Abstract
Nonsteroidal anti-inflammatory drugs are commonly used in the treatment of inflammatory conditions, and have potential value in the treatment of thrombotic disease in the horse. This study compares the potency of three nonsteroidal anti-inflammatory drugs phenylbutazone, naproxen (equiproxen) and flunixin meglumine (banamine) with respect to their effects on equine platelets. Two functional responses of horse platelets were evaluated in vitro: their ability to aggregate and their ability to make available platelet factor 3 procoagulant activity.
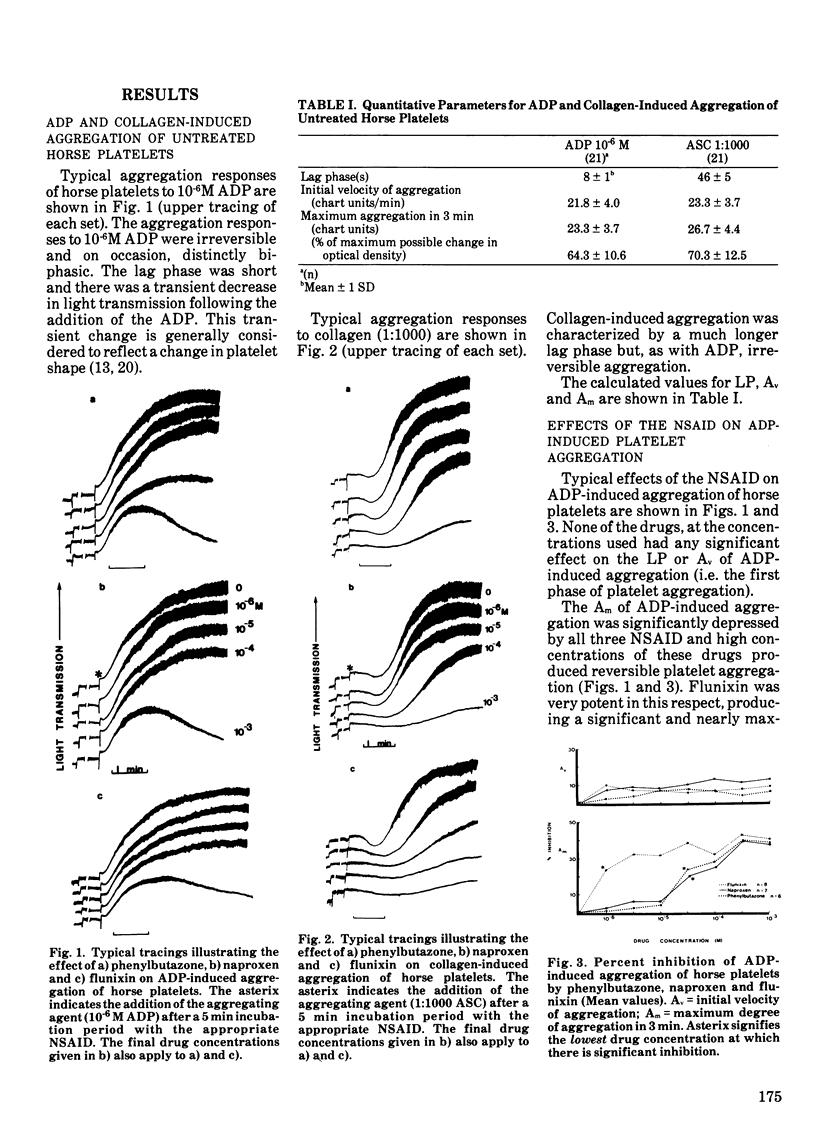
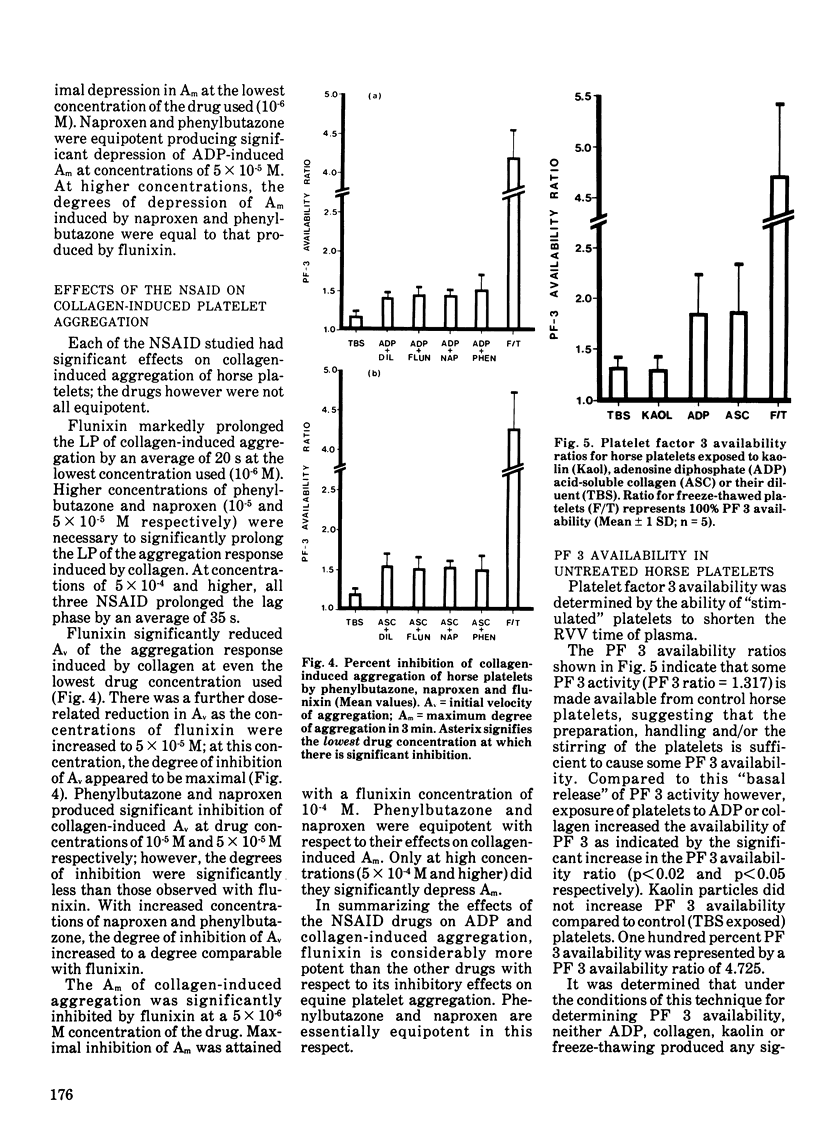
Flunixin at a concentration of 10-6 M significantly depressed the maximum degree of adenosine diphosphate-induced (10-6M) aggregation while much higher concentrations of phenylbutazone and naproxen (5 X 105M) were required to produce similar effects. None of the non-steriodal anti-inflammatory drugs significantly affected the duration of the lag phase or the initial velocity of adenosine diphosphate-induced aggregation within the range of drug concentrations used (10-6-10-3M). The lag phase and initial velocity of acid-soluble collagen-induced aggregation were significantly affected by 10-6 M flunixin and 10-4 M phenylbutazone or naproxen was required to produce equivalent effects. Concentrations of 5 X 10-6 M flunixin and 5 X 10-4 M phenylbutazone or naproxen were required to significantly depress the degree of collaen-induced aggregation of horse platelets.
Although the effects of the nonsteroidal anti-inflammatory drugs were qualitatively similar, flunixin was a much more potent inhibitor of platelet aggregation than either of the other two drugs (which were equipotent). At very high drug concentrations (5 X 10-4 M and greater), all three drugs produced the same degree of inhibition of equine platelet aggregation.
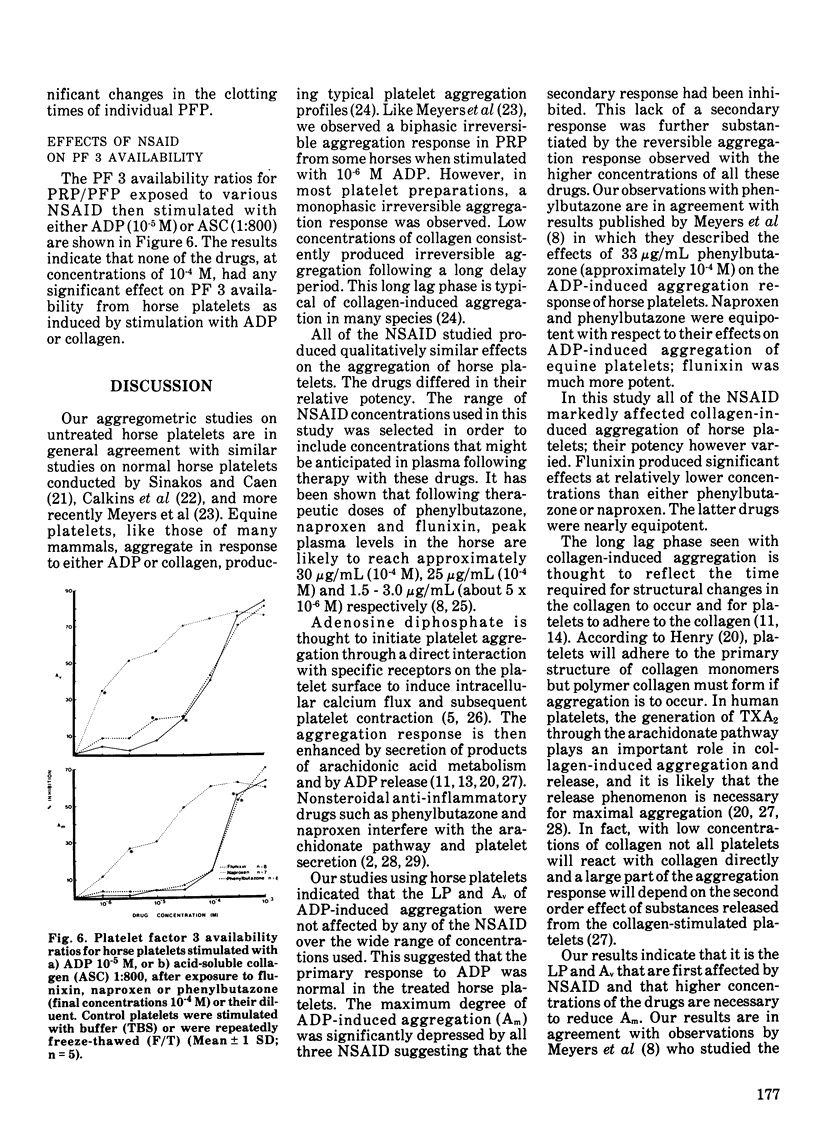
Platelet factor 3 activity was made available by exposing horse platelets to 10-5 M adenosine diphosphate or 1:800 acid-soluble collagen; but not by exposure to a suspension of kaolin particles. Only a small portion of the total platelet factor 3 activity was made available on stimulation with either adenosine diphosphate or collagen. Pretreatment of horse platelets with any of the nonsteroidal anti-inflammatory drugs (10-4 M concentration) had no significant effect on adenosine diphosphate or collagen-induced platelet factor 3 availability.
Keywords: Nonsteroidal anti-inflammatory drugs, equine platelets, platelet aggregation, platelet factor 3 availability
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., McDonald J. W. Reversible and irreversible inhibition of platelet cyclooxygenase and serotonin release by nonsteroidal antinflammatory drugs. Thromb Res. 1978 Dec;13(6):1057–1065. doi: 10.1016/0049-3848(78)90234-7. [DOI] [PubMed] [Google Scholar]
- Calkins J., Lane K. P., LoSasso B., Thurber L. E. Comparative study of platelet aggregation in various species. J Med. 1974;5(5):292–296. [PubMed] [Google Scholar]
- Cazenave J. P., Packham M. A., Guccione M. A., Mustard J. F. Inhibition of platelet adherence to a collagen-coated surface by nonsteroidal anti-inflammatory drugs, pyrimido-pyrimidine and tricyclic compounds, and lidocaine. J Lab Clin Med. 1974 May;83(5):797–806. [PubMed] [Google Scholar]
- Cazenave J. P., Packham M. A., Mustard J. F. Adherence of platelets to a collagen-coated surface: development of a quantitative method. J Lab Clin Med. 1973 Dec;82(6):978–990. [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell C. N., Kahn R. A., Cooper R. G., Muhrer M. E. Platelet factor 3 availability in bleeder swine. Comp Biochem Physiol A Comp Physiol. 1972 Aug 1;42(4):817–822. doi: 10.1016/0300-9629(72)90386-6. [DOI] [PubMed] [Google Scholar]
- Dawson W. Mechanisms of action of antiinflammatory drugs. Adv Prostaglandin Thromboxane Res. 1980;8:1741–1745. [PubMed] [Google Scholar]
- Dodds W. J. Canine von Willebrand's disease. J Lab Clin Med. 1970 Nov;76(5):713–721. [PubMed] [Google Scholar]
- Fuster V., Chesebro J. H. 10. Antithrombotic therapy: role of platelet-inhibitor drugs. II. Pharmacologic effects of platelet-inhibitor drugs (second of three parts). Mayo Clin Proc. 1981 Mar;56(3):185–195. [PubMed] [Google Scholar]
- Fuster V., Chesebro J. H. Antithrombotic therapy: role of platelet-inhibitor drugs. I. Current concepts of thrombogenesis: role of platelets. (first of three parts). Mayo Clin Proc. 1981 Feb;56(2):102–112. [PubMed] [Google Scholar]
- HARDISTY R. M., HUTTON R. A. THE KAOLIN CLOTTING TIME OF PLATELET-RICH PLASMA: A TEST OF PLATELET FACTOR-3 AVAILABILITY. Br J Haematol. 1965 May;11:258–268. doi: 10.1111/j.1365-2141.1965.tb06586.x. [DOI] [PubMed] [Google Scholar]
- Horowitz H. I., Cohen B. D., Martinez P., Papayoanou M. F. Defective ADP-induced platelet factor 3 activation in uremia. Blood. 1967 Sep;30(3):331–340. [PubMed] [Google Scholar]
- Johnstone I. B., Lotz F. Ristocetin-Willebrand factor activity in dog plasma. Can J Physiol Pharmacol. 1980 Sep;58(9):1128–1134. doi: 10.1139/y80-170. [DOI] [PubMed] [Google Scholar]
- Judson D. G., Barton M. Effect of aspirin on haemostasis in the horse. Res Vet Sci. 1981 Mar;30(2):241–242. [PubMed] [Google Scholar]
- McIntyre B. A., Philp R. B. Effect of three nonsteroidal anti-inflammatory agents on platelet function and prostaglandin synthesis in vitro. Thromb Res. 1978 Jan;12(1):67–77. doi: 10.1016/0049-3848(78)90086-5. [DOI] [PubMed] [Google Scholar]
- Meyers K. M., Katz J. B., Clemmons R. M., Smith J. B., Holmsen H. An evaluation of the arachidonate pathway of platelets from companion and food-producing animals, mink, and man. Thromb Res. 1980 Oct 1;20(1):13–24. doi: 10.1016/0049-3848(80)90052-3. [DOI] [PubMed] [Google Scholar]
- Meyers K. M., Lindner C., Grant B. Characterization of the equine platelet aggregation response. Am J Vet Res. 1979 Feb;40(2):260–264. [PubMed] [Google Scholar]
- Meyers K. M., Lindner C., Katz J., Grant B. Phenylbutazone inhibition of equine platelet function. Am J Vet Res. 1979 Feb;40(2):265–270. [PubMed] [Google Scholar]
- Packham M. A., Mustard J. F. Clinical pharmacology of platelets. Blood. 1977 Oct;50(4):555–573. [PubMed] [Google Scholar]
- Pérez-Requejo J. L. A standardized bioassay for platelet factor 3 released by kaolin. Br J Haematol. 1976 May;33(1):39–51. doi: 10.1111/j.1365-2141.1976.tb00970.x. [DOI] [PubMed] [Google Scholar]
- SPAET T. H., CINTRON J. STUDIES ON PLATELET FACTOR-3 AVAILABILITY. Br J Haematol. 1965 May;11:269–275. doi: 10.1111/j.1365-2141.1965.tb06587.x. [DOI] [PubMed] [Google Scholar]
- Saleem A., Kreig A. F., Cieslica R. Platelet activity ratio. A new approach for quantitation of platelet factor 3. Am J Clin Pathol. 1975 Jan;63(1):68–73. doi: 10.1093/ajcp/63.3.68. [DOI] [PubMed] [Google Scholar]
- Schafer A. I., Handin R. I. The role of platelets in thrombotic and vascular disease. Prog Cardiovasc Dis. 1979 Jul-Aug;22(1):31–52. doi: 10.1016/0033-0620(79)90002-1. [DOI] [PubMed] [Google Scholar]
- Sinakos Z., Caen J. P. Platelet aggregation in mammalians (human, rat, rabbit, guinea-pig, horse, dog). A comparative study. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):99–111. [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Prostaglandins in platelet function. Thromb Res. 1974 Jun;4(0):suppl 1–1:55. doi: 10.1016/0049-3848(74)90148-0. [DOI] [PubMed] [Google Scholar]


