Abstract
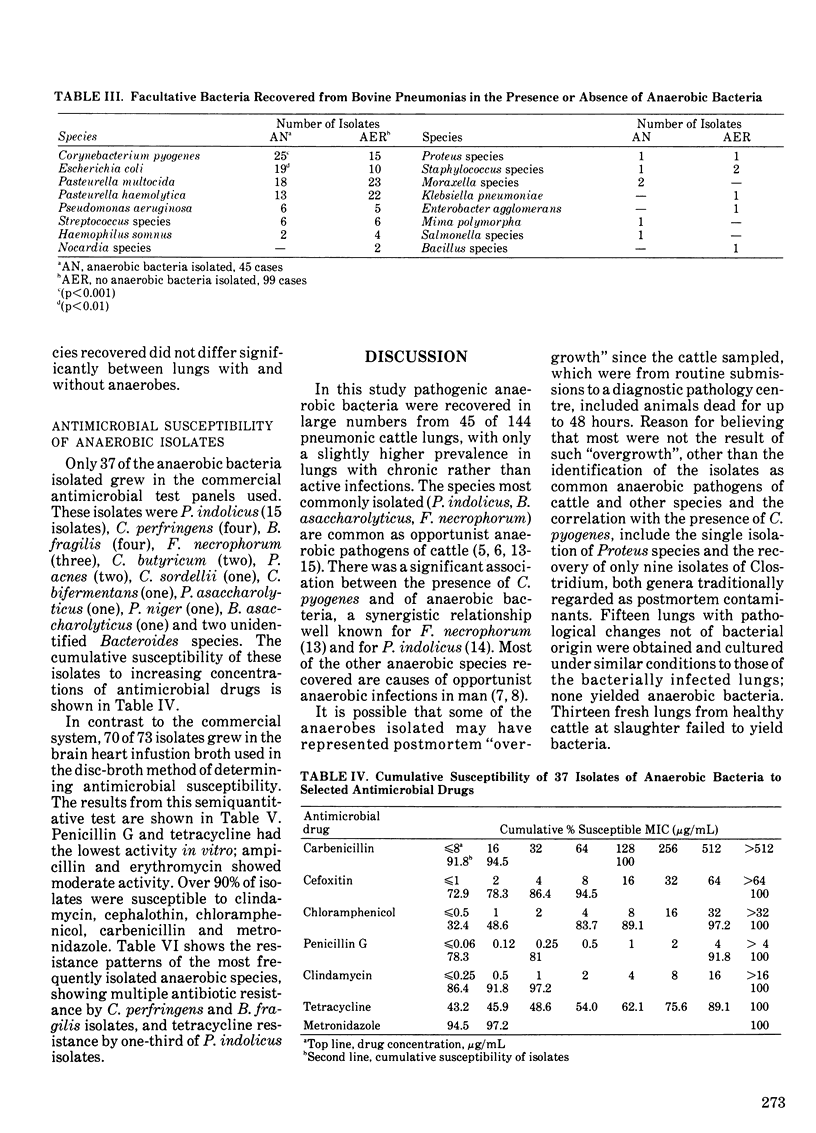
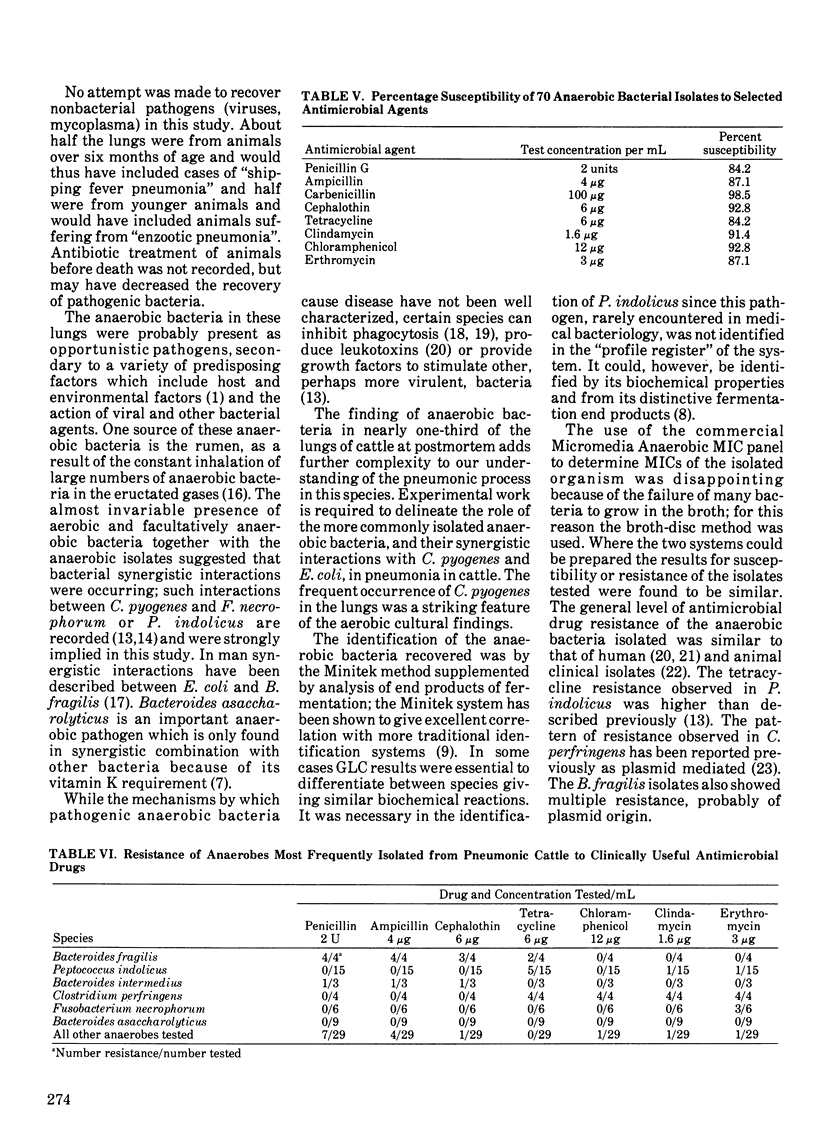
One hundred and forty-four lungs obtained postmortem from cattle with pneumonia were cultured for anaerobic bacteria. Forty-five lungs yielded 73 anaerobic isolates belonging to 20 species. The number of isolations of anaerobes from acute fibrinous or suppurative bronchopneumonias (32.5%) was slightly lower than from similar chronic bronchopneumonias (36.5%). Anaerobes were not recovered from 15 lungs showing macroscopic changes not of bacterial origin, nor from 13 healthy lungs. The predominant genera isolated were Bacteroides, Peptococcus, Fusobacterium and Clostridium. The most common species were P. indolicus (15 isolates), B. asaccharolyticus (nine), F. necrophorum (six), C. perfringens (four) and B. fragilis (four). There was a significant correlation between the presence of Corynebacterium pyogenes (p less than 0.001) or Escherichia coli (p less than 0.01) and the presence of anaerobes in the lungs. The isolated anaerobic bacteria were generally susceptible to ampicillin, penicillin G, cefoxitin, cephalothin, clindamycin, chloramphenicol, erythromycin, tetracycline and metronidazole. The B. fragilis and C. perfringens isolates showed multiple antibiotic resistance, and five P. indolicus isolates were resistant to tetracycline.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamu S. A., Sperry J. F. Polymorphonuclear neutrophil chemotaxis induced and inhibited by Bacteroides spp. Infect Immun. 1981 Sep;33(3):806–810. doi: 10.1128/iai.33.3.806-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Chatterton S. A. Susceptibility of anaerobic bacteria to ten antimicrobial agents. Antimicrob Agents Chemother. 1978 Sep;14(3):371–376. doi: 10.1128/aac.14.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefort G., Magot M., Ionesco H., Sebald M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid. 1977 Nov;1(1):52–66. doi: 10.1016/0147-619x(77)90008-7. [DOI] [PubMed] [Google Scholar]
- Coyle-Dennis J. E., Lauerman L. H. Biological and biochemical characteristics of Fusobacterium necrophorum leukocidin. Am J Vet Res. 1978 Nov;39(11):1790–1793. [PubMed] [Google Scholar]
- Du Preez J. H., Greeff A. S., Eksteen N. Isolation and significance of anaerobic bacteria isolated from cases of bovine mastitis. Onderstepoort J Vet Res. 1981 Jun;48(2):123–126. [PubMed] [Google Scholar]
- Hirsh D. C., Biberstein E. L., Jang S. S. Obligate anaerobes in clinical veterinary practice. J Clin Microbiol. 1979 Aug;10(2):188–191. doi: 10.1128/jcm.10.2.188-191.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jericho K. W. Update on pasteurellosis in young cattle. Can Vet J. 1979 Nov;20(11):333–335. [PMC free article] [PubMed] [Google Scholar]
- Lopez A., Maxie M. G., Savan M., Ruhnke H. L., Thomson R. G., Barnum D. A., Geissinger H. D. The pulmonary clearance of Pasteurella haemolytica in calves infected with bovine virus diarrhea or Mycoplasma bovis. Can J Comp Med. 1982 Jul;46(3):302–306. [PMC free article] [PubMed] [Google Scholar]
- MULLENAX C. H., ALLISON M. J., SONGER J. R. TRANSPORT OF AEROSOLIZED MICROORGANISMS FROM THE RUMEN TO THE RESPIRATORY SYSTEM DURING ERUCTATION. Am J Vet Res. 1964 Nov;25:1583–1594. [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Johnson J. A., Curtis R. A. Factors associated with morbidity and mortality in feedlot calves: the Bruce County beef project, year two. Can J Comp Med. 1981 Apr;45(2):103–112. [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F. Identification of some anaerobic bacteria in nonspecific anaerobic infections in animals. Can J Comp Med. 1979 Apr;43(2):194–199. [PMC free article] [PubMed] [Google Scholar]
- Roberts D. S. The pathogenic synergy of Fusiformis necrophorus and Corynebacterium pyogenes. II. The response of F. necrophorus to a filterable product of C. pyogenes. Br J Exp Pathol. 1967 Dec;48(6):674–679. [PMC free article] [PubMed] [Google Scholar]
- Stargel M. D., Lombard G. L., Dowell V. R., Jr Alternative procedures for identification of anaerobic bacteria. Am J Med Technol. 1978 Jul;44(7):709–722. [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]


