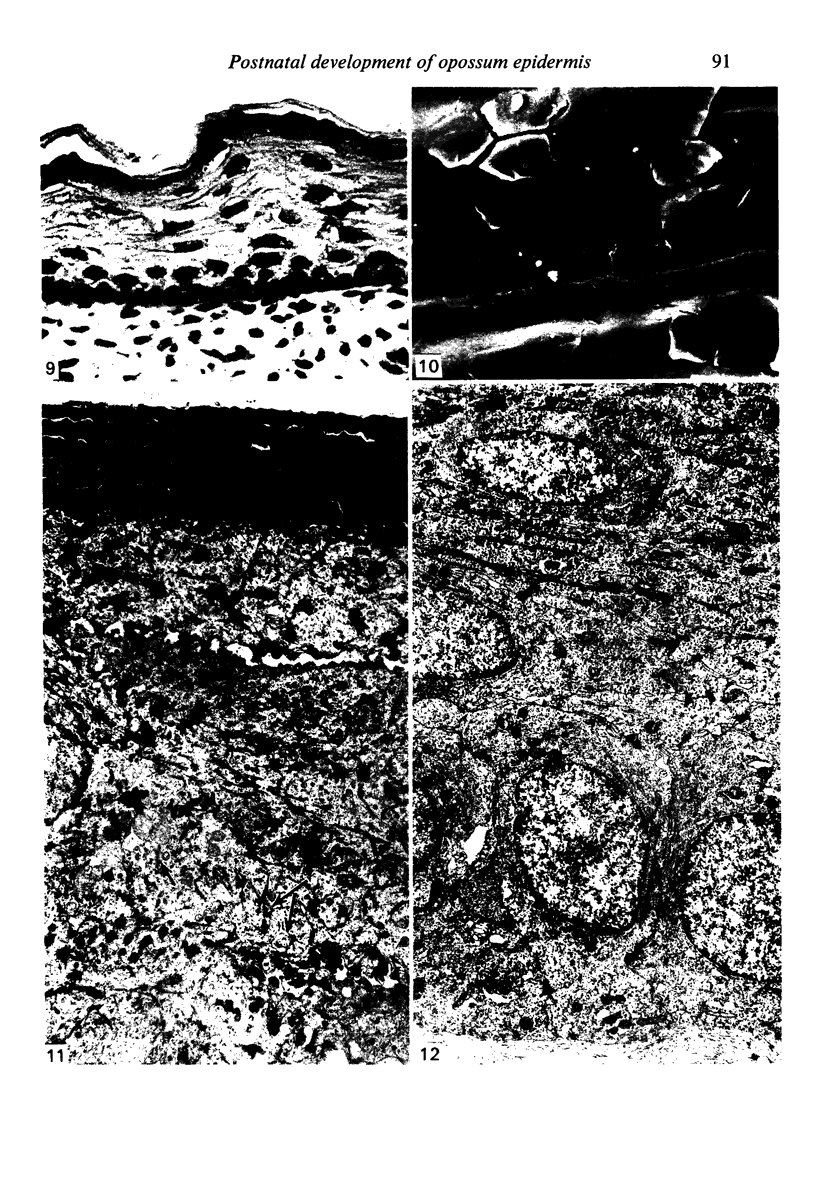
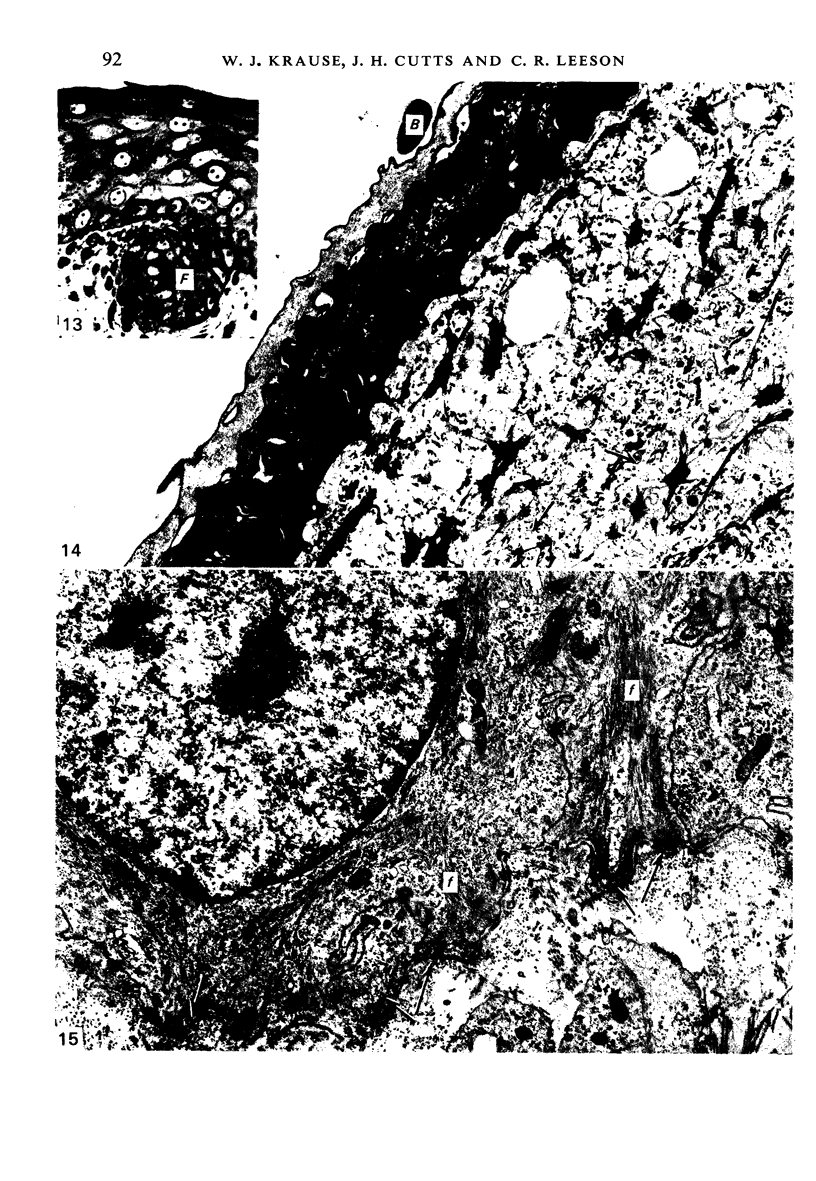
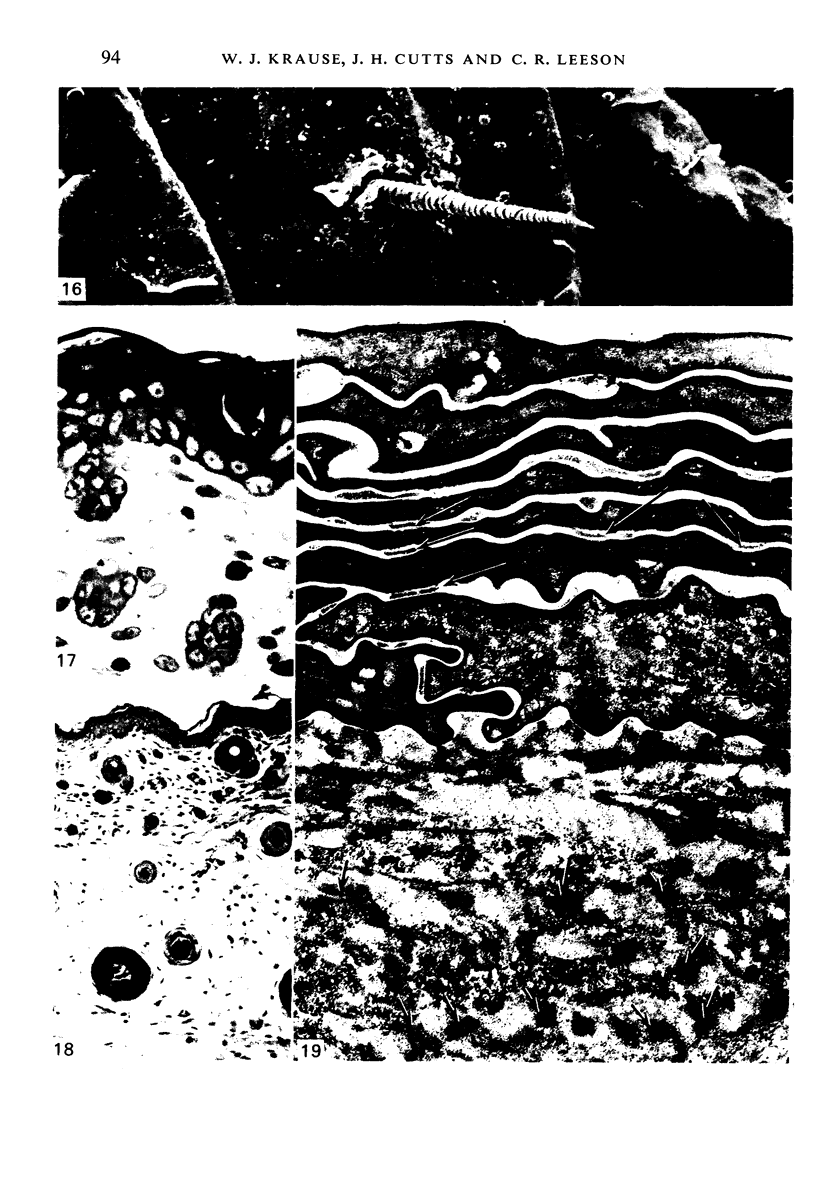
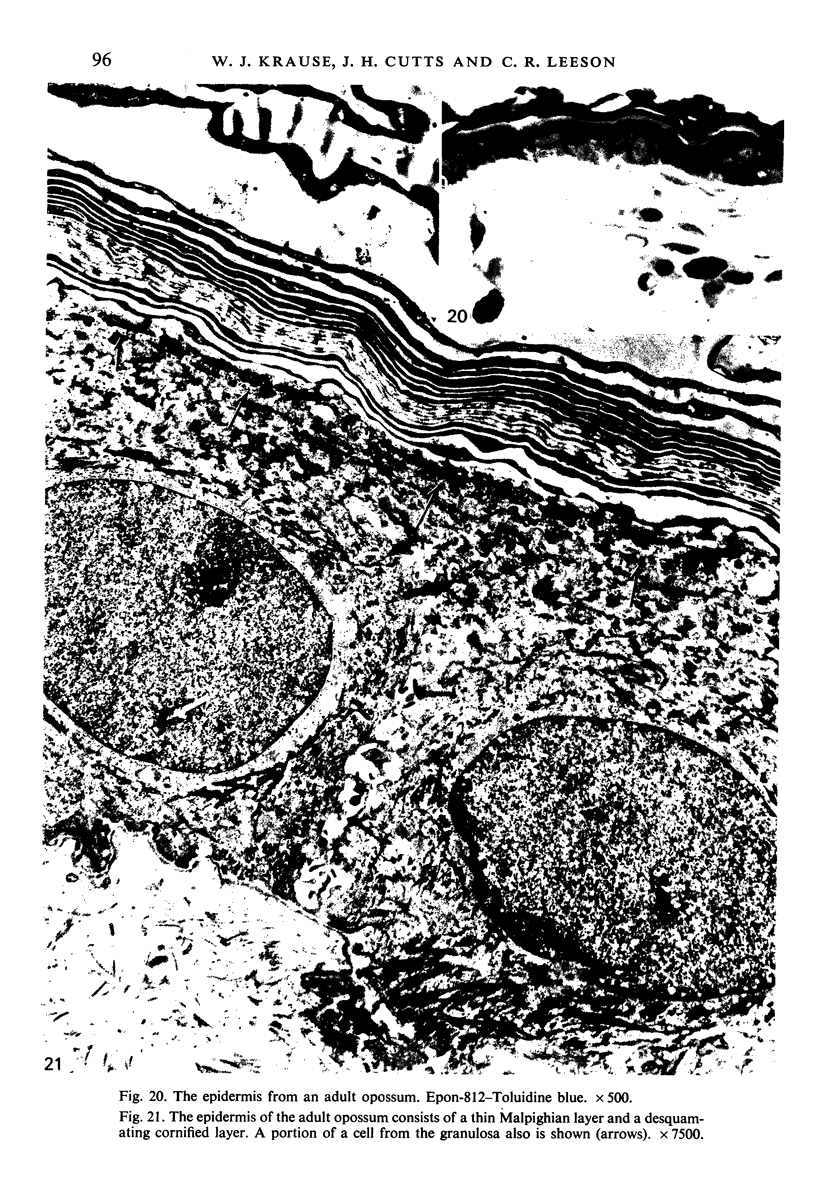
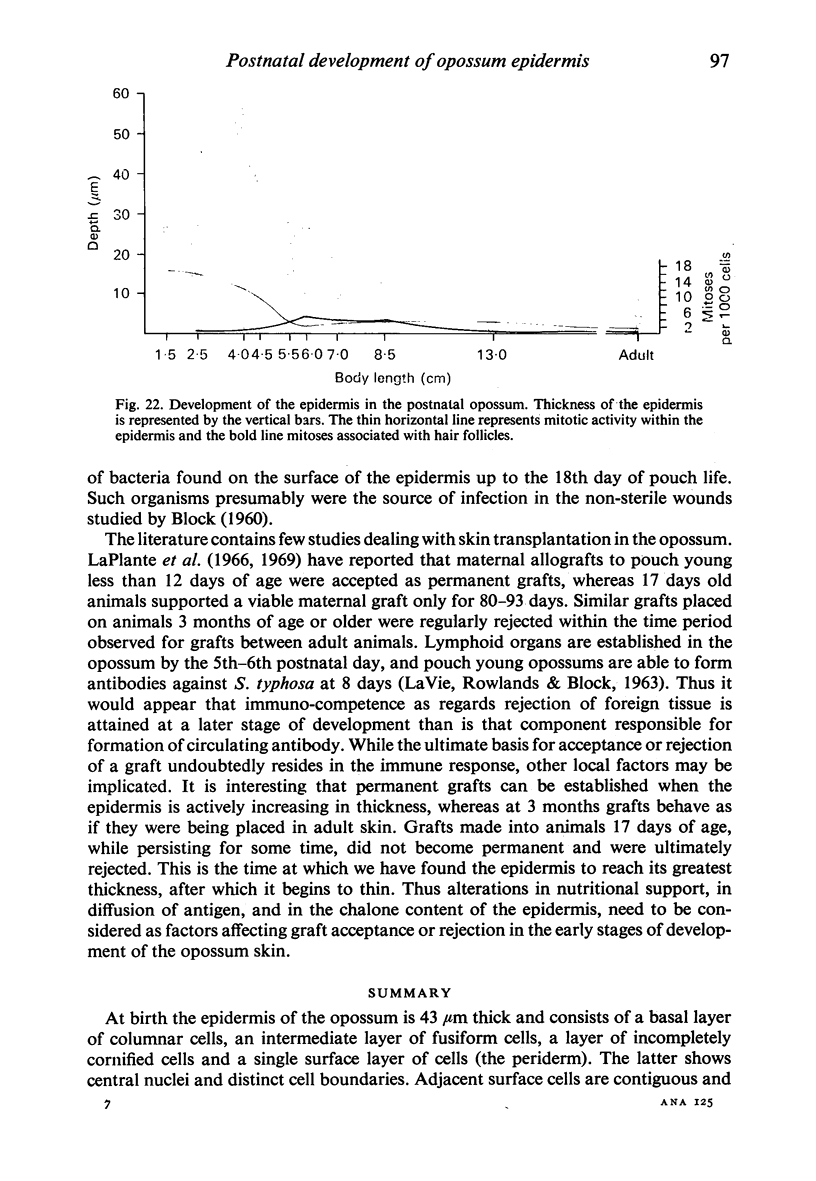
At birth the epidermis of the opossum is 43 micron thick and consists of a basal layer of columnar cells, an intermediate layer of fusiform cells, a layer of incompletely cornified cells and a single surface layer of cells (the periderm). The latter shows central nuclei and distinct cell boundaries. Adjacent surface cells. are contiguous and show extensive interdigitations of the lateral cell membranes. The periderm is lost during the first week of postnatal development. The epidermis attains its greatest thickness (58 micron) at the 4.0 cm stage (18 days postnatum), and this is due primarily to an increase in thickness of the spinous layer. After this the epidermis thins to 14 micron in the adult. The epidermis of the adult consists of a thin Malphighian layer and a desquamating cornified layer. Hair follicles begin to differentiate at the 2.5 cm stage (7 days postnatum). They continue to differentiate and develop while the epidermis is increasing, and then decreasing, in thickness. The young are fully furred prior to the time they first venture from the protection of the pouch.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Potten C. S. Desmosomal form, fate, and function in mammalian epidermis. J Ultrastruct Res. 1975 Apr;51(1):94–105. doi: 10.1016/s0022-5320(75)80011-6. [DOI] [PubMed] [Google Scholar]
- Allen T. D., Potten C. S. Ultrastructural site variations in mouse epidermal organization. J Cell Sci. 1976 Jul;21(2):341–359. doi: 10.1242/jcs.21.2.341. [DOI] [PubMed] [Google Scholar]
- BLOCK M. Wound healing in the new-born opossum (Didelphis virginianam). Nature. 1960 Jul 23;187:340–341. doi: 10.1038/187340a0. [DOI] [PubMed] [Google Scholar]
- Bonneville M. A. Observations on epidermal differentiation in the fetal rat. Am J Anat. 1968 Jul;123(1):147–164. doi: 10.1002/aja.1001230107. [DOI] [PubMed] [Google Scholar]
- Breathnach A. S., Wyllie L. M. Fine structure of cells forming the surface layer of the epidermis in human fetuses at fourteen and twelve weeks. J Invest Dermatol. 1965 Sep;45(3):179–189. doi: 10.1038/jid.1965.116. [DOI] [PubMed] [Google Scholar]
- Cutts J. H., Leeson C. R., Krause W. J. The postnatal development of the liver in a marsupial, Didelphis virginiana. 1. Light microscopy. J Anat. 1973 Sep;115(Pt 3):327–346. [PMC free article] [PubMed] [Google Scholar]
- Hanson J. The histogenesis of the epidermis in the rat and mouse. J Anat. 1947 Apr;81(Pt 2):174–197. [PMC free article] [PubMed] [Google Scholar]
- Holbrook K. A., Odland G. F. The fine structure of developing human epidermis: light, scanning, and transmission electron microscopy of the periderm. J Invest Dermatol. 1975 Jul;65(1):16–38. doi: 10.1111/1523-1747.ep12598029. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H., Leeson C. R. The postnatal development of the alimentary canal in the opossum. I. Oesophagus. J Anat. 1976 Nov;122(Pt 2):293–314. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H., Leeson C. R. The postnatal development of the alimentary canal in the opossum. II. Stomach. J Anat. 1976 Dec;122(Pt 3):499–519. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H., Leeson C. R. The postnatal development of the alimentary canal in the opossum. III. Small intestine and colon. J Anat. 1977 Feb;123(Pt 1):21–45. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H., Leeson C. R. The postnatal development of the liver in a marsupial, Didelphis virginiana. 2. Electron microscopy. J Anat. 1975 Sep;120(Pt 1):191–205. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H., Leeson C. R. Type II pulmonary epithelial cells of the newborn opossum lung. Am J Anat. 1976 Jun;146(2):181–187. doi: 10.1002/aja.1001460206. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Leeson C. R. Postnatal development of the respiratory system of the opossum. II. Electron microscopy of the epithelium and pleura. Acta Anat (Basel) 1975;92(1):28–44. doi: 10.1159/000144426. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Leeson C. R. The postnatal development of the respiratory system of the opossum. I. Light and scanning electron microscopy. Am J Anat. 1973 Jul;137(3):337–355. doi: 10.1002/aja.1001370306. [DOI] [PubMed] [Google Scholar]
- La Via M. F., Rowlands D. T., Jr, Block M. Antibody Formation in Embryos. Science. 1963 Jun 14;140(3572):1219–1220. doi: 10.1126/science.140.3572.1219. [DOI] [PubMed] [Google Scholar]
- LaPlante E. S., Burrell R. G., Watne A. L., Zimmermann B. Permanent survival of skin allografts applied to the pouch young of the opossum. Surg Forum. 1966;17:200–201. [PubMed] [Google Scholar]
- LaPlante E. S., Burrell R., Watne A. L., Taylor D. L., Zimmermann B. Skin allograft studies in the pouch young of the opossum. Transplantation. 1969 Jan;7(1):67–72. doi: 10.1097/00007890-196901000-00006. [DOI] [PubMed] [Google Scholar]
- Lyne A. G., Henrikson R. C., Hollis D. E. Development of the epidermis of the marsupial Trichosurus vulpecula. Aust J Biol Sci. 1970 Oct;23(5):1067–1075. [PubMed] [Google Scholar]
- Lyne A. G. The development of hair follicles in the marsupial Trichosurus vulpecula. Aust J Biol Sci. 1970 Dec;23(6):1241–1253. doi: 10.1071/bi9701241. [DOI] [PubMed] [Google Scholar]
- Mann S. J. The tylotrich (hair) follicle of the American opossum. Anat Rec. 1968 Feb;160(2):171–179. doi: 10.1002/ar.1091600203. [DOI] [PubMed] [Google Scholar]
- Montagna W., Macpherson E. Similarities in cutaneous nerve receptors. Arch Dermatol. 1973 Mar;107(3):383–385. [PubMed] [Google Scholar]
- Munger B. L. The intraepidermal innervation of the snout skin of the opossum. A light and electron microscope study, with observations on the nature of Merkel's Tastzellen. J Cell Biol. 1965 Jul;26(1):79–97. doi: 10.1083/jcb.26.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNELL R. S. THE FATE OF EPIDERMAL DESMOSOMES IN MAMMALIAN SKIN. Z Zellforsch Mikrosk Anat. 1965 May 26;66(4):471–477. doi: 10.1007/BF00368240. [DOI] [PubMed] [Google Scholar]