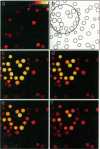
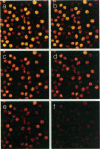
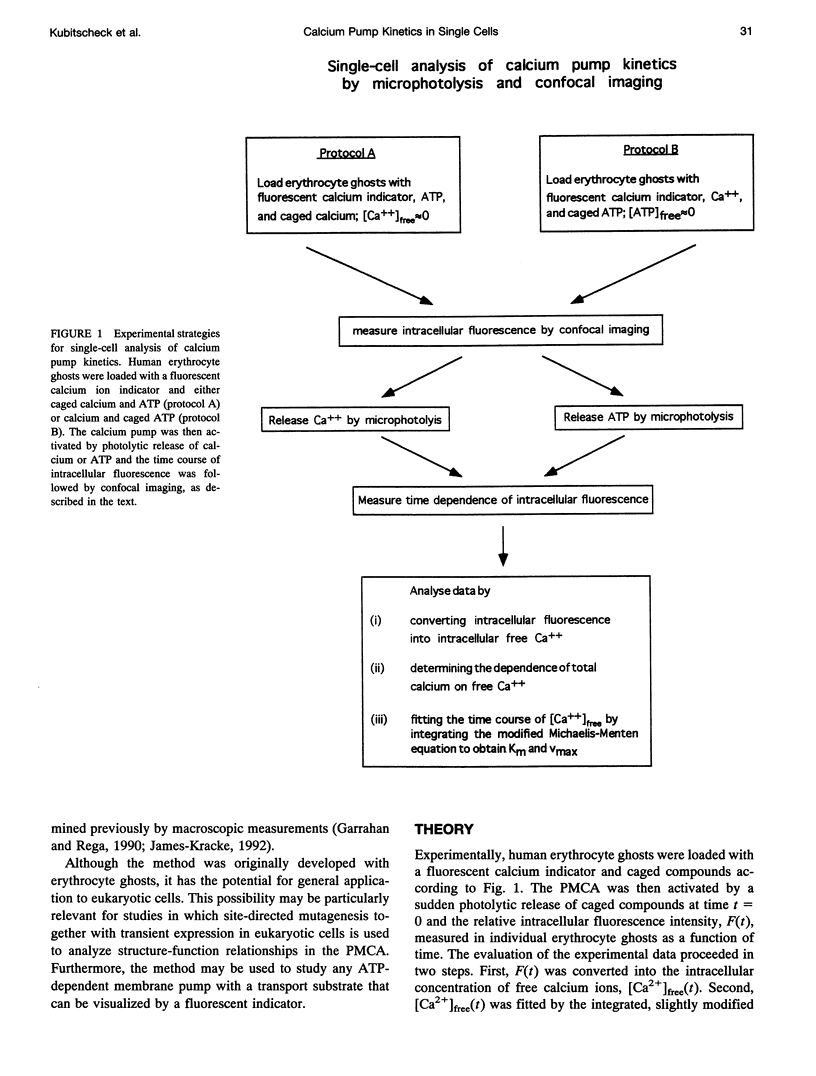
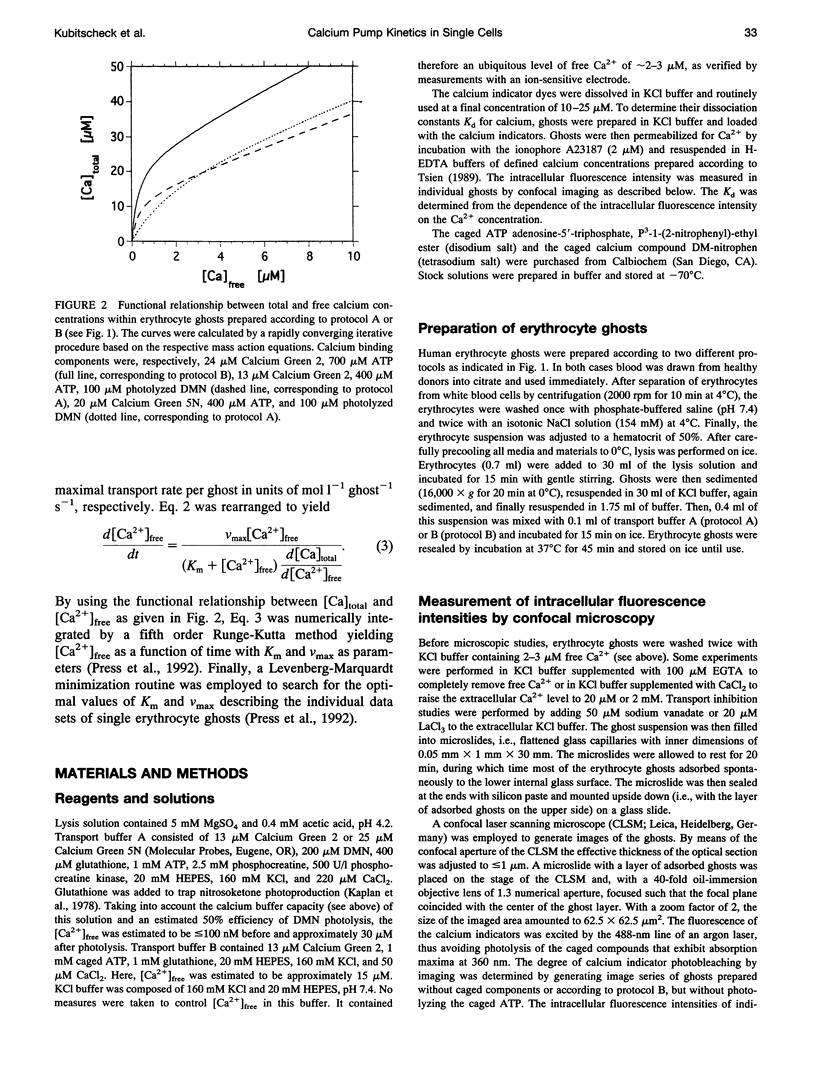
Abstract
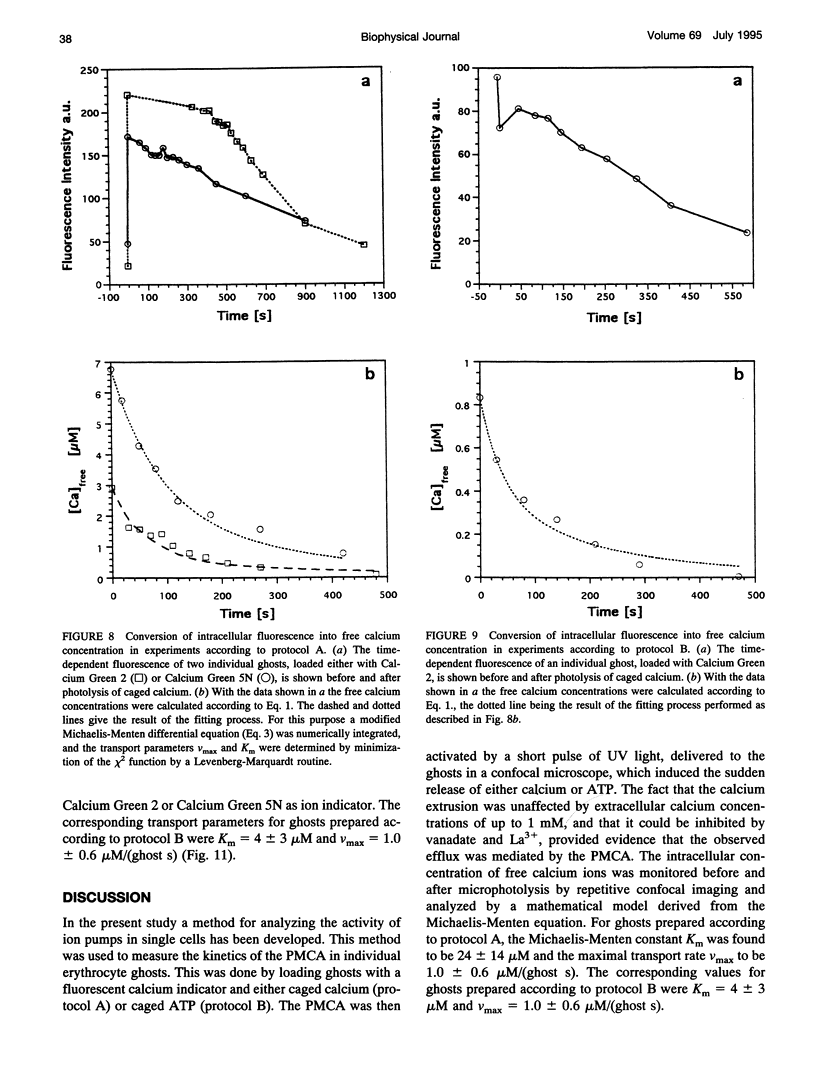
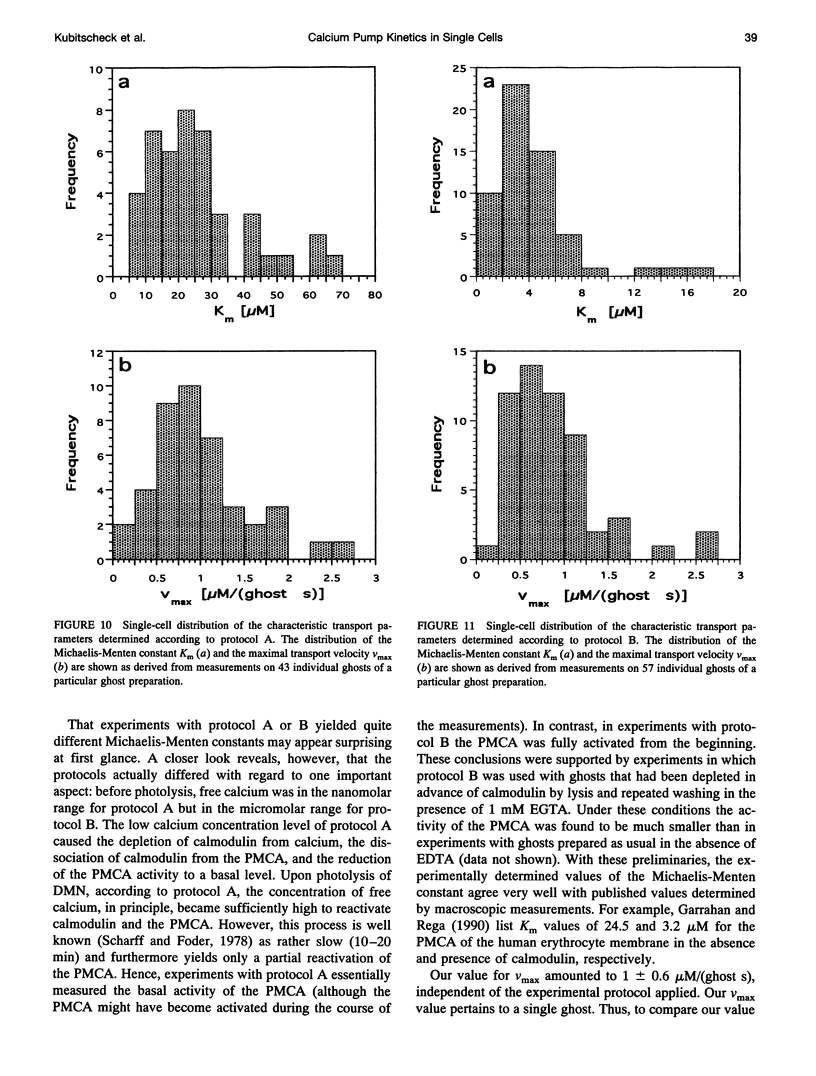
The activity of the plasma membrane calcium pump was measured in single cells. Human red blood cell ghosts were loaded with a fluorescent calcium indicator and either caged calcium and ATP (protocol A) or caged ATP and calcium (protocol B). In a suitably modified laser scanning microscope either calcium or ATP were released by a short UV light pulse. The time-dependent fluorescence intensity of the calcium indicator was then followed in single ghosts by repetitive confocal imaging. The fluorescence intensity was converted into calcium concentration, which in turn was used to derive the kinetic parameters of the calcium pump, the Michaelis-Menten constant Km, and the maximal transport rate vmax. Km and vmax values derived in this manner were 24 +/- 14 microM and 1.0 +/- 0.6 microM/(ghost s) for protocol A, and 4 +/- 3 microM and 1.0 +/- 0.6 microM/(ghost s) for protocol B, respectively. The difference between A and B is presumably caused by calmodulin, which is inactive in the experiments with protocol A. The possibilities to extend the new method to living nucleus-containing cells transiently transfected with mutants of the plasma membrane calcium pump are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo H. P., Verma A. K., Sanders M. A., Heim R., Salisbury J. L., Wieben E. D., Penniston J. T. Overexpression of the erythrocyte plasma membrane Ca2+ pump in COS-1 cells. Biochem J. 1992 Aug 1;285(Pt 3):791–797. doi: 10.1042/bj2850791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrabin H., Garrahan P. J., Rega A. F. Vanadate inhibition of the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1980 Aug 14;600(3):796–804. doi: 10.1016/0005-2736(80)90482-4. [DOI] [PubMed] [Google Scholar]
- Becker E. W. Biomechanical model of the P-type ion pumps of the cell. Naturwissenschaften. 1994 Jan;81(1):21–27. doi: 10.1007/BF01138556. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992 Feb 5;267(4):2115–2118. [PubMed] [Google Scholar]
- Ellis-Davies G. C., Kaplan J. H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Verma A. K., Filoteo A. G., Penniston J. T. A highly active 120-kDa truncated mutant of the plasma membrane Ca2+ pump. J Biol Chem. 1993 May 15;268(14):10621–10626. [PubMed] [Google Scholar]
- Föhr K. J., Warchol W., Gratzl M. Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods Enzymol. 1993;221:149–157. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- James-Kracke M. R. Calmodulin activation of the Ca2+ pump revealed by fluorescent chelator dyes in human red blood cell ghosts. J Gen Physiol. 1992 Jan;99(1):41–62. doi: 10.1085/jgp.99.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks W. P. How does a calcium pump pump calcium? J Biol Chem. 1989 Nov 15;264(32):18855–18858. [PubMed] [Google Scholar]
- Jencks W. P. On the mechanism of ATP-driven Ca2+ transport by the calcium ATPase of sarcoplasmic reticulum. Ann N Y Acad Sci. 1992 Nov 30;671:49–57. doi: 10.1111/j.1749-6632.1992.tb43783.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Kratje R. B., Garrahan P. J., Rega A. F. Two modes of inhibition of the Ca2+ pump in red cells by Ca2+. Biochim Biophys Acta. 1985 Jun 27;816(2):365–378. doi: 10.1016/0005-2736(85)90504-8. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Toyofuku T., Lytton J. Structure-function relationships in sarcoplasmic or endoplasmic reticulum type Ca2+ pumps. Ann N Y Acad Sci. 1992 Nov 30;671:1–10. doi: 10.1111/j.1749-6632.1992.tb43779.x. [DOI] [PubMed] [Google Scholar]
- Maruyama K., MacLennan D. H. Mutation of aspartic acid-351, lysine-352, and lysine-515 alters the Ca2+ transport activity of the Ca2+-ATPase expressed in COS-1 cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3314–3318. doi: 10.1073/pnas.85.10.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G. Ca2+i versus [Ca2+]i. Biophys J. 1993 Aug;65(2):561–562. doi: 10.1016/S0006-3495(93)81087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Reversible shift between two states of Ca2+-ATPase in human erythrocytes mediated by Ca2+ and a membrane-bound activator. Biochim Biophys Acta. 1978 May 4;509(1):67–77. doi: 10.1016/0005-2736(78)90008-1. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Strehler E. E. Recent advances in the molecular characterization of plasma membrane Ca2+ pumps. J Membr Biol. 1991 Feb;120(1):1–15. doi: 10.1007/BF01868586. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K., Enyedi A., Filoteo A. G., Penniston J. T. Regulatory region of plasma membrane Ca2+ pump. 28 residues suffice to bind calmodulin but more are needed for full auto-inhibition of the activity. J Biol Chem. 1994 Jan 21;269(3):1687–1691. [PubMed] [Google Scholar]
- Verma A. K., Filoteo A. G., Stanford D. R., Wieben E. D., Penniston J. T., Strehler E. E., Fischer R., Heim R., Vogel G., Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988 Oct 5;263(28):14152–14159. [PubMed] [Google Scholar]
- Zucker R. S. Effects of photolabile calcium chelators on fluorescent calcium indicators. Cell Calcium. 1992 Jan;13(1):29–40. doi: 10.1016/0143-4160(92)90027-p. [DOI] [PubMed] [Google Scholar]