Abstract
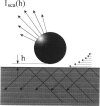
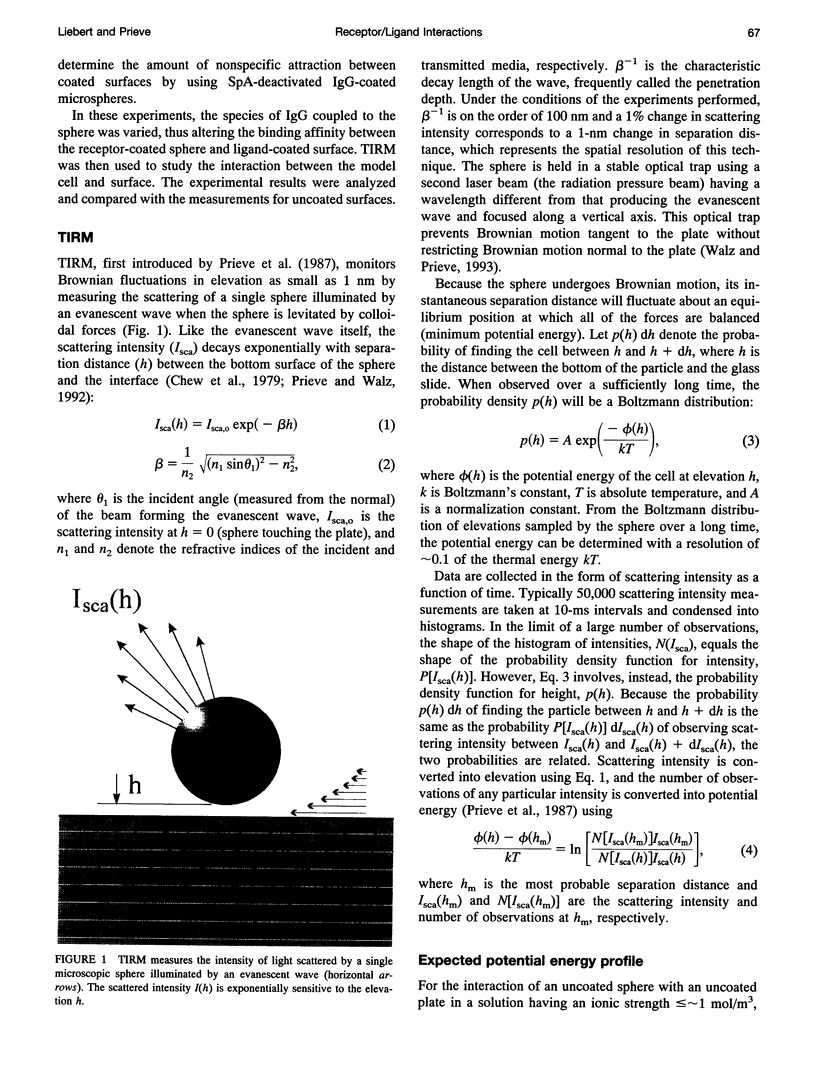
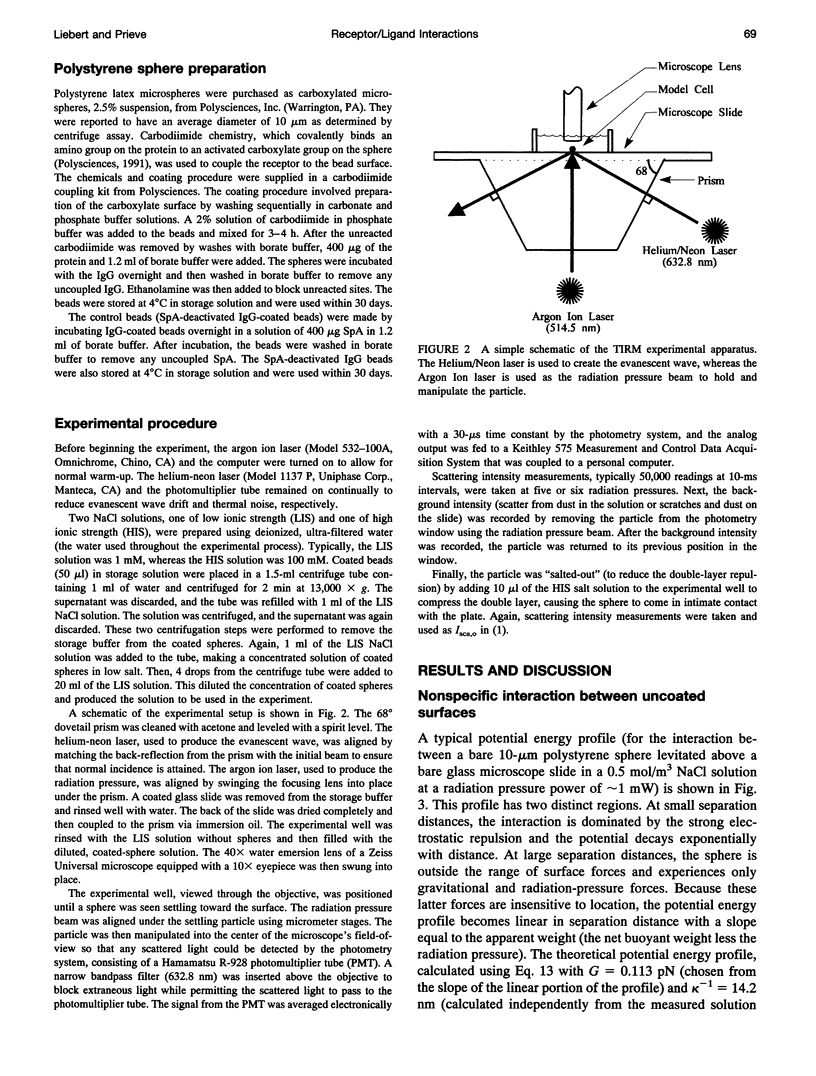
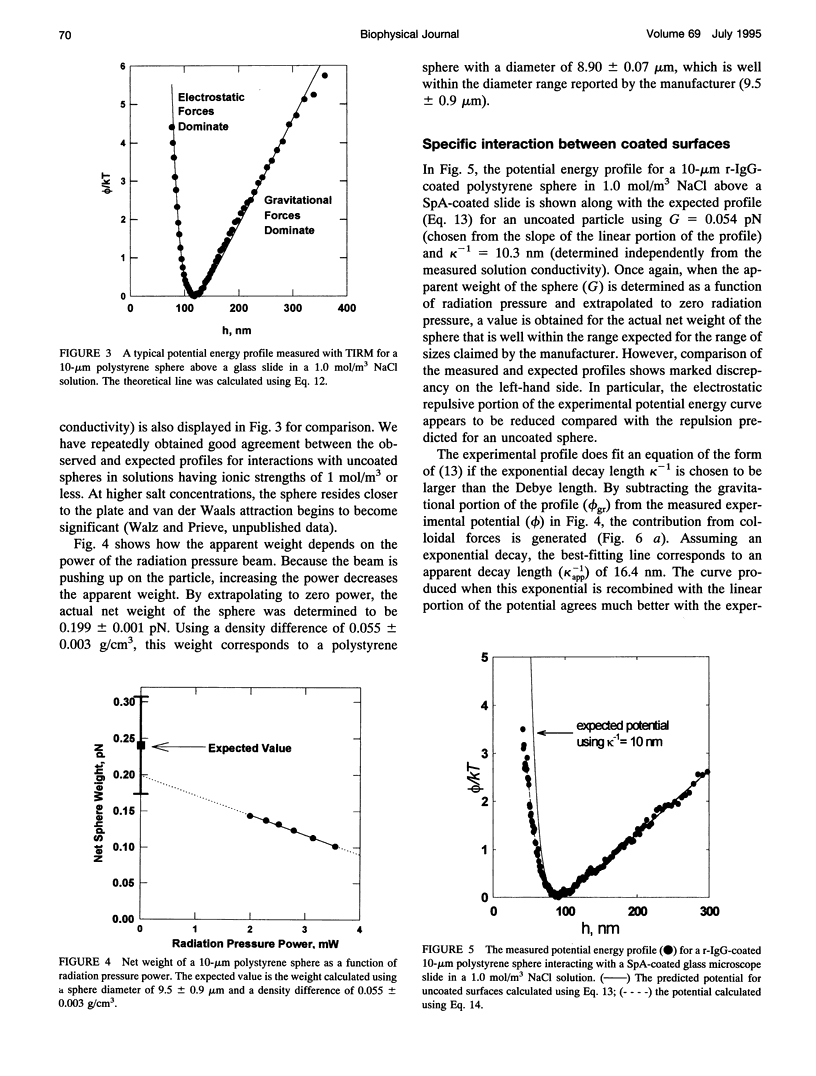
Total internal reflection microscopy (TIRM) monitors Brownian fluctuations in elevation as small as 1 nm by measuring the scattering of a single sphere illuminated by an evanescent wave when the sphere is levitated by colloidal forces such as electrostatic double-layer repulsion. From the Boltzmann distribution of elevations sampled by the sphere over time, the potential energy profile can be determined with a resolution of approximately 0.1 of the thermal energy kT. Thus, the interaction between a receptor-coated (goat, horse, or rabbit immunoglobulin G (IgG)) latex sphere and a protein A (SpA)-coated glass microscope slide was studied. A typical TIRM potential energy profile measured between a bare sphere and a bare glass plate, where the sphere fluctuates around the secondary potential energy minimum formed between double-layer repulsion and gravitational attraction, agrees well with DLVO theory. The interactions measured between IgG-coated spheres and SpA-coated slides, on the other hand, displayed a weaker repulsion compared with that observed between bare surfaces under the same conditions. Analysis of the results obtained between the coated surfaces suggests an additional attractive force. The decay length of this attraction correlates with the known dissociation constants for the binding of IgG with SpA in free solution.
Full text
PDF

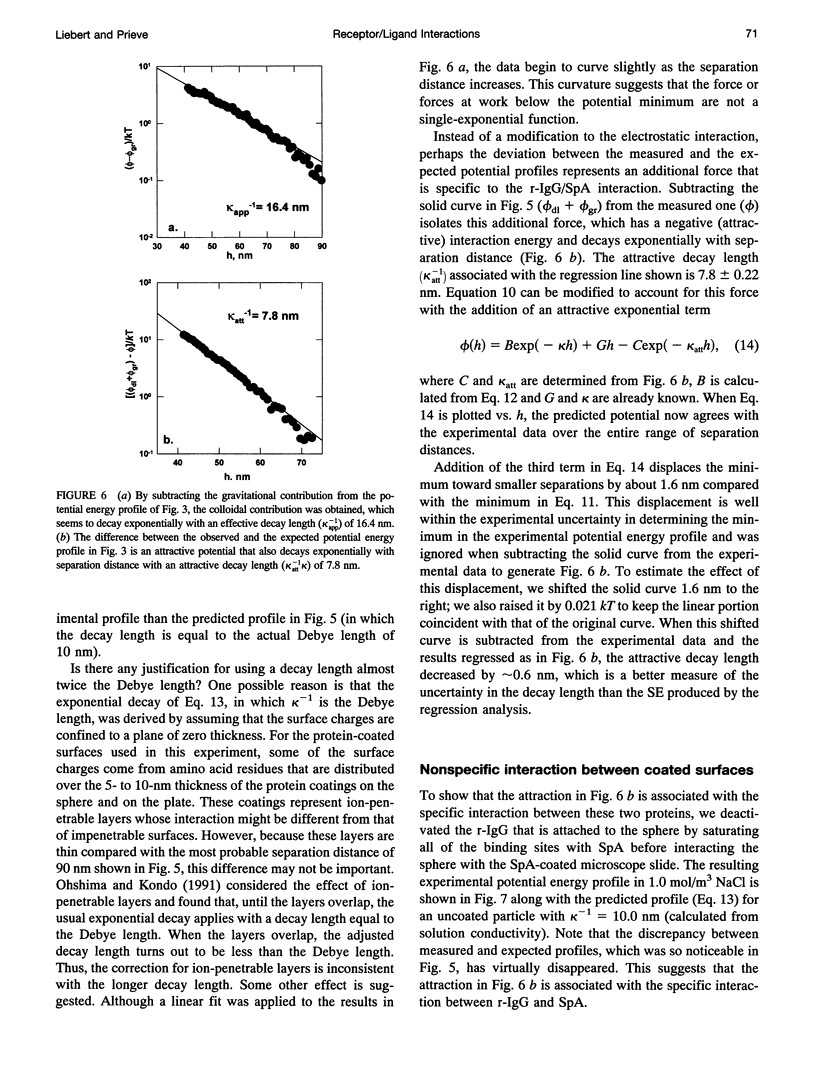
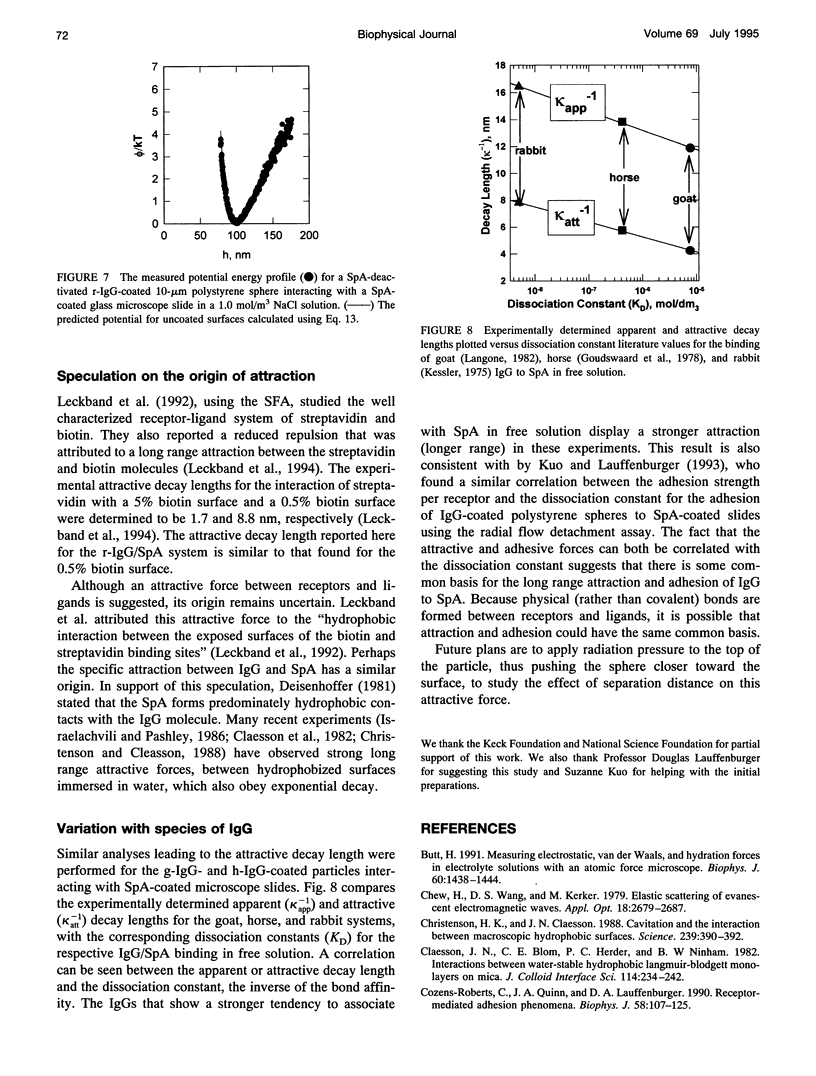

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butt H. J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys J. 1991 Dec;60(6):1438–1444. doi: 10.1016/S0006-3495(91)82180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson H. K., Claesson P. M. Cavitation and the interaction between macroscopic hydrophobic surfaces. Science. 1988 Jan 22;239(4838):390–392. doi: 10.1126/science.239.4838.390. [DOI] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenberger D. A. Receptor-mediated adhesion phenomena. Model studies with the Radical-Flow Detachment Assay. Biophys J. 1990 Jul;58(1):107–125. doi: 10.1016/S0006-3495(90)82357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- EASTY G. C., EASTY D. M., AMBROSE E. J. Studies of cellular adhesiveness. Exp Cell Res. 1960 Apr;19:539–548. doi: 10.1016/0014-4827(60)90062-8. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Lackie J. M. Adhesion of neutrophil leucocytes under conditions of flow. J Cell Sci. 1984 Aug;70:93–110. doi: 10.1242/jcs.70.1.93. [DOI] [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Israelachvili J., Pashley R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature. 1982 Nov 25;300(5890):341–342. doi: 10.1038/300341a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kuo S. C., Lauffenburger D. A. Relationship between receptor/ligand binding affinity and adhesion strength. Biophys J. 1993 Nov;65(5):2191–2200. doi: 10.1016/S0006-3495(93)81277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langone J. J. Applications of immobilized protein A in immunochemical techniques. J Immunol Methods. 1982 Dec 30;55(3):277–296. doi: 10.1016/0022-1759(82)90088-6. [DOI] [PubMed] [Google Scholar]
- Leckband D. E., Israelachvili J. N., Schmitt F. J., Knoll W. Long-range attraction and molecular rearrangements in receptor-ligand interactions. Science. 1992 Mar 13;255(5050):1419–1421. doi: 10.1126/science.1542789. [DOI] [PubMed] [Google Scholar]
- Leckband D. E., Schmitt F. J., Israelachvili J. N., Knoll W. Direct force measurements of specific and nonspecific protein interactions. Biochemistry. 1994 Apr 19;33(15):4611–4624. doi: 10.1021/bi00181a023. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Wessel G. M., Marchase R. B. Intercellular recognition: quantitation of initial binding events. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4975–4979. doi: 10.1073/pnas.78.8.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima H., Kondo T. On the electrophoretic mobility of biological cells. Biophys Chem. 1991 Feb;39(2):191–198. doi: 10.1016/0301-4622(91)85021-h. [DOI] [PubMed] [Google Scholar]
- WEISS L. The measurement of cell adhesion. Exp Cell Res. 1961;Suppl 8:141–153. doi: 10.1016/0014-4827(61)90345-7. [DOI] [PubMed] [Google Scholar]
- Weetall H. H. Covalent coupling methods for inorganic support materials. Methods Enzymol. 1976;44:134–148. doi: 10.1016/s0076-6879(76)44012-0. [DOI] [PubMed] [Google Scholar]