Abstract
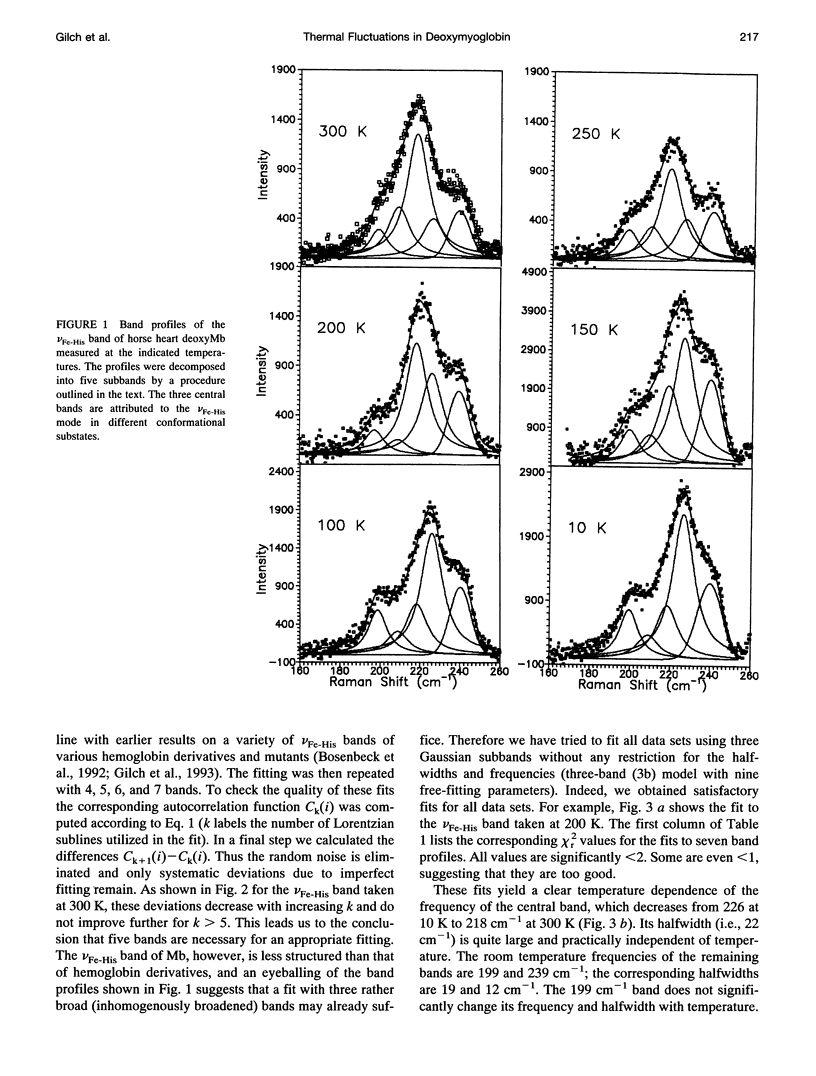
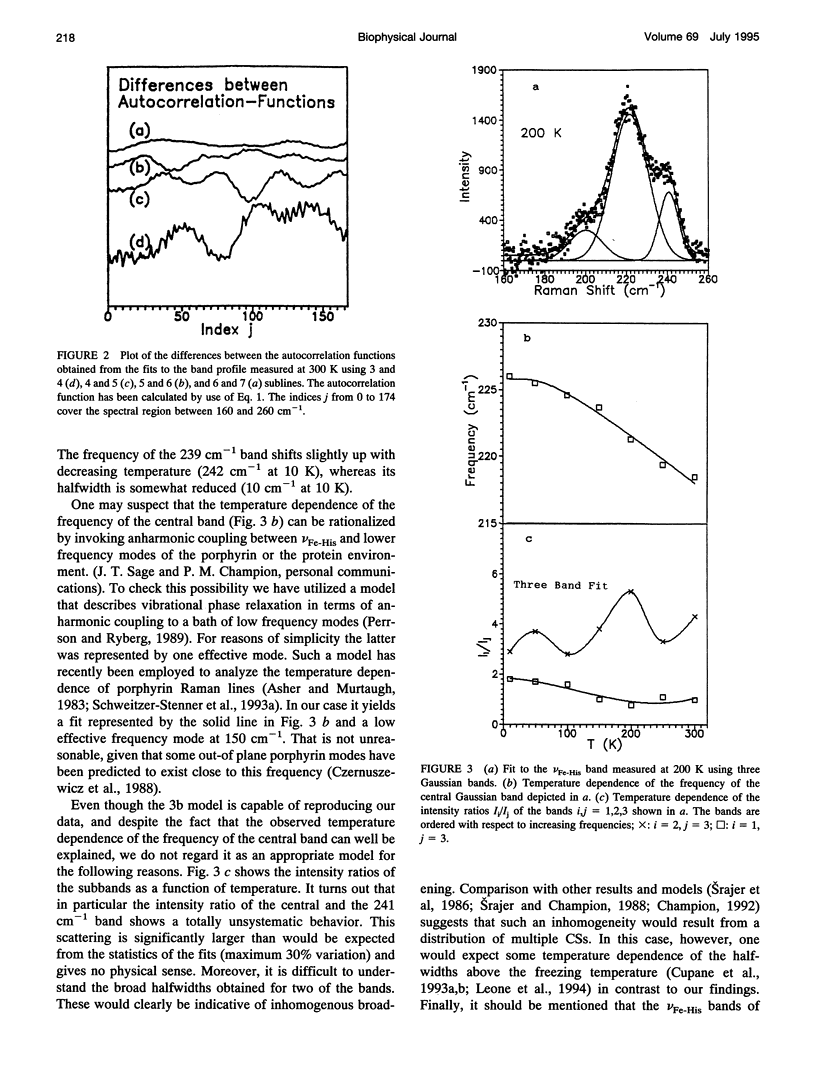
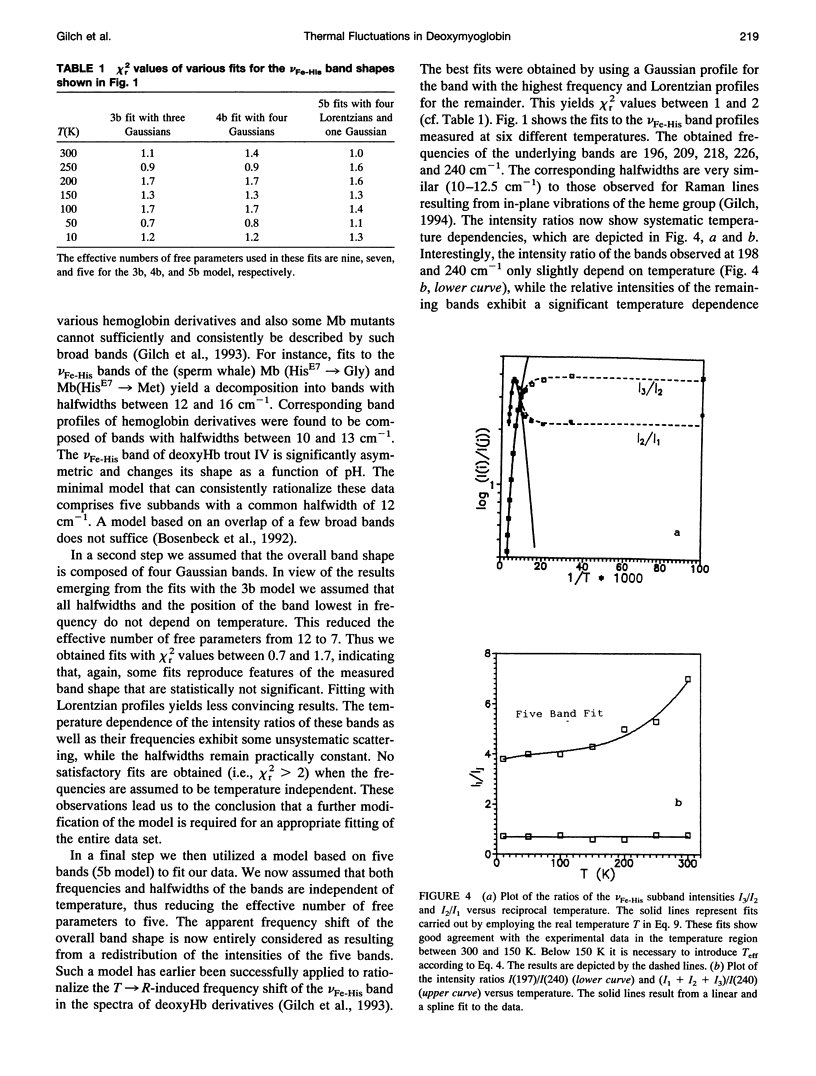
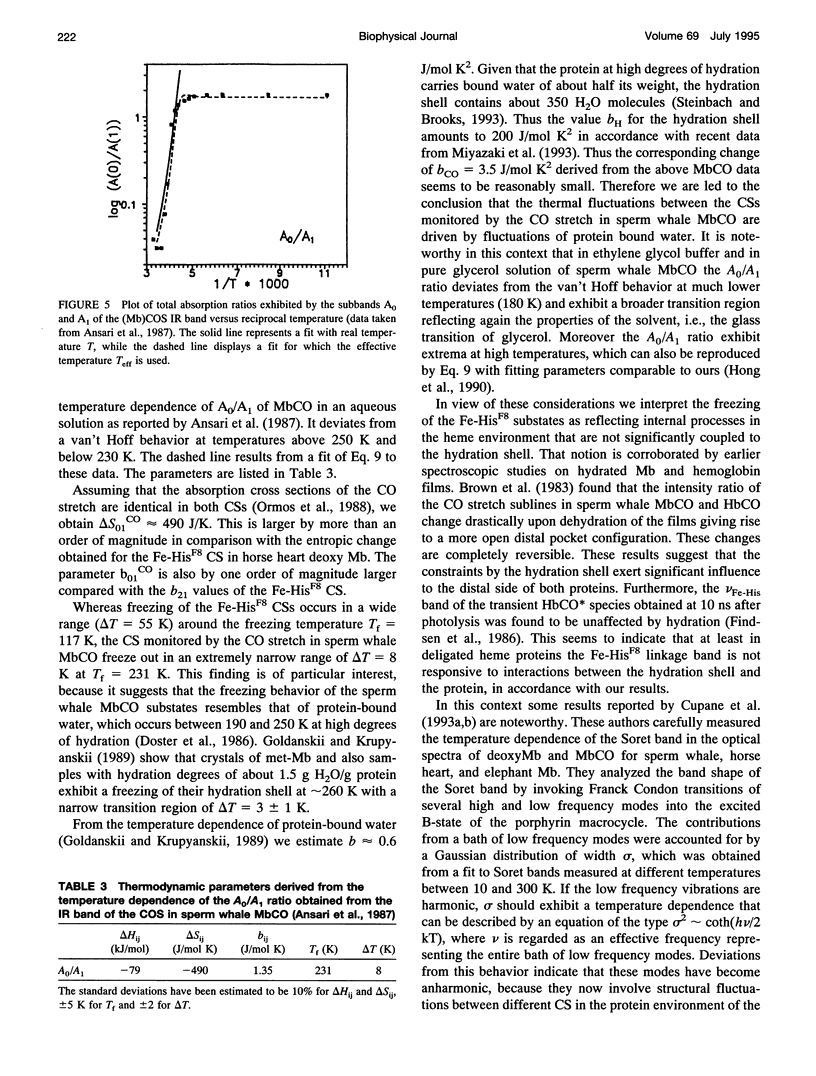
We have measured the VFe-His Raman band of horse heart deoxymyoglobin dissolved in an aqueous solution as a function of temperature between 10 and 300 K. The minimal model to which these data can be fitted in a statistically significant and physically meaningful way comprises four different Lorentzian bands with frequencies at 197, 209, 218, and 226 cm-1, and a Gaussian band at 240 cm-1, which exhibit halfwidths between 10 and 12.5 cm-1. All these parameters were assumed to be independent of temperature. The temperature dependence of the apparent total band shape's frequency is attributed to an intensity redistribution of the subbands at omega 1 = 209 cm-1, omega 2 = 218 cm-1, and omega 3 = 226 cm-1, which are assigned to Fe-N epsilon (HisF8) stretching modes in different conformational substrates of the Fe-HisF8 linkage. They comprise different out-of-plane displacements of the heme iron. The two remaining bands at 197 and 240 cm-1 result from porphyrin modes. Their intensity ratio is nearly temperature independent. The intensity ratio I3/I2 of the vFe-His subbands exhibits a van't Hoff behavior between 150 and 300 K, bending over in a region between 150 and 80 K, and remains constant between 80 and 10 K, whereas I2/I1 shows a maximum at 170 K and approaches a constant value at 80 K. These data can be fitted by a modified van't Hoff expression, which accounts for the freezing into a non-equilibrium distribution of substates below a distinct temperature Tf and also for the linear temperature dependence of the specific heat of proteins. The latter leads to a temperature dependence of the entropic and enthalpic differences between conformational substates. The fits to the intensity ratios of the vFe-His subbands yield a freezing temperature of Tf = 117 K and a transition region of delta T = 55 K. In comparison we have utilized the above thermodynamic model to reanalyze earlier data on the temperature dependence of the ratio Ao/A1 of two subbands underlying the infrared absorption band of the CO stretching vibration in CO-ligated myoglobin (A. Ansari, J. Berendzen, D. Braunstein, B. R. Cowen, H. Frauenfelder, M. K. Kong, I. E. T. Iben, J. Johnson, P. Ormos, T. B. Sauke, R. Scholl, A. Schulte, P. J. Steinbach, R. D. Vittitow, and R. D. Young, 1987, Biophys. Chem. 26:237-335). This yields thermodynamic parameters, in particular the freezing temperature (Tf = 231 K) and the width of the transition region (AT =8 K), which are significantly different from the corresponding parameters obtained from the above vFe-His data, but very close to values describing the transition of protein bound water from a liquid into an amorphous state. These findings and earlier reported data on the temperature dependence exhibited by the Soret absorption bands of various deoxy and carbonmonoxymyoglobins led us to the conclusion that the fluctuations between conformational substates of the heme environment in carbonmonoxymyoglobin are strongly coupled to motions within the hydration shell, whereas the thermal motions between the substates of the Fe-HisF8 linkage in deoxymyoglobin proceed on an energy landscape that is mainly determined by the intrinsic properties of the protein. The latter differ from protein fluctuations monitored by Mossbauer experiments ondeoxymyoglobin crystals which exhibit a strong coupling to the protein bound water and most probably reflect a higher tier in the hierarchical arrangement of substates and equilibrium fluctuations.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Astl G., Mayer E. Alkali cation effect on carbonyl-hemoglobin's and -myoglobin's conformer populations when exposed to freeze-concentration of their phosphate-buffered aqueous solutions. Biochim Biophys Acta. 1991 Oct 25;1080(2):155–159. doi: 10.1016/0167-4838(91)90143-n. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bosenbeck M., Schweitzer-Stenner R., Dreybrodt W. pH-induced conformational changes of the Fe(2+)-N epsilon (His F8) linkage in deoxyhemoglobin trout IV detected by the Raman active Fe(2+)-N epsilon (His F8) stretching mode. Biophys J. 1992 Jan;61(1):31–41. doi: 10.1016/S0006-3495(92)81813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein D. P., Chu K., Egeberg K. D., Frauenfelder H., Mourant J. R., Nienhaus G. U., Ormos P., Sligar S. G., Springer B. A., Young R. D. Ligand binding to heme proteins: III. FTIR studies of His-E7 and Val-E11 mutants of carbonmonoxymyoglobin. Biophys J. 1993 Dec;65(6):2447–2454. doi: 10.1016/S0006-3495(93)81310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. E., 3rd, Sutcliffe J. W., Pulsinelli P. D. Multiple internal reflectance infrared spectra of variably hydrated hemoglobin and myoglobin films: effects of globin hydration on ligand conformer dynamics and reactivity at the heme. Biochemistry. 1983 Jun 7;22(12):2914–2923. doi: 10.1021/bi00281a021. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Linkage of functional and structural heterogeneity in proteins: dynamic hole burning in carboxymyoglobin. Science. 1987 Oct 16;238(4825):373–376. doi: 10.1126/science.3659921. [DOI] [PubMed] [Google Scholar]
- Chavez M. D., Courtney S. H., Chance M. R., Kiula D., Nocek J., Hoffman B. M., Friedman J. M., Ondrias M. R. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe-Mn hybrid hemoglobins. Biochemistry. 1990 May 22;29(20):4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
- Chu K, Ernst RM, Frauenfelder H, Mourant JR, Nienhaus GU, Philipp R. Light-induced and thermal relaxation in a protein. Phys Rev Lett. 1995 Mar 27;74(13):2607–2610. doi: 10.1103/PhysRevLett.74.2607. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L., Hiltpold U. R., Winterhalter K. H., Yu W., Di Iorio E. E. Structure-dynamics-function relationships in Asian elephant (Elephas maximus) myoglobin. An optical spectroscopy and flash photolysis study on functionally important motions. Biophys J. 1993 Dec;65(6):2461–2472. doi: 10.1016/S0006-3495(93)81311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E. Protein dynamics: conformational disorder, vibrational coupling and anharmonicity in deoxy-hemoglobin and myoglobin. Eur Biophys J. 1993;21(6):385–391. doi: 10.1007/BF00185865. [DOI] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Findsen E. W., Simons P., Ondrias M. R. Response of the local heme environment of (carbonmonoxy)hemoglobin to protein dehydration. Biochemistry. 1986 Dec 2;25(24):7912–7917. doi: 10.1021/bi00372a019. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Gratton E. Protein dynamics and hydration. Methods Enzymol. 1986;127:207–216. doi: 10.1016/0076-6879(86)27017-2. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Campbell B. F., Noble R. W. A possible new control mechanism suggested by resonance Raman spectra from a deep ocean fish hemoglobin. Biophys Chem. 1990 Aug 31;37(1-3):43–59. doi: 10.1016/0301-4622(90)88006-e. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- Gilch H., Schweitzer-Stenner R., Dreybrodt W. Structural heterogeneity of the Fe(2+)-N epsilon (HisF8) bond in various hemoglobin and myoglobin derivatives probed by the Raman-active iron histidine stretching mode. Biophys J. 1993 Oct;65(4):1470–1485. doi: 10.1016/S0006-3495(93)81216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldanskii V. I., Krupyanskii Y. F. Protein and protein-bound water dynamics studied by Rayleigh scattering of Mössbauer radiation (RSMR). Q Rev Biophys. 1989 Feb;22(1):39–92. doi: 10.1017/s003358350000336x. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Hong M. K., Braunstein D., Cowen B. R., Frauenfelder H., Iben I. E., Mourant J. R., Ormos P., Scholl R., Schulte A., Steinbach P. J. Conformational substates and motions in myoglobin. External influences on structure and dynamics. Biophys J. 1990 Aug;58(2):429–436. doi: 10.1016/S0006-3495(90)82388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iben IE, Braunstein D, Doster W, Frauenfelder H, Hong MK, Johnson JB, Luck S, Ormos P, Schulte A, Steinbach PJ. Glassy behavior of a protein. Phys Rev Lett. 1989 Apr 17;62(16):1916–1919. doi: 10.1103/PhysRevLett.62.1916. [DOI] [PubMed] [Google Scholar]
- Leone M., Cupane A., Militello V., Cordone L. Thermal broadening of the Soret band in heme complexes and in heme-proteins: role of iron dynamics. Eur Biophys J. 1994;23(5):349–352. doi: 10.1007/BF00188658. [DOI] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. Temperature dependence of dynamics of hydrated myoglobin. Comparison of force field calculations with neutron scattering data. J Mol Biol. 1990 Oct 5;215(3):439–455. doi: 10.1016/s0022-2836(05)80363-8. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Mourant J. R., Braunstein D. P., Chu K., Frauenfelder H., Nienhaus G. U., Ormos P., Young R. D. Ligand binding to heme proteins: II. Transitions in the heme pocket of myoglobin. Biophys J. 1993 Oct;65(4):1496–1507. doi: 10.1016/S0006-3495(93)81218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Chu K., Frauenfelder H. Ligand binding to heme proteins: the effect of light on ligand binding in myoglobin. Biochemistry. 1994 Nov 15;33(45):13413–13430. doi: 10.1021/bi00249a030. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F. Correlation of protein dynamics with water mobility: Mössbauer spectroscopy and microwave absorption methods. Methods Enzymol. 1986;127:196–206. doi: 10.1016/0076-6879(86)27016-0. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- Persson BN, Ryberg R. Vibrational line shapes of low-frequency adsorbate modes: CO on Pt(111). Phys Rev B Condens Matter. 1989 Nov 15;40(15):10273–10281. doi: 10.1103/physrevb.40.10273. [DOI] [PubMed] [Google Scholar]
- Post F., Doster W., Karvounis G., Settles M. Structural relaxation and nonexponential kinetics of CO-binding to horse myoglobin. Multiple flash photolysis experiments. Biophys J. 1993 Jun;64(6):1833–1842. doi: 10.1016/S0006-3495(93)81554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers L., Chance B., Chance M., Campbell B., Friedman J., Khalid S., Kumar C., Naqui A., Reddy K. S., Zhou Y. Kinetic, structural, and spectroscopic identification of geminate states of myoglobin: a ligand binding site on the reaction pathway. Biochemistry. 1987 Jul 28;26(15):4785–4796. doi: 10.1021/bi00389a028. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Dasgupta S., Rousseau D. L. Cryogenic stabilization of myoglobin photoproducts. J Biol Chem. 1986 Oct 15;261(29):13704–13713. [PubMed] [Google Scholar]
- Schweitzer-Stenner R. Allosteric linkage-induced distortions of the prosthetic group in haem proteins as derived by the theoretical interpretation of the depolarization ratio in resonance Raman scattering. Q Rev Biophys. 1989 Nov;22(4):381–479. doi: 10.1017/s0033583500003164. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Bosenbeck M., Dreybrodt W. Raman dispersion spectroscopy probes heme distortions in deoxyHb-trout IV involved in its T-state Bohr effect. Biophys J. 1993 Apr;64(4):1194–1209. doi: 10.1016/S0006-3495(93)81485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srajer V., Champion P. M. Investigations of optical line shapes and kinetic hole burning in myoglobin. Biochemistry. 1991 Jul 30;30(30):7390–7402. doi: 10.1021/bi00244a005. [DOI] [PubMed] [Google Scholar]
- Srajer V., Reinisch L., Champion P. M. Investigation of laser-induced long-lived states of photolyzed MbCO. Biochemistry. 1991 May 21;30(20):4886–4895. doi: 10.1021/bi00234a008. [DOI] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Stavrov S. S. The effect of iron displacement out of the porphyrin plane on the resonance Raman spectra of heme proteins and iron porphyrins. Biophys J. 1993 Nov;65(5):1942–1950. doi: 10.1016/S0006-3495(93)81265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Brooks B. R. Protein hydration elucidated by molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9135–9139. doi: 10.1073/pnas.90.19.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff H. J., Kramm B., Hess G., Owerdieck C., Redhardt A. Rotational and translational water diffusion in the hemoglobin hydration shell: dielectric and proton nuclear relaxation measurements. Biophys J. 1993 Oct;65(4):1486–1495. doi: 10.1016/S0006-3495(93)81217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian WD, Sage JT, Srajer V, V, Champion PM. Relaxation dynamics of myoglobin in solution. Phys Rev Lett. 1992 Jan 20;68(3):408–411. doi: 10.1103/PhysRevLett.68.408. [DOI] [PubMed] [Google Scholar]


