Abstract
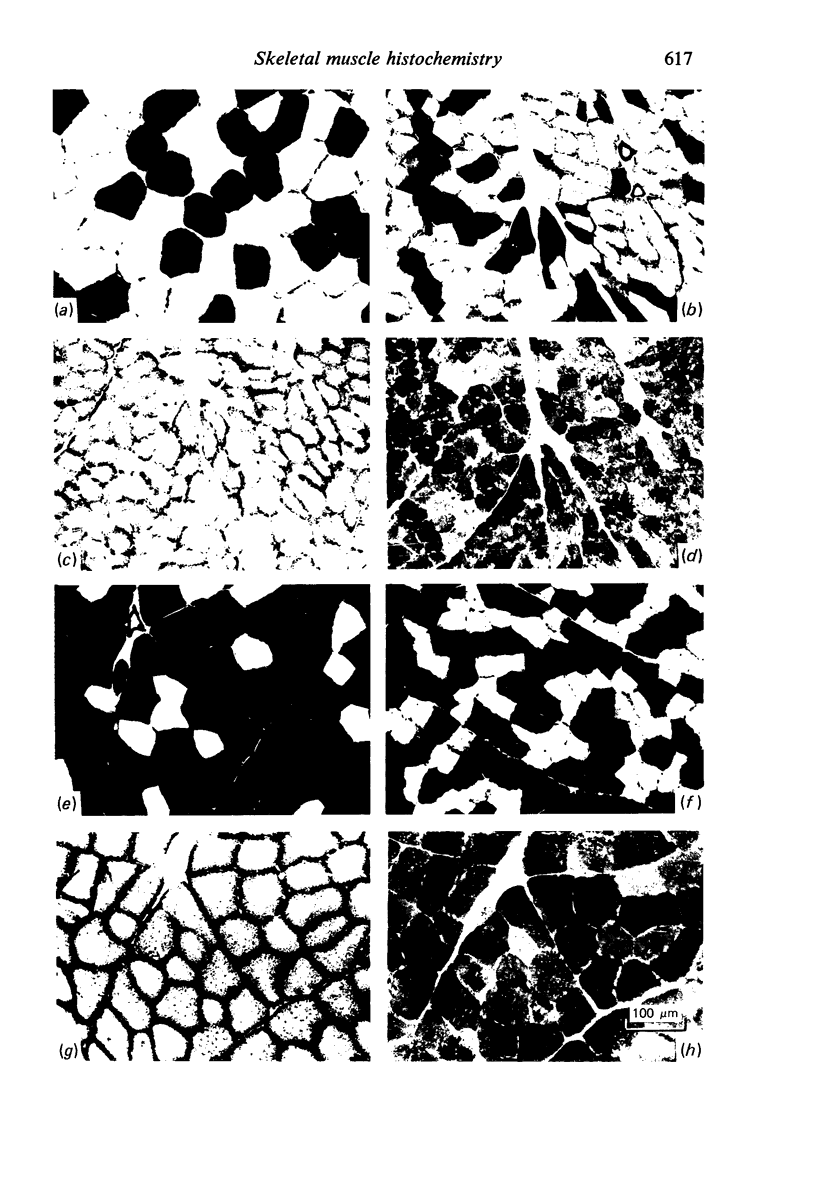
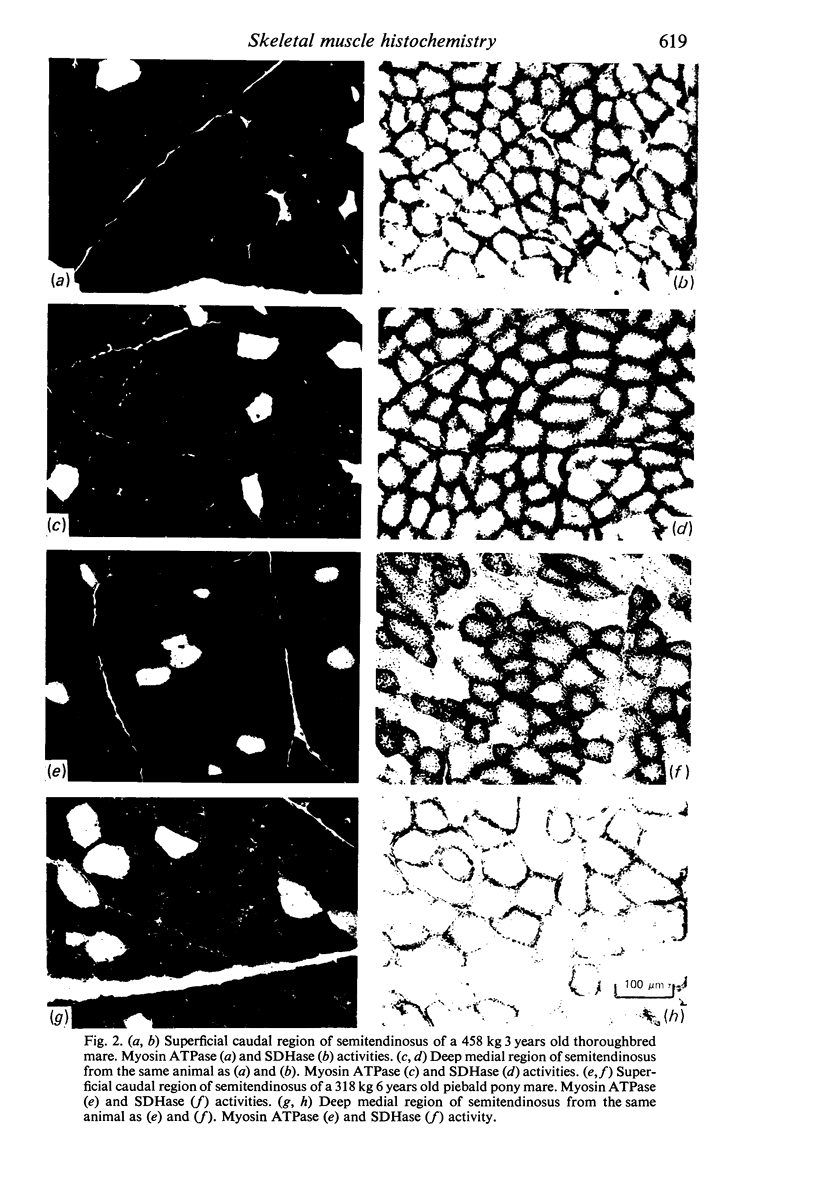
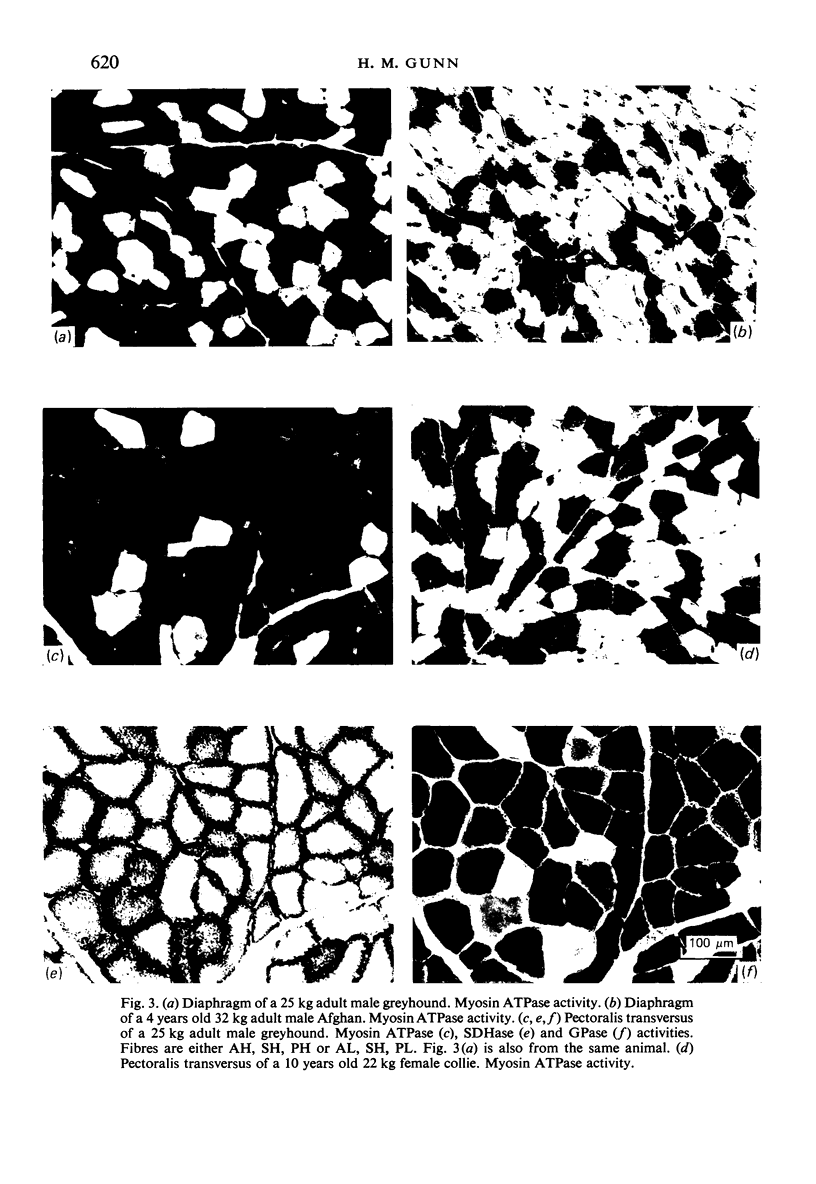
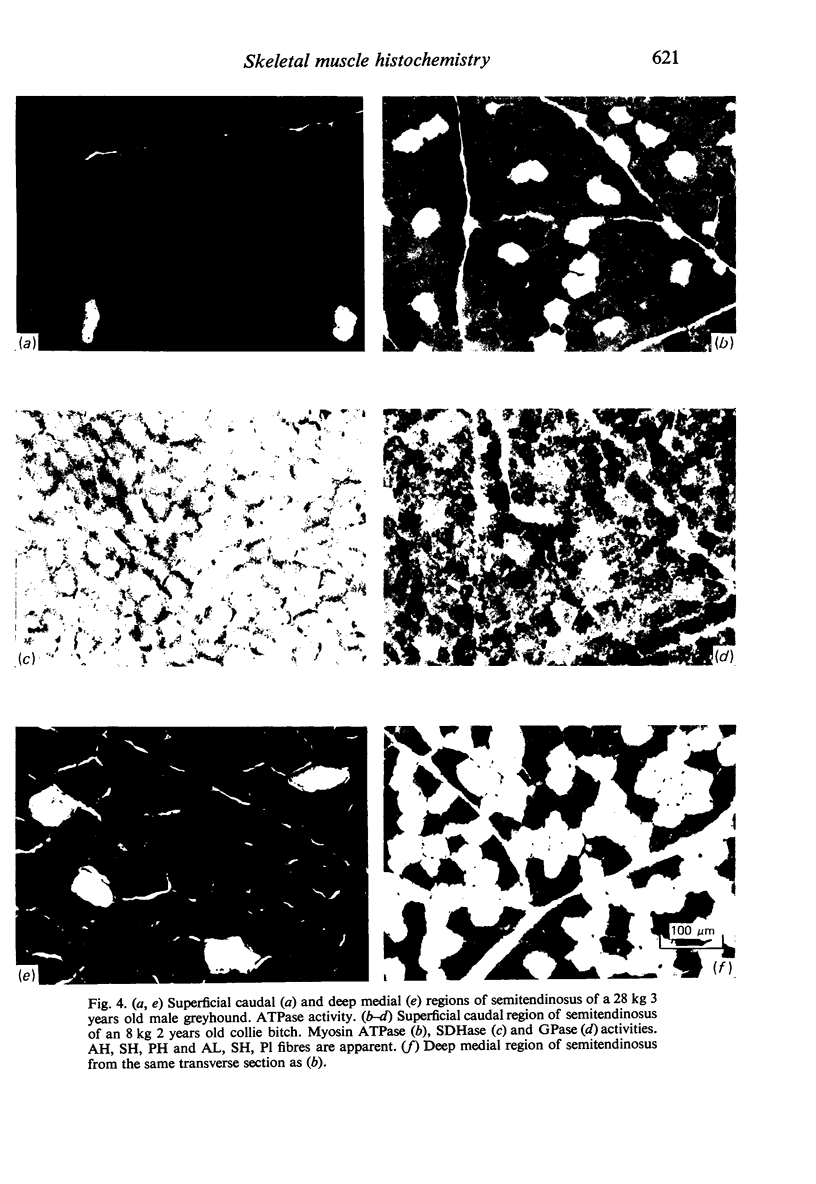
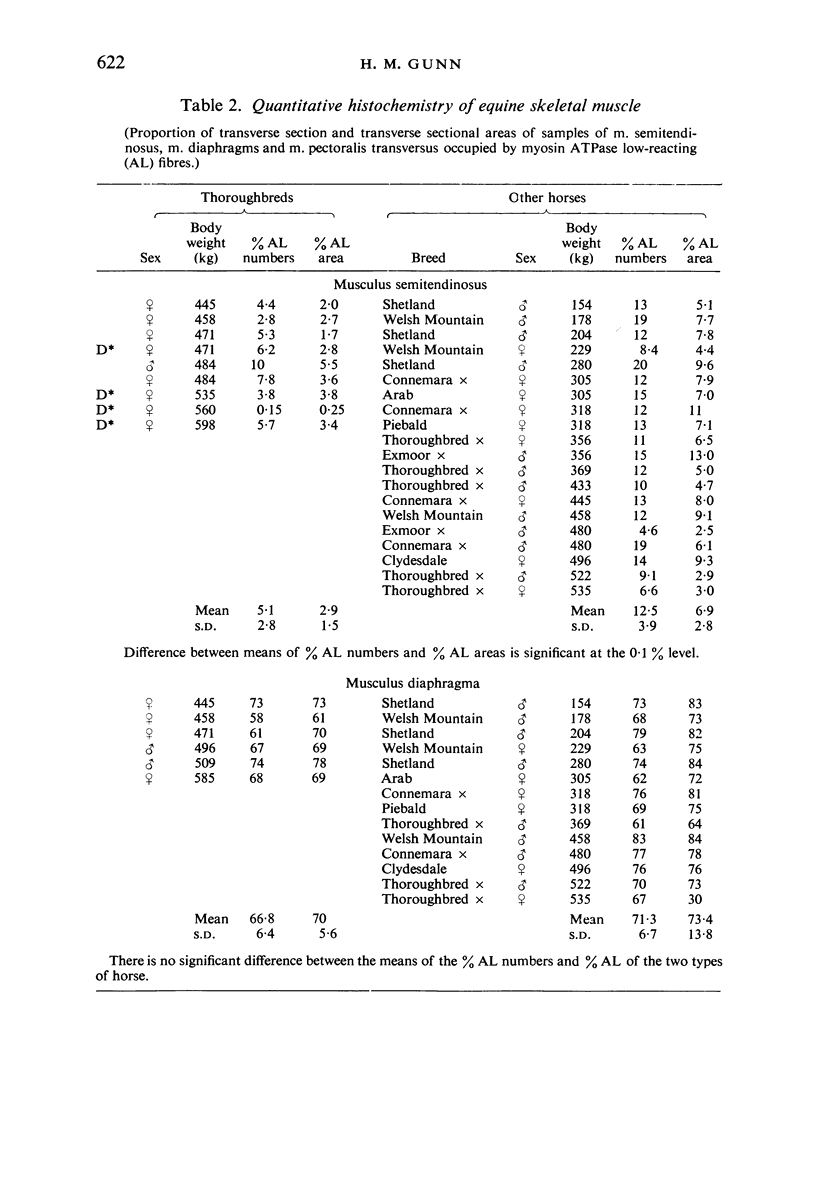
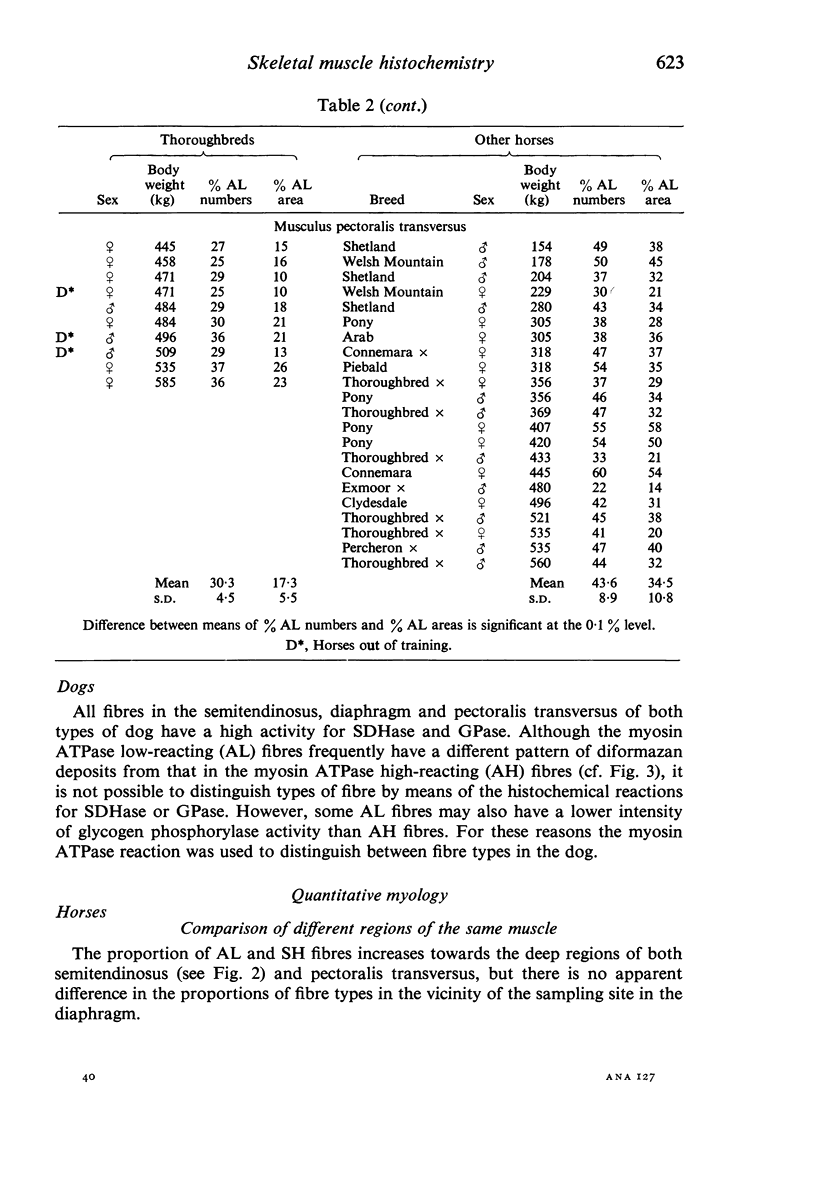
Histochemical profiles of individual muscle fibres were established using myosin adenosine triphosphatase (myosin ATPase), succinate dehydrogenase (SDHase), and glycogen phosphorylase (GPase) reactions in three muscles (semitendinosus, diaphragm, and pectoralis transversus) of the horse and dog. The major histochemical difference between fibres lies in their myosin ATPase activity; fibres can be subdivided into those with a high and those with a low activity. In horse muscle, all fibres have a high activity of GPase. In the diaphragm and pectoralis transversus, all fibres have a high SDHase activity, but fibres with a low activity of SDHase are also present in samples of the semitendinosus. In dog muscle, all fibres have a high SDHase activity; myosin ATPase low-reacting fibres also have a low activity of GPase. There is a greater fractional area of myosin ATPase high-reacting fibres in the pectoralis transversus and semitendinosus of thoroughbred horses and greyhounds (breeds selected for high speed running) and in the diaphragm of greyhounds. In adults this feature does not appear to be due to training, as are the differences in aerobic and anaerobic capacity (shown in other studies). The preponderance of myosin Atpase high-reacting fibres suggests that there may be differences in the nervous systems of athletes and non-athletes. It is concluded that the proportions of fibre types in muscles are related to the functions of muscles and of their parts. No sex differences or detraining effects were apparent, although the value for the proportion of fibre types (as differentiated by the myosin ATPase reaction) in the limb muscles of thoroughbred crosses lies between those of thoroughbreds and non-thoroughbreds.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. J., Sembrowich W. L., Gollnick P. D. Myosin ATPase and fiber composition from trained and untrained rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1415–1417. doi: 10.1152/ajplegacy.1972.223.6.1415. [DOI] [PubMed] [Google Scholar]
- Barnard R. J., Edgerton V. R., Peter J. B. Effect of exercise on skeletal muscle. I. Biochemical and histochemical properties. J Appl Physiol. 1970 Jun;28(6):762–766. doi: 10.1152/jappl.1970.28.6.762. [DOI] [PubMed] [Google Scholar]
- Barnard R. J., Edgerton V. R., Peter J. B. Effect of exercise on skeletal muscle. II. Contractile properties. J Appl Physiol. 1970 Jun;28(6):767–770. doi: 10.1152/jappl.1970.28.6.767. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Campbell A. M., Onan G., Thomas D., Weirich W., Will J. A., Cassens R. G., Briskey E. J. The effect of exercise on muscle ATPase. Histochemie. 1971;25(4):373–375. doi: 10.1007/BF00278229. [DOI] [PubMed] [Google Scholar]
- Cardinet G. H., 3rd, Fedde M. R., Tunell G. L. Correlates of histochemical and physiologic properties in normal and hypotrophic pectineus muscles of the dog. Lab Invest. 1972 Jul;27(1):32–38. [PubMed] [Google Scholar]
- Cardinet G. H., 3rd, Wallace L. J., Fedde M. R., Guffy M. M., Bardens J. W. Developmental myopathy in the canine with type II muscle fiber hypotrophy. Arch Neurol. 1969 Dec;21(6):620–630. doi: 10.1001/archneur.1969.00480180076007. [DOI] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. S., Gunn H. M. Histochemical fibre types in the mammalian diaphragm. J Anat. 1972 May;112(Pt 1):41–60. [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saltin B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973 Jan;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Karlsson J., Piehl K., Saltin B. Selective glycogen depletion in skeletal muscle fibres of man following sustained contractions. J Physiol. 1974 Aug;241(1):59–67. doi: 10.1113/jphysiol.1974.sp010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths I. R., Duncan I. D., McQueen A., Quirk C., Miller R. Neuromuscular disease in dogs: some aspects of its investigation and diagnosis. J Small Anim Pract. 1973 Sep;14(9):533–554. doi: 10.1111/j.1748-5827.1973.tb06495.x. [DOI] [PubMed] [Google Scholar]
- Gunn H. M. Adaptations of skeletal muscle that favour athletic ability. N Z Vet J. 1975 Nov;23(11):249–254. doi: 10.1080/00480169.1975.34253. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J., Albers R. W. The neural regulation of some phenotypic differences between the fiber types of mammalian skeletal muscle. Exp Neurol. 1970 Jan;26(1):126–135. doi: 10.1016/0014-4886(70)90094-4. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- HEARN G. R., GOLLNICK P. D. Effects of exercise on the adenosinetriphosphatase activity in skeletal and heart muscle of rats. Int Z Angew Physiol. 1961;19:23–26. doi: 10.1007/BF00693625. [DOI] [PubMed] [Google Scholar]
- Hendricks H. B., Aberle E. D., Jones D. J., Martin T. G. Muscle fiber type, rigor development and bone strength in dobule muscled cattle. J Anim Sci. 1973 Dec;37(6):1305–1311. doi: 10.2527/jas1973.3761305x. [DOI] [PubMed] [Google Scholar]
- Holmes J. H., Ashmore C. R. A histochemical study of development of muscle fiber type and size in normal and "double muscled" cattle. Growth. 1972 Dec;36(4):351–372. [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. Transformation of the histochemical profile of skeletal muscle by "foreign" innervation. Nature. 1967 Sep 30;215(5109):1509–1510. doi: 10.1038/2151509a0. [DOI] [PubMed] [Google Scholar]
- Khan M. A., Papadimitriou J. M., Kakulas B. A. The effect of temperature on the pH stability of myosin ATPase as demonstrated histochemically. Histochemie. 1974 Feb 6;38(2):181–194. doi: 10.1007/BF00499665. [DOI] [PubMed] [Google Scholar]
- LAWRIE R. A. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J. 1953 Sep;55(2):298–305. doi: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm A., Saltin B. The physiological and biochemical response of standardbred horses to exercise of varying speed and duration. Acta Vet Scand. 1974;15(3):310–324. doi: 10.1186/BF03547461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Maxwell L. C., Faulkner J. A., Lieberman D. A. Histochemical manifestations of age and endurance training in skeletal muscle fibers. Am J Physiol. 1973 Feb;224(2):356–361. doi: 10.1152/ajplegacy.1973.224.2.356. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- RAWLINSON W. A., GOULD M. K. Biochemical adaptation as a response to exercise. 2. Adenosine triphosphatase and creatine phosphokinase activity in muscles of exercised rats. Biochem J. 1959 Sep;73:44–48. doi: 10.1042/bj0730044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Karpati G., Engel W. K. Histochemical and contractile properties in the cross-innervated guinea pig soleus muscle. Arch Neurol. 1969 Mar;20(3):318–329. doi: 10.1001/archneur.1969.00480090106015. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha F. J., Guth L., Albers R. W. Differences between slow and fast muscle myosin. Adenosine triphosphatase activity and release of associated proteins by p-chloromercuriphenylsulfonate. J Biol Chem. 1970 Jan 25;245(2):219–224. [PubMed] [Google Scholar]
- Samaha F. J., Guth L., Albers R. W. Phenotypic differences between the actomyosin ATPase of the three fiber types of mammalian skeletal muscle. Exp Neurol. 1970 Jan;26(1):120–125. doi: 10.1016/0014-4886(70)90093-2. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Gergely J. The effect of cross reinnervation on the synthesis of myosin light chains. Biochem Biophys Res Commun. 1974 Jan;56(1):84–89. doi: 10.1016/s0006-291x(74)80318-9. [DOI] [PubMed] [Google Scholar]
- Straub R., Howald H., Gerber H., Diehl M., Pauli B. Ultrastrukturelle und enzymatische Untersuchungen an trainierten und untrainierten Pferdeskelettmuskeln. Schweiz Arch Tierheilkd. 1975 Aug;117(8):453–457. [PubMed] [Google Scholar]
- Streter F. A., Gergely J., Salmons S., Romanul F. Synthesis by fast muscle of myosin light chains characteristic of slow muscle in response to long-term stimulation. Nat New Biol. 1973 Jan 3;241(105):17–19. doi: 10.1038/newbio241017a0. [DOI] [PubMed] [Google Scholar]
- Syrový I., Gutmann E., Melichna J. Effect of exercise on skeletal muscle myosin ATP-ase activity. Physiol Bohemoslov. 1972;21(6):633–638. [PubMed] [Google Scholar]
- TAKEUCHI T., KURIAKI H. Histochemical detection of phosphorylase in animal tissues. J Histochem Cytochem. 1955 May;3(3):153–160. doi: 10.1177/3.3.153. [DOI] [PubMed] [Google Scholar]
- Tarrant P. J., Hegarty P. V., McLoughlin J. V. A study on the high energy phosphates and anaerobic glycolysis in the red and white fibres of porcine semitendinosus muscle. Proc R Ir Acad B. 1972;72(14):229–251. [PubMed] [Google Scholar]
- Trevino G. S., Demaree R. S., Jr, Sanders B. V., O'Donnell T. A. Needle biopsy of skeletal muscle in dogs: light and electron microscopy of resting muscle. Am J Vet Res. 1973 Apr;34(4):507–514. [PubMed] [Google Scholar]
- Vaughan H. S., Aziz-Ullah, Goldspink G., Nowell N. W. Sex and stock differences in the histochemical myofibrillar adenosine triphosphatase reaction in the soleus muscle of the mouse. J Histochem Cytochem. 1974 Mar;22(3):155–159. doi: 10.1177/22.3.155. [DOI] [PubMed] [Google Scholar]
- Walker M. G. Effect of training on the properties of isolated skeletal muscles. Experientia. 1968 Apr 15;24(4):360–360. doi: 10.1007/BF02140821. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- West R. L. Red to white fiber ratios as an index of double muscling in beef cattle. J Anim Sci. 1974 May;38(5):1165–1175. doi: 10.2527/jas1974.3851165x. [DOI] [PubMed] [Google Scholar]
- Yellin H. Differences in histochemical attributes between diaphragm and hindleg muscles of the rat. Anat Rec. 1972 Jul;173(3):333–339. doi: 10.1002/ar.1091730308. [DOI] [PubMed] [Google Scholar]