Abstract
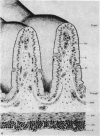
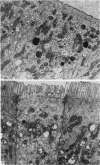
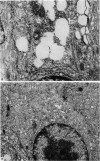
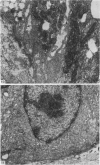
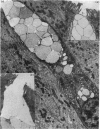
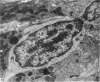
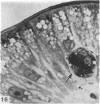
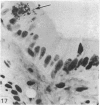
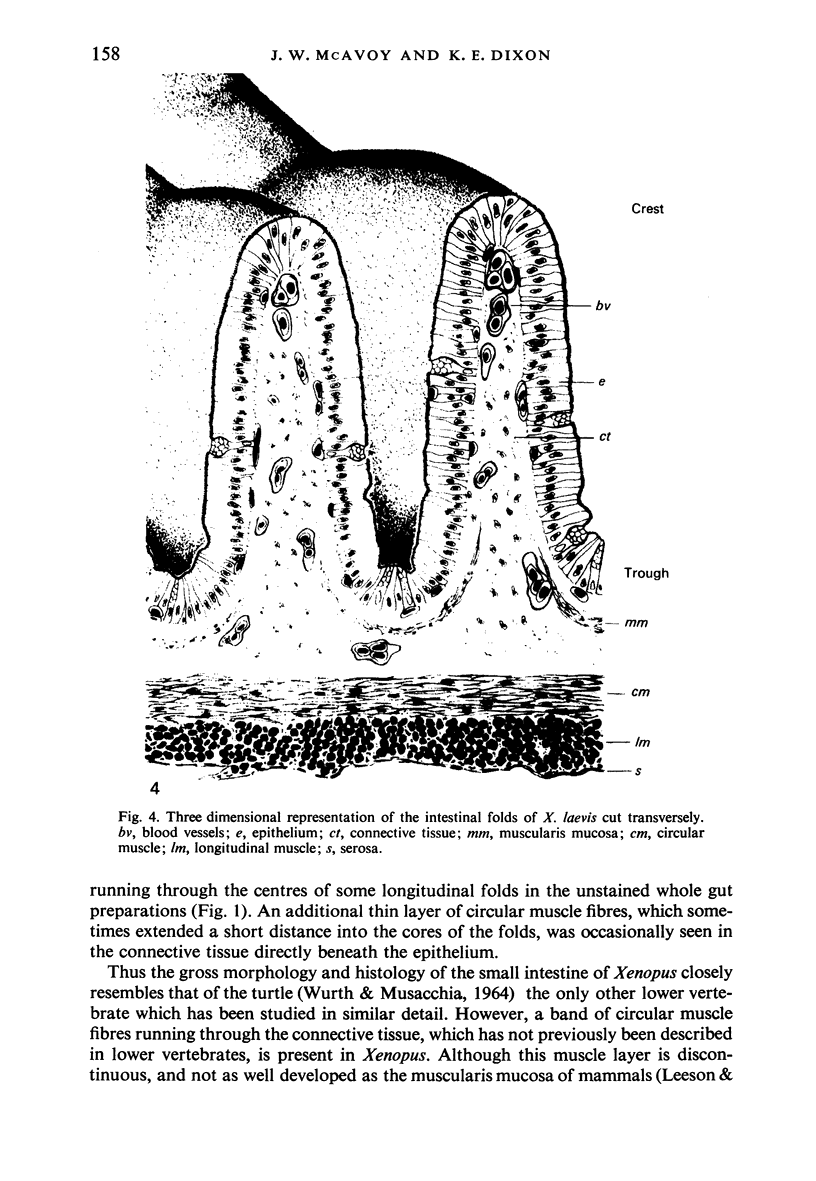
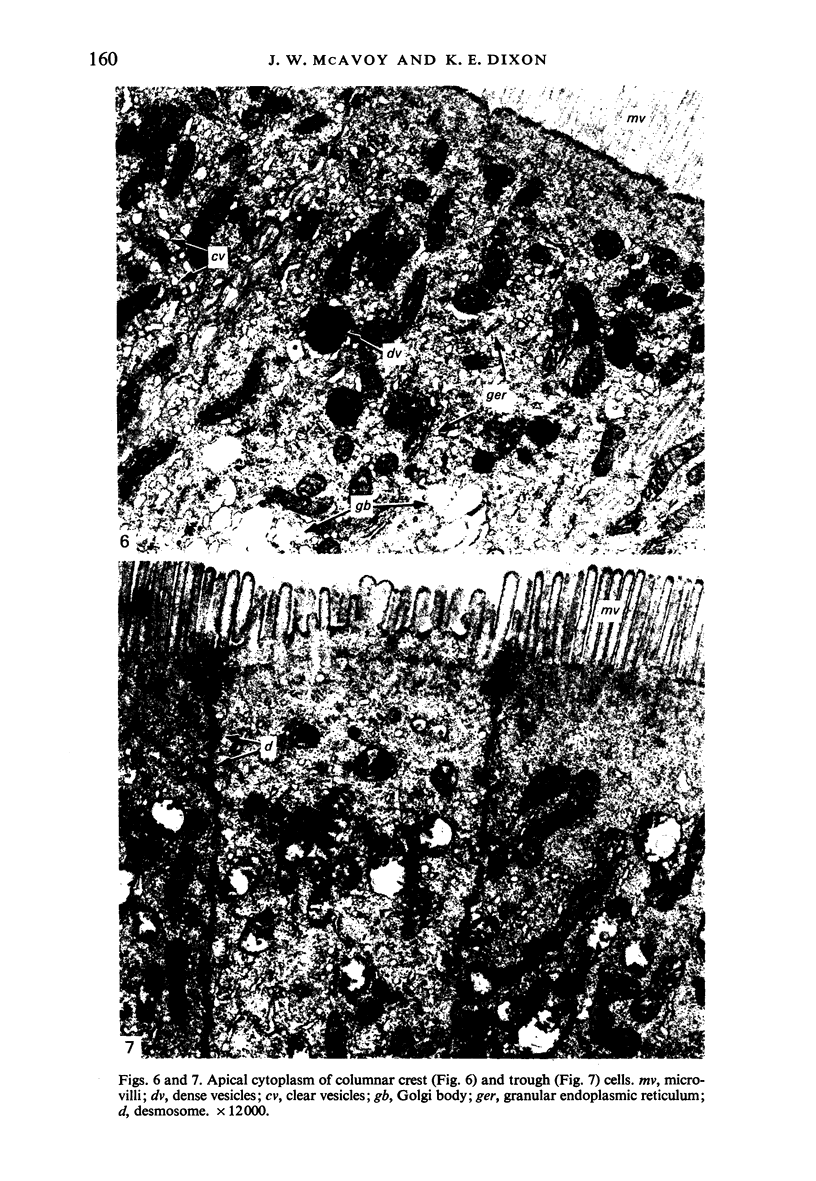
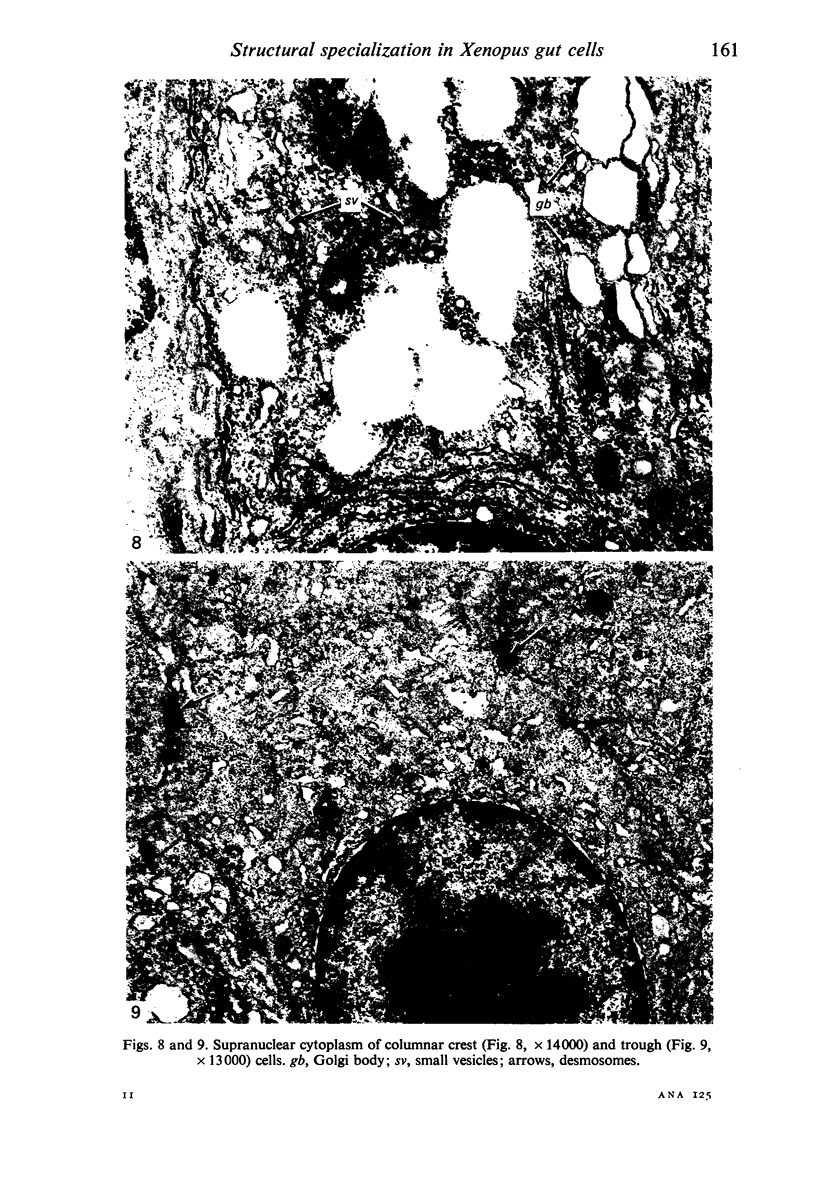
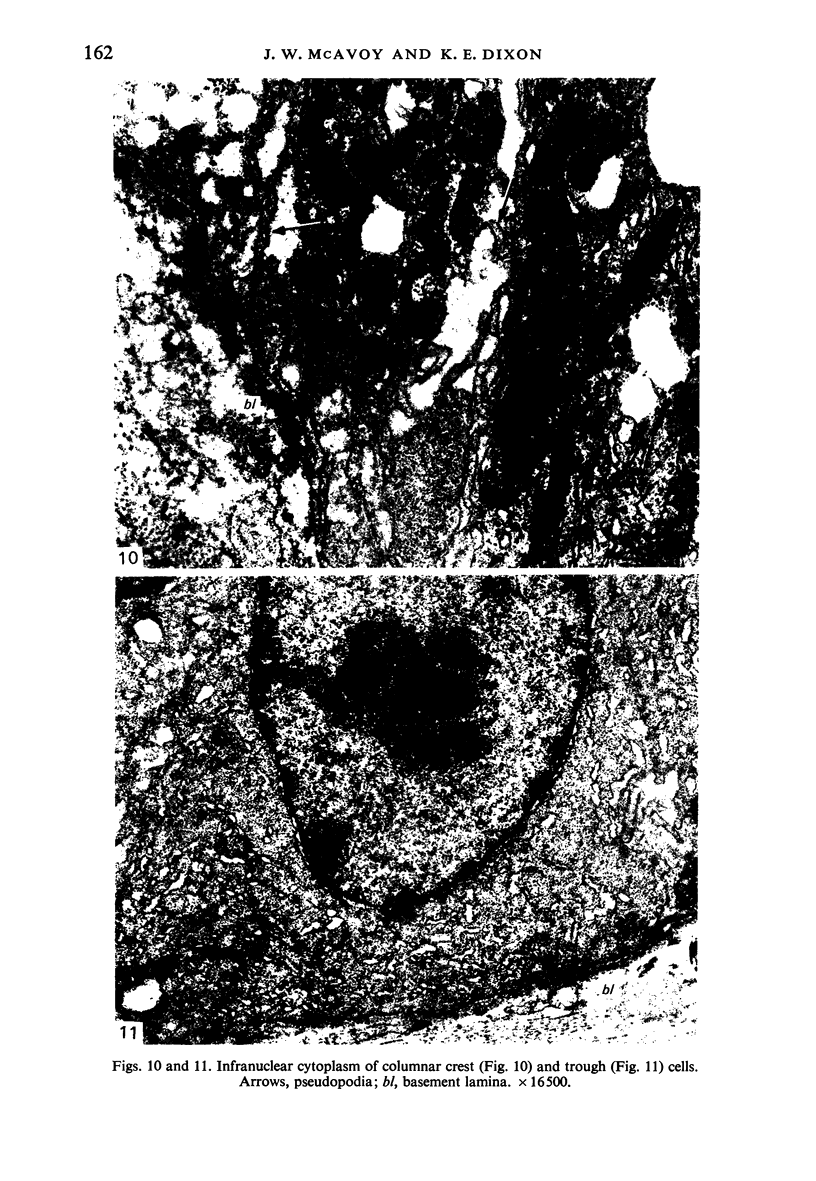
The structure, cellular composition and organization of the small intestinal epithelium of adult Xenopus laevis are described. The mucosa is thrown into a system of longitudinal folds and is composed mainly of columnar cells and goblet cells. Leucocytes comprise about 28% of the mucosal cells. The degree of structural specialization of columnar cells varies according to their position in the folds. Cells at the crest of the folds display the greastest degree of specialization, evidenced by the degree of development of their inter- and intracellular membrane systems. Cells in the trough regions between the folds are much less specialized. These cell types are structurally analogous to mammalian villous and crypt cells respectively, although they are not segregated to the same degree.
Full text
PDF




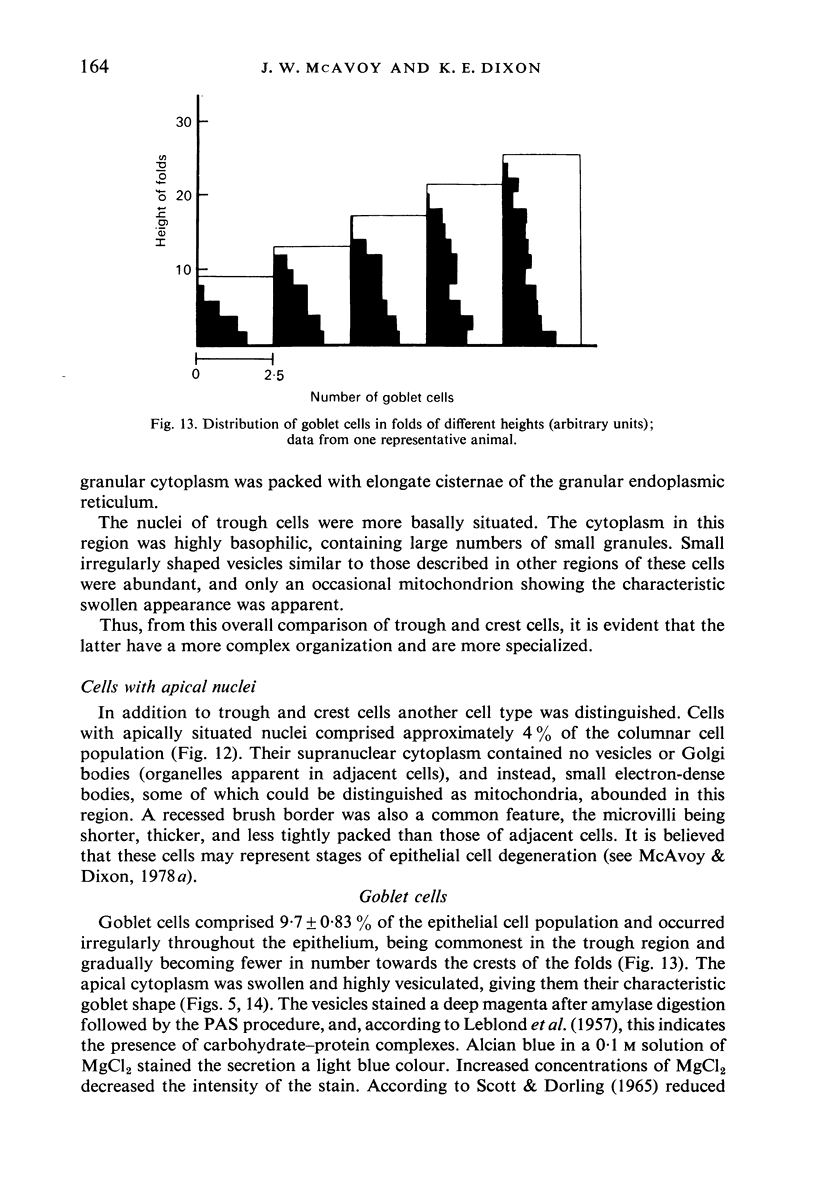
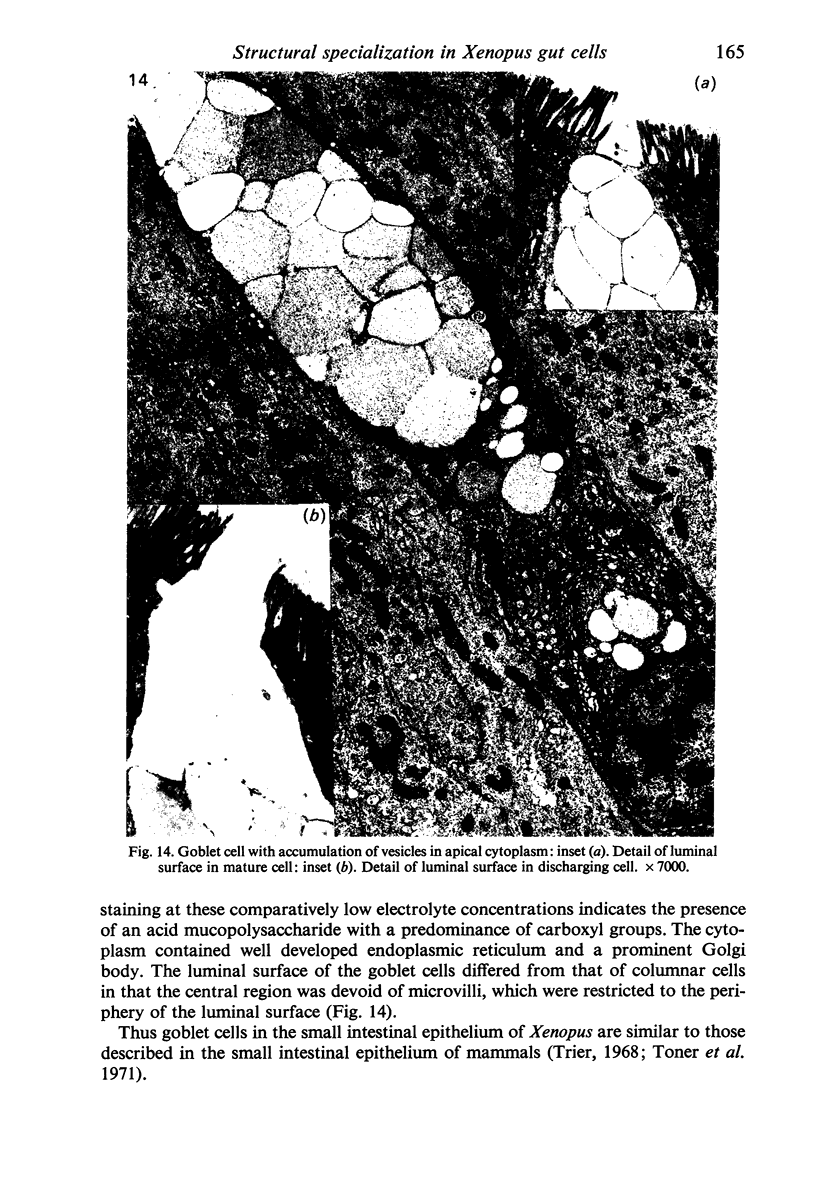




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREW W. Mucus secretion in cell nests and surface epithelium. Ann N Y Acad Sci. 1963 Mar 30;106:502–517. doi: 10.1111/j.1749-6632.1963.tb16662.x. [DOI] [PubMed] [Google Scholar]
- ARCHER G. T., AIR G., JACKAS M., MORELL D. B. STUDIES ON RAT EOSINOPHIL PEROXIDASE. Biochim Biophys Acta. 1965 Apr 26;99:96–101. doi: 10.1016/s0926-6593(65)80010-8. [DOI] [PubMed] [Google Scholar]
- CASON J. E. A rapid one-step Mallory-Heidenhain stain for connective tissue. Stain Technol. 1950 Oct;25(4):225–226. doi: 10.3109/10520295009110996. [DOI] [PubMed] [Google Scholar]
- DODGE J. D. CYTOCHEMICAL STAINING OF SECTIONS FROM PLASTIC-EMBEDDED FLAGELLATES. Stain Technol. 1964 Nov;39:381–386. doi: 10.3109/10520296409063310. [DOI] [PubMed] [Google Scholar]
- Ferreira M. N. Argentaffin and other "endocrine" cells of the small intestine in the adult mouse. I. Ultrastructure and classification. Am J Anat. 1971 Jul;131(3):315–329. doi: 10.1002/aja.1001310304. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., GLEGG R. E., EIDINGER D. Presence of carbohydrates with free 1,2-glycol groups in sites stained by the periodic acid-Schiff technique. J Histochem Cytochem. 1957 Sep;5(5):445–458. doi: 10.1177/5.5.445. [DOI] [PubMed] [Google Scholar]
- LEHRER G. M., ORNSTEIN L. A diazo coupling method for the electron microscopic localization of cholinesterase. J Biophys Biochem Cytol. 1959 Dec;6:399–406. doi: 10.1083/jcb.6.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipetz J., Cristofalo V. J. Ultrastructural changes accompanying the aging of human diploid cells in culture. J Ultrastruct Res. 1972 Apr;39(1):43–56. doi: 10.1016/s0022-5320(72)80005-4. [DOI] [PubMed] [Google Scholar]
- Martin R. Etude autoradiographique du renouvellement de l'épithélium intestinal de l'axolotl (amphibien urodèle) C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 2;272(22):2816–2819. [PubMed] [Google Scholar]
- McAvoy J. W., Dixon K. E. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: functional aspects. J Anat. 1978 Feb;125(Pt 2):237–245. [PMC free article] [PubMed] [Google Scholar]
- McAvoy J. W., Dixon K. E., Marshall J. A. Effects of differences in mitotic activity, stage of cell cycle, and degree of specialization of donor cells on nuclear transplantation in Xenopus laevis. Dev Biol. 1975 Aug;45(2):330–339. doi: 10.1016/0012-1606(75)90070-6. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Dixon K. E. Nuclear transplantation from specialized and unspecialized gut epithelial cells of adult Xenopus laevis. J Exp Zool. 1974 Aug;189(2):243–248. doi: 10.1002/jez.1401890212. [DOI] [PubMed] [Google Scholar]
- Nabeyama A., Leblond C. P. "Caveolated cells" characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974 Jun;140(2):147–165. doi: 10.1002/aja.1001400203. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B. Peroxisomes in absorptive cells of mammalian small intestine. J Cell Biol. 1972 May;53(2):532–560. doi: 10.1083/jcb.53.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADYKULA H. A. Recent functional interpretations of intestinal morphology. Fed Proc. 1962 Nov-Dec;21:873–879. [PubMed] [Google Scholar]
- PATTEN S. F., Jr Renewal of the intestinal epithelium of the Urodele. Exp Cell Res. 1960 Sep;20:638–641. doi: 10.1016/0014-4827(60)90146-4. [DOI] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Strum J. M., Karnovsky M. J. Ultrastructural localization of peroxidase in submaxillary acinar cells. J Ultrastruct Res. 1970 May;31(3):323–336. doi: 10.1016/s0022-5320(70)90135-8. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WURTH M. A., MUSACCHIA X. J. RENEWAL OF INTESTINAL EPITHELIUM IN THE FRESHWATER TURTLE, CHRYSEMYS PICTA. Anat Rec. 1964 Mar;148:427–439. doi: 10.1002/ar.1091480302. [DOI] [PubMed] [Google Scholar]