Abstract
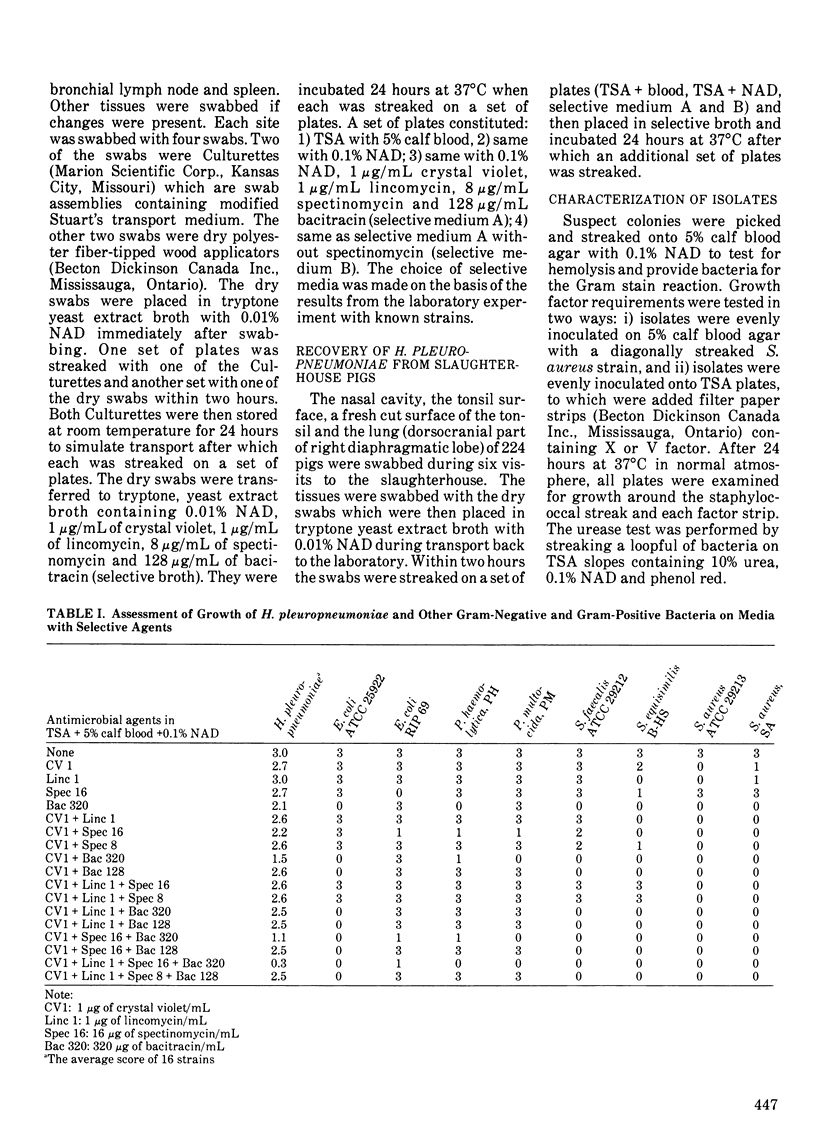
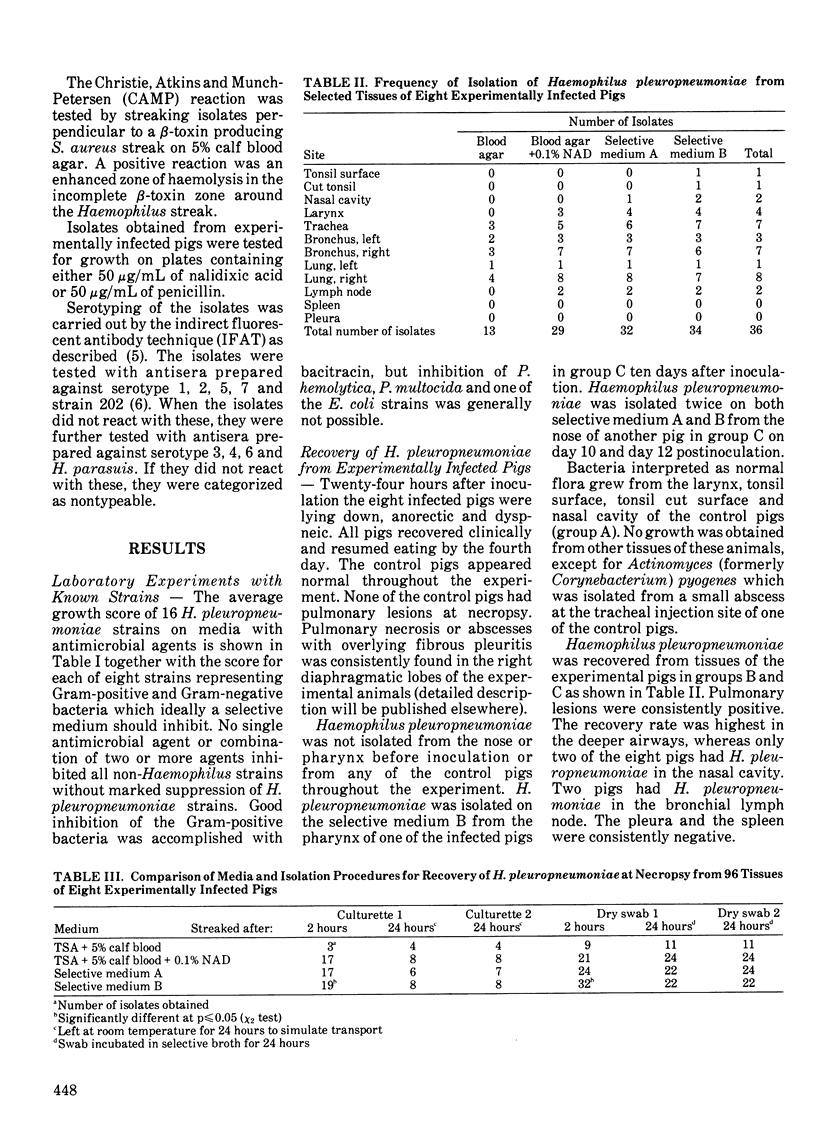
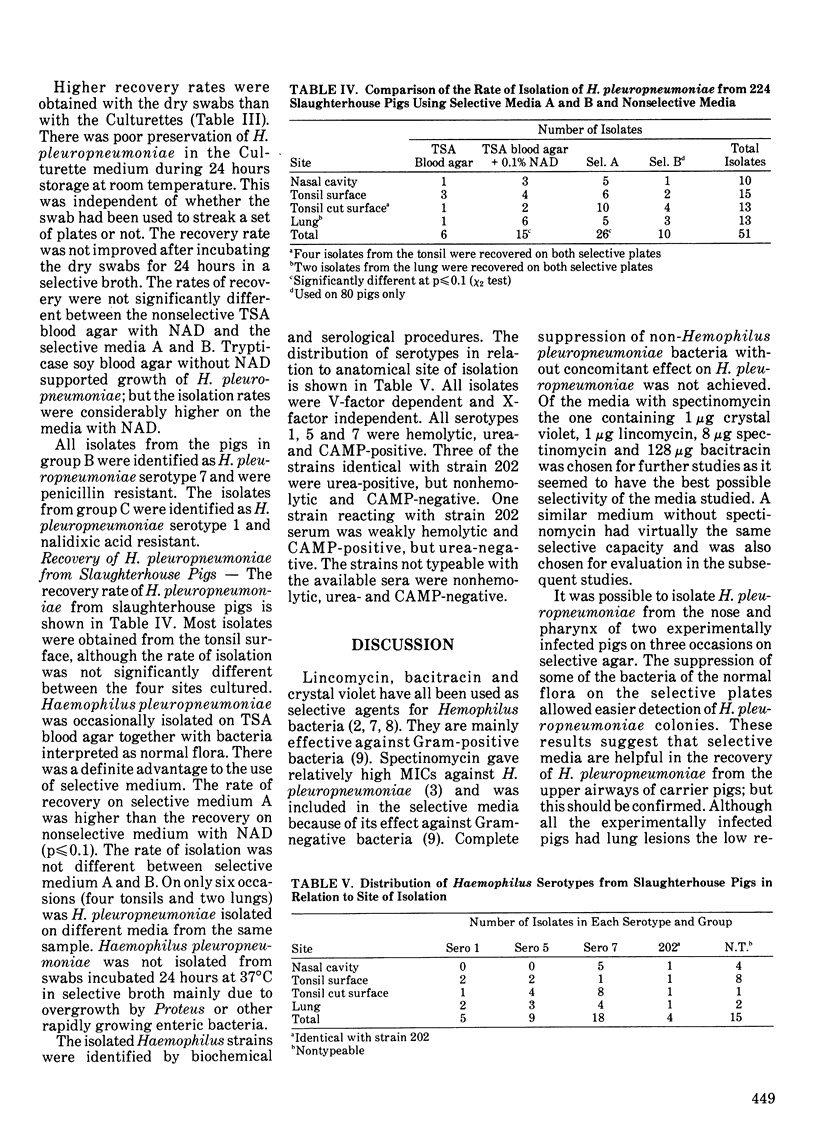
Crystal violet, lincomycin, spectinomycin and bacitracin were evaluated as selective agents in media for isolation of Haemophilus pleuropneumoniae. No single antimicrobial agent or combination of two or more inhibited all non-Haemophilus strains (Escherichia coli, Pasteurella haemolytica, Pasteurella multocida, Streptococcus faecalis, Streptococcus equisimilis and Staphylococcus aureus) without marked suppression of 16 H. pleuropneumoniae strains. A medium containing 1 micrograms/mL of crystal violet, 1 microgram/mL of lincomycin, 8 micrograms/mL of spectinomycin and 128 micrograms/mL of bacitracin inhibited one E. coli strain and the Gram-positive strains while H. pleuropneumoniae strains were suppressed to a minor degree only. Haemophilus pleuropneumoniae was isolated on the selective medium on three occasions from the nose or pharynx of two out of eight experimentally inoculated pigs. Haemophilus pleuropneumoniae was recovered from the nose of only two pigs at necropsy and from tonsil of one, whereas the lower airways in most pigs and the lung lesions in all pigs were positive. There was no advantage to using the selective medium for the recovery of H. pleuropneumoniae at necropsy from these eight experimentally infected pigs, probably because other bacteria were absent or present in very low numbers in the tissues with H. pleuropneumoniae. The isolation rate on selective medium was higher than the rate on non-selective medium (p less than or equal to 0.1; chi 2 test) when the airways of slaughtered pigs were cultured. This was likely due to a high degree of contamination. Dry swabs placed in tryptone yeast extract with nicotinamide-adenine-dinucleotide gave a significantly higher recovery rate than commercial Culturette swabs in modified Stuart's transport medium.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Csukás Z. New selective medium for the isolation of Haemophilus species. Acta Microbiol Acad Sci Hung. 1980;27(2):141–145. [PubMed] [Google Scholar]
- Kilian M. The haemolytic activity of Haemophilus species. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):339–341. doi: 10.1111/j.1699-0463.1976.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Little T. W. Haemophilus infection in pigs. Vet Rec. 1970 Oct 3;87(14):399–402. doi: 10.1136/vr.87.14.399. [DOI] [PubMed] [Google Scholar]
- Little T. W., Harding J. D. The comparative pathogenicity of two porcine haemophilus species. Vet Rec. 1971 May 22;88(21):540–545. doi: 10.1136/vr.88.21.540. [DOI] [PubMed] [Google Scholar]
- Nielsen R., Mandrup M. Pleuropneumonia in swine caused by Haemophilus parahaemolyticus. A study of the epidemiology of the infection. Nord Vet Med. 1977 Nov;29(11):465–473. [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A. Haemophilus pleuropneumoniae serotyping. J Clin Microbiol. 1982 Nov;16(5):840–843. doi: 10.1128/jcm.16.5.840-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Lombin L., DeMoor J. Serotyping and detection of Haemophilus pleuropneumoniae by indirect fluorescent antibody technique. Can J Comp Med. 1981 Jul;45(3):271–274. [PMC free article] [PubMed] [Google Scholar]


