Abstract
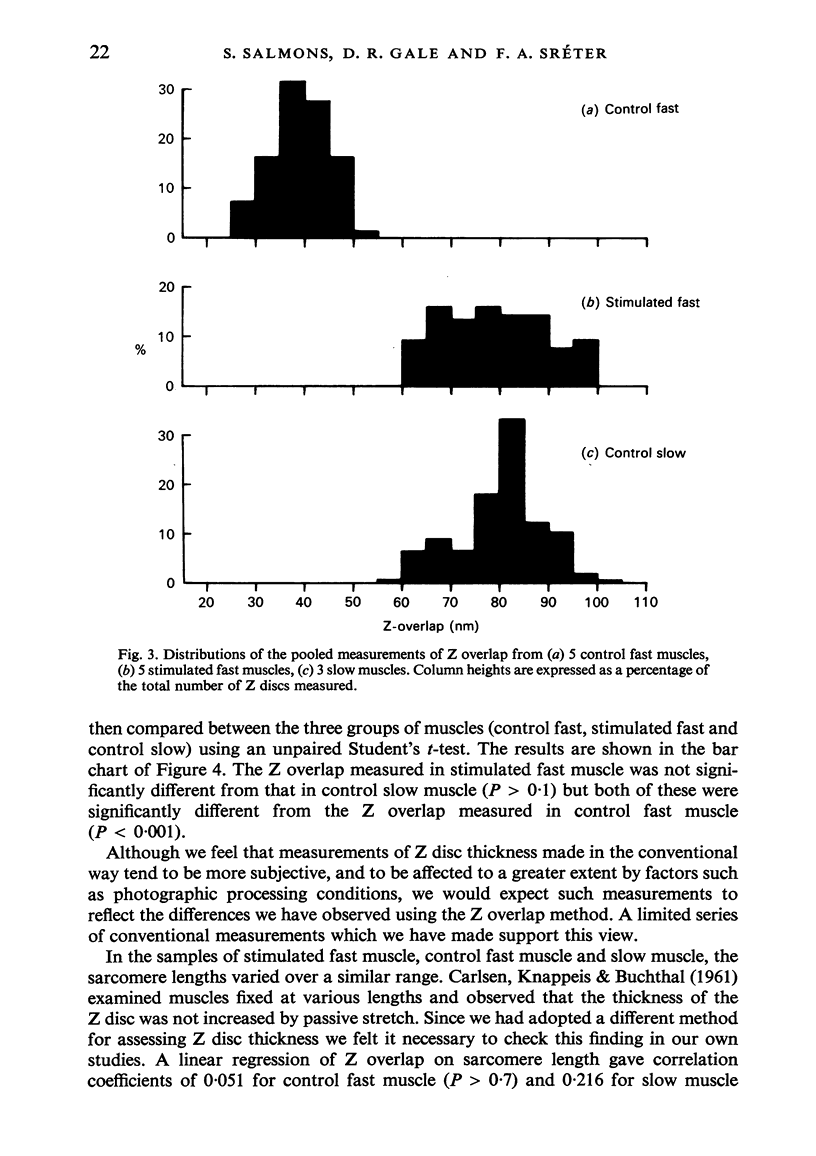
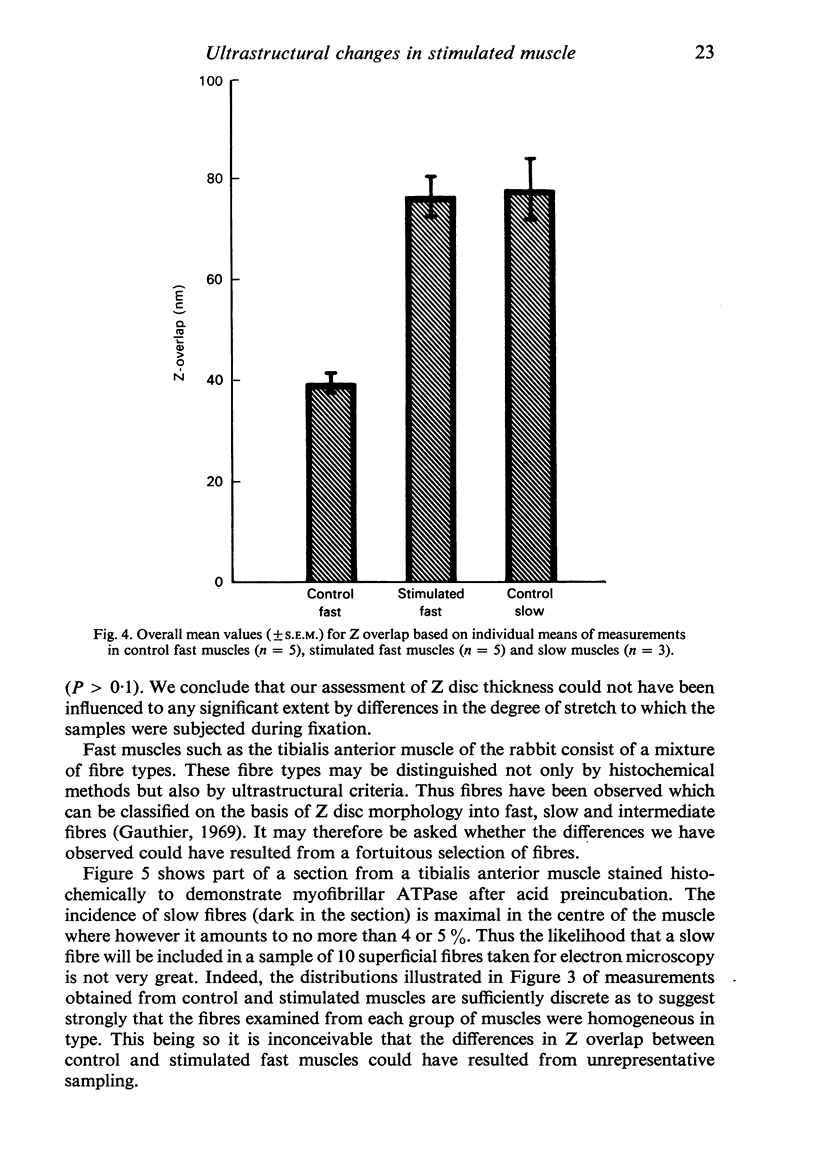
The criteria which serve to distinguish the slow from the fast type of mammalian skeletal muscle include differences at the ultrastructural level, in particular differences in Z disc morphology and mitochondrial content. If a fast muscle is subjected to sustained low frequency impulse activity similar to that normally received by a slow muscle, its physiological and biochemical properties become indistinguishable from those of a slow muscle. In the present study we have examined the fine structure of fast muscles stimulated in this way. The thickness of Z discs was significantly greater than that of control fast muscles and indistinguishable from that of slow muscles. Mitochondrial profiles, seen only infrequently in control fast muscles, were abundant in their stimulated counterparts. The regulatory influence of impulse activity on the differentiation of skeletal muscle thus extends to some of its most characteristic ultrastructural features.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Perry S. V., Syska H., Brown M. D., Vrbova G. Cross innervation and the regulatory protein system of rabbit soleus muscle. Nature. 1975 Oct 16;257(5527):602–604. doi: 10.1038/257602a0. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSEN F., KNAPPEIS G. G., BUCHTHAL F. Ultrastructure of the resting and contracted striated muscle fiber at different degrees of stretch. J Biophys Biochem Cytol. 1961 Oct;11:95–117. doi: 10.1083/jcb.11.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Cohen C., Longley W. Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol. 1969 Apr 14;41(1):87–107. doi: 10.1016/0022-2836(69)90128-4. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Crews J., Kaiser K. K., Brooke M. H. Muscle pathology of myotonia congenita. J Neurol Sci. 1976 Aug;28(4):449–457. doi: 10.1016/0022-510x(76)90116-7. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V. Cross-innervated mammalian skeletal muscle: histochemical, physiological and biochemical observations. J Physiol. 1967 Dec;193(3):481–496.3. doi: 10.1113/jphysiol.1967.sp008373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F. On the relationship of ultrastructural and cytochemical features of color in mammalian skeletal muscle. Z Zellforsch Mikrosk Anat. 1969;95(3):462–482. doi: 10.1007/BF00995217. [DOI] [PubMed] [Google Scholar]
- Goll D. E., Mommaerts W. F., Reedy M. K., Seraydarian K. Studies on alpha-actinin-like proteins liberated during trypsin digestion of alpha-actinin and of myofibrils. Biochim Biophys Acta. 1969 Feb 4;175(1):174–194. doi: 10.1016/0005-2795(69)90156-1. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Jirmanová I., Zelená J. Ultrastructural transformation of fast chicken muscle fibres induced by nerve cross-union. Z Zellforsch Mikrosk Anat. 1973 Dec 31;146(1):103–121. doi: 10.1007/BF00306761. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. Transformation of the histochemical profile of skeletal muscle by "foreign" innervation. Nature. 1967 Sep 30;215(5109):1509–1510. doi: 10.1038/2151509a0. [DOI] [PubMed] [Google Scholar]
- Kelly D. E., Cahill M. A. Filamentous and matrix components of skeletal muscle Z-disks. Anat Rec. 1972 Apr;172(4):623–642. doi: 10.1002/ar.1091720403. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Landon D. N. The influence of fixation upon the fine structure of the Z-disk of rat striated muscle. J Cell Sci. 1970 Jan;6(1):257–276. doi: 10.1242/jcs.6.1.257. [DOI] [PubMed] [Google Scholar]
- Lane B. P., Elias J., Drummond E. Immunoelectronmicroscopic localization of alpha-actinin in skeletal muscle cells. J Histochem Cytochem. 1977 Jan;25(1):69–72. doi: 10.1177/25.1.833423. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Engel A. G. Observations on organization of Z-disk components and on rod-bodies of Z-disk origin. J Cell Biol. 1971 Feb;48(2):431–437. doi: 10.1083/jcb.48.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T., Endo M., Ebashi S. Localization of 6S component of a alpha-actinin at Z-band. J Biochem. 1967 Nov;62(5):630–632. doi: 10.1093/oxfordjournals.jbchem.a128717. [DOI] [PubMed] [Google Scholar]
- Munsat T. L., McNeal D., Waters R. Effects of nerve stimulation on human muscle. Arch Neurol. 1976 Sep;33(9):608–617. doi: 10.1001/archneur.1976.00500090014004. [DOI] [PubMed] [Google Scholar]
- Peckham P. H., Mortimer J. T., Van Der Meulen J. P. Physiologic and metabolic changes in white muscle of cat following induced exercise. Brain Res. 1973 Feb 28;50(2):424–429. doi: 10.1016/0006-8993(73)90745-2. [DOI] [PubMed] [Google Scholar]
- Pelloni-Müller G., Ermini M., Jenny E. Changes in myosin light and heavy chain stoichiometry during development of rabbit fast, slow, and cardiac muscle. FEBS Lett. 1976 Nov;70(1):113–117. doi: 10.1016/0014-5793(76)80738-7. [DOI] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. Some aspects of the structural organization of the myofibril as revealed by antibody--staining methods. J Cell Biol. 1966 Mar;28(3):505–525. doi: 10.1083/jcb.28.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Riley D. A., Allin E. F. The effects of inactivity, programmed stimulation, and denervation on the histochemistry of skeletal muscle fiber types. Exp Neurol. 1973 Aug;40(2):391–413. doi: 10.1016/0014-4886(73)90082-4. [DOI] [PubMed] [Google Scholar]
- Riley D. A. Factors affecting the conversion of cross-reinnervated skeletal muscles. Am J Anat. 1974 Aug;140(4):609–615. doi: 10.1002/aja.1001400414. [DOI] [PubMed] [Google Scholar]
- Robbins N., Karpati G., Engel W. K. Histochemical and contractile properties in the cross-innervated guinea pig soleus muscle. Arch Neurol. 1969 Mar;20(3):318–329. doi: 10.1001/archneur.1969.00480090106015. [DOI] [PubMed] [Google Scholar]
- Robson R. M., Zeece M. G. Comparative studies of -actinin from porcine cardiac and skeletal muscle. Biochim Biophys Acta. 1973 Jan 25;295(1):208–224. doi: 10.1016/0005-2795(73)90088-3. [DOI] [PubMed] [Google Scholar]
- Romanul F. C., Sreter F. A., Salmons S. Reversal of energy metabolism and myosin characteristics of white muscles after chronic stimulation. Trans Am Neurol Assoc. 1973;98:297–298. [PubMed] [Google Scholar]
- Romanul F. C., Van der Meulen J. P. Slow and fast muscles after cross innervation. Enzymatic and physiological changes. Arch Neurol. 1967 Oct;17(4):387–402. doi: 10.1001/archneur.1967.00470280053006. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. The ultrastructure of Z disks from white, intermediate, and red fibers of mammalian striated muscles. J Cell Biol. 1973 May;57(2):261–277. doi: 10.1083/jcb.57.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R. W. Ultrastructure of the Z line of skeletal muscle fibers. J Cell Biol. 1971 Dec;51(3):674–685. doi: 10.1083/jcb.51.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S. An implantable muscle stimulator. J Physiol. 1967 Jan;188(2):13P–14P. doi: 10.1113/jphysiol.1967.sp008150. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Hanzlíková V., Pierobon S. Relations between structure and function in rat skeletal muscle fibers. J Cell Biol. 1970 Oct;47(1):107–119. doi: 10.1083/jcb.47.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H., Kamieniecka Z. Fiber types in the human brachial biceps muscle. Exp Neurol. 1974 Aug;44(2):313–328. doi: 10.1016/0014-4886(74)90069-7. [DOI] [PubMed] [Google Scholar]
- Sreter F. A., Elzinga M., Mabuchi K. The Ntau-methylhistidine content of myosin in stimulated and cross-reinnervated skeletal muscles of the rabbit. FEBS Lett. 1975 Sep 1;57(1):107–111. doi: 10.1016/0014-5793(75)80163-3. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Luff A. R., Gergely J. Effect of cross-reinnervation on physiological parameters and on properties of myosin and sarcoplasmic reticulum of fast and slow muscles of the rabbit. J Gen Physiol. 1975 Dec;66(6):811–821. doi: 10.1085/jgp.66.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer M. H., Hartshorne D. J., Mueller H., Rice R. V. The effect of various protein fractions on Z- and M-line reconstitution. J Cell Biol. 1969 Jan;40(1):167–178. doi: 10.1083/jcb.40.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Stromer M. H., Temple J. -actinin from red and white porcine muscle. Biochim Biophys Acta. 1973 Jan 25;295(1):188–207. doi: 10.1016/0005-2795(73)90087-1. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Zelená J., Jirmanová I. Ultrastructure of chicken slow muscle after nerve cross union. Exp Neurol. 1973 Feb;38(2):272–285. doi: 10.1016/0014-4886(73)90151-9. [DOI] [PubMed] [Google Scholar]