Abstract
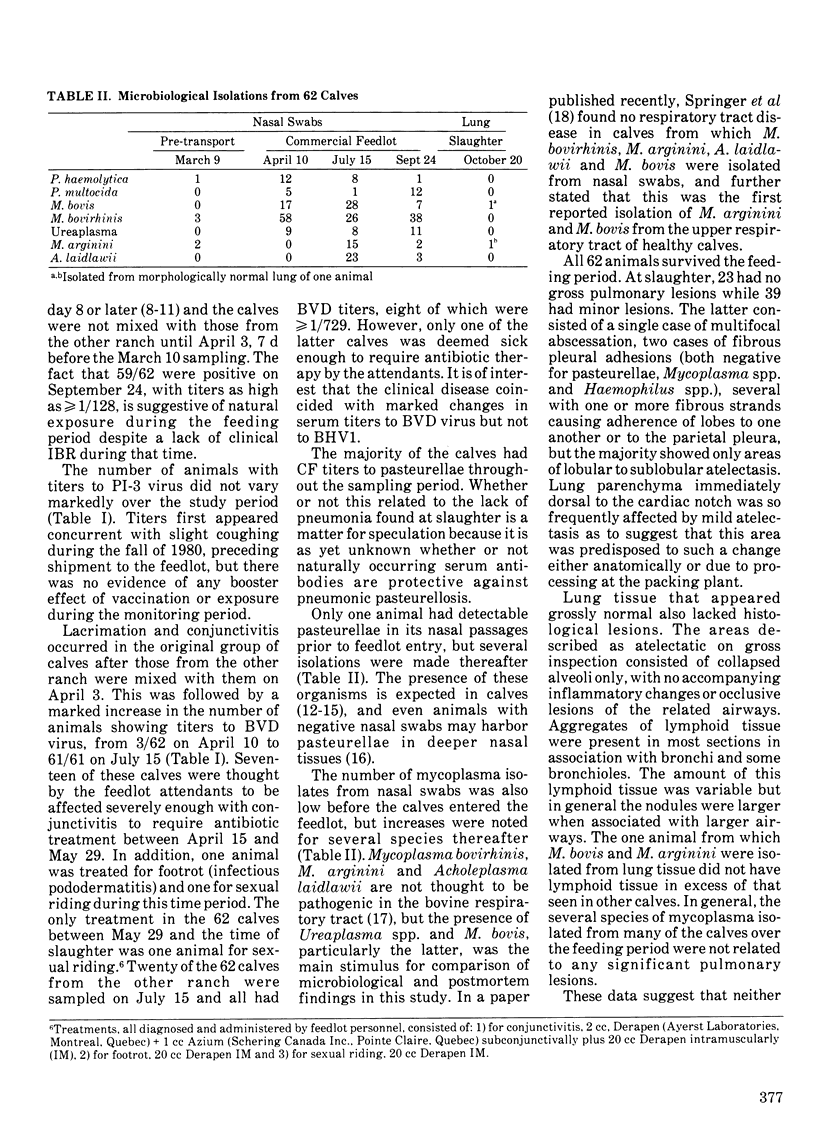
A group of 62 beef calves, born and raised in an institutional herd, were transferred at nine months of age to a commercial feedlot where they remained until slaughter seven months later. Clinical, immunological and microbiological monitoring was carried out during this period. No serious clinical illness occurred. One hundred percent seroconversion to bovine virus diarrhea virus took place after introduction of the calves into the feedlot as well as almost complete (59/62) seroconversion to bovine herpesvirus 1, a proportion of which could be related to a single vaccination. Significant increases in recoveries of Mycoplasma spp. from nasal swabs also occurred in the feedlot. At slaughter, the lungs of all animals were recovered and examined for pathological lesions: 23 were completely normal and 39 showed minor histological changes chiefly characterized by areas of lobular to sublobular atelectasis. For this group of calves, no relationship was found between the presence of potential pathogens in nasal mucus and the occurrence of lesions in the lung. The serological results are discussed in terms of vaccinations and other known events that occurred during the study period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cho H. J. Demonstration of Complement Fixing Antibody in the Sera of Cattle Vaccinated with Combined Living Blackleg-anthrax Vaccine. Can J Comp Med. 1971 Apr;35(2):155–160. [PMC free article] [PubMed] [Google Scholar]
- Collier J. R. Significance of bacteria in bovine respiratory disease. J Am Vet Med Assoc. 1968 Dec 15;153(12):1645–1651. [PubMed] [Google Scholar]
- Darcel C. Q. Some factors influencing the microtest method for neutralizing antibodies to the virus for neutralizing antibodies to the virus of infectious bovine rhinotracheitis. Can Vet J. 1975 Feb;16(2):59–62. [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Thomas L. H., Wyld S. G. Pathogenicity of some Mycoplasma and Acholeplasma species in the lungs of gnotobiotic calves. Res Vet Sci. 1979 Sep;27(2):233–237. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Jericho K. W., Yates W. D., Babiuk L. A. Bovine herpesvirus-1 vaccination against experimental bovine herpesvirus-1 and Pasteurella haemolytica respiratory tract infection: onset of protection. Am J Vet Res. 1982 Oct;43(10):1776–1780. [PubMed] [Google Scholar]
- Magwood S. E., Barnum D. A., Thomson R. G. Nasal bacterial flora of calves in healthy and in pneumonia-prone herds. Can J Comp Med. 1969 Oct;33(4):237–243. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Johnson J. A., Curtis R. A. Factors associated with mortality and treatment costs in feedlot calves: the Bruce County Beef Project, years 1978, 1979, 1980. Can J Comp Med. 1982 Oct;46(4):341–349. [PMC free article] [PubMed] [Google Scholar]
- Pass D. A., Thompson R. G. Wide distribution of Pasteurella haemolytica type 1 over the nasal mucosa of cattle. Can J Comp Med. 1971 Jul;35(3):181–186. [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C. Differential methods for identification of T-mycoplasmas based on demonstration of urease. J Infect Dis. 1973 Mar;127(Suppl):S22–S25. doi: 10.1093/infdis/127.supplement_1.s22. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Differential agar medium (A7) for identification of Ureaplasma urealyticum (human T mycoplasmas) in primary cultures of clinical material. J Clin Microbiol. 1976 Jun;3(6):613–625. doi: 10.1128/jcm.3.6.613-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. T., Fulton R. W., Hagstad H. V., Nicholson S. S., Garton J. D. Prevalence of Mycoplasma and Chlamydia in the nasal flora of dairy calves. Vet Microbiol. 1982 Sep;7(4):351–357. doi: 10.1016/0378-1135(82)90015-3. [DOI] [PubMed] [Google Scholar]
- Stockdale P. H., Langford E. V., Darcel C. Experimental bovine pneumonic pasteurellosis. I. Prevention of the disease. Can J Comp Med. 1979 Jul;43(3):262–271. [PMC free article] [PubMed] [Google Scholar]
- Todd J. D. Development of intranasal vaccination for the immunization of cattle against infectious bovine rhinotracheitis. Can Vet J. 1974 Sep;15(9):257–259. [PMC free article] [PubMed] [Google Scholar]
- Todd J. D. Intranasal vaccination of cattle against IBR and PI3: field and laboratory observations in dairy, beef, and neonatal calf populations. Dev Biol Stand. 1976;33:391–395. [PubMed] [Google Scholar]
- Todd J. D., Volenec F. J., Paton I. M. Intranasal vaccination against infectious bovine rhinotracheitis: studies on early onset of protection and use of the vaccine in pregnant cows. J Am Vet Med Assoc. 1971 Dec 1;159(11):1370–1374. [PubMed] [Google Scholar]
- Wessman G. E., Hilker G. Characterization of Pasteurella hemolytica isolated from the respiratory tract of cattle. Can J Comp Med. 1968 Jul;32(3):498–504. [PMC free article] [PubMed] [Google Scholar]
- Witte K. H., Easterday B. C. Stained monolayer test: a color test in disposable plastic trays for titrating transmissible gastroenteritis virus and neutralizing antibodies. Am J Vet Res. 1968 Jul;29(7):1409–1417. [PubMed] [Google Scholar]


