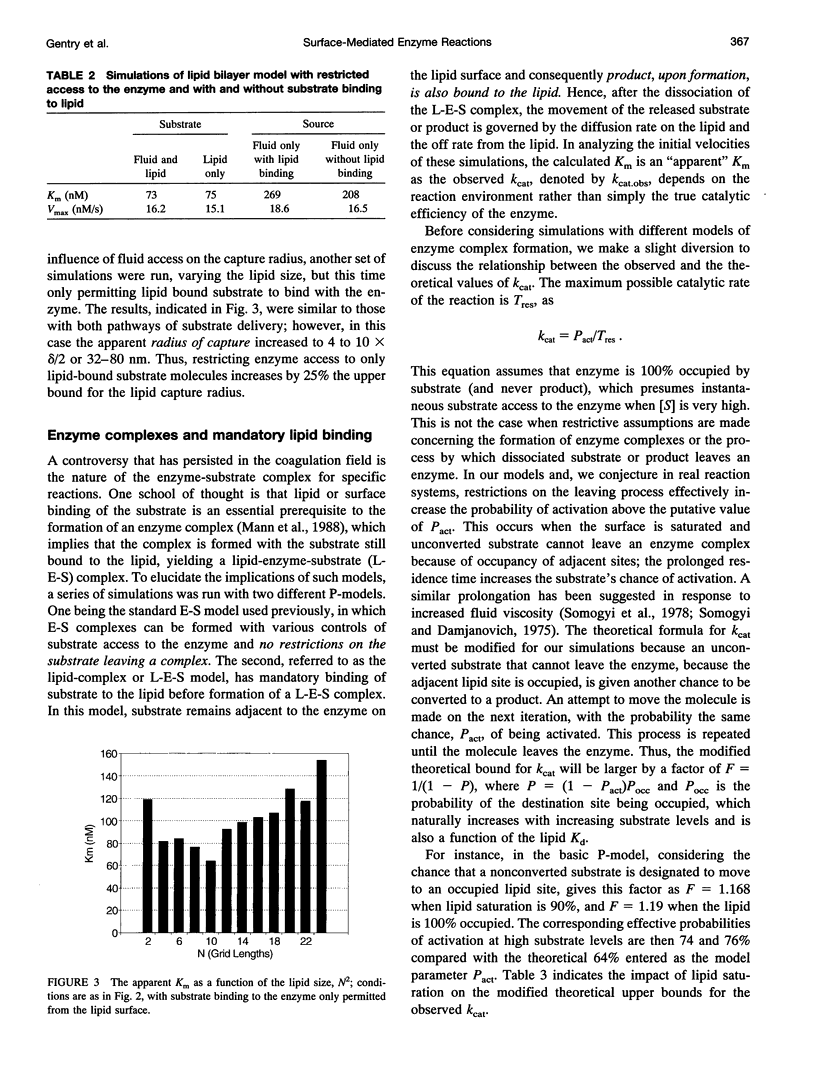
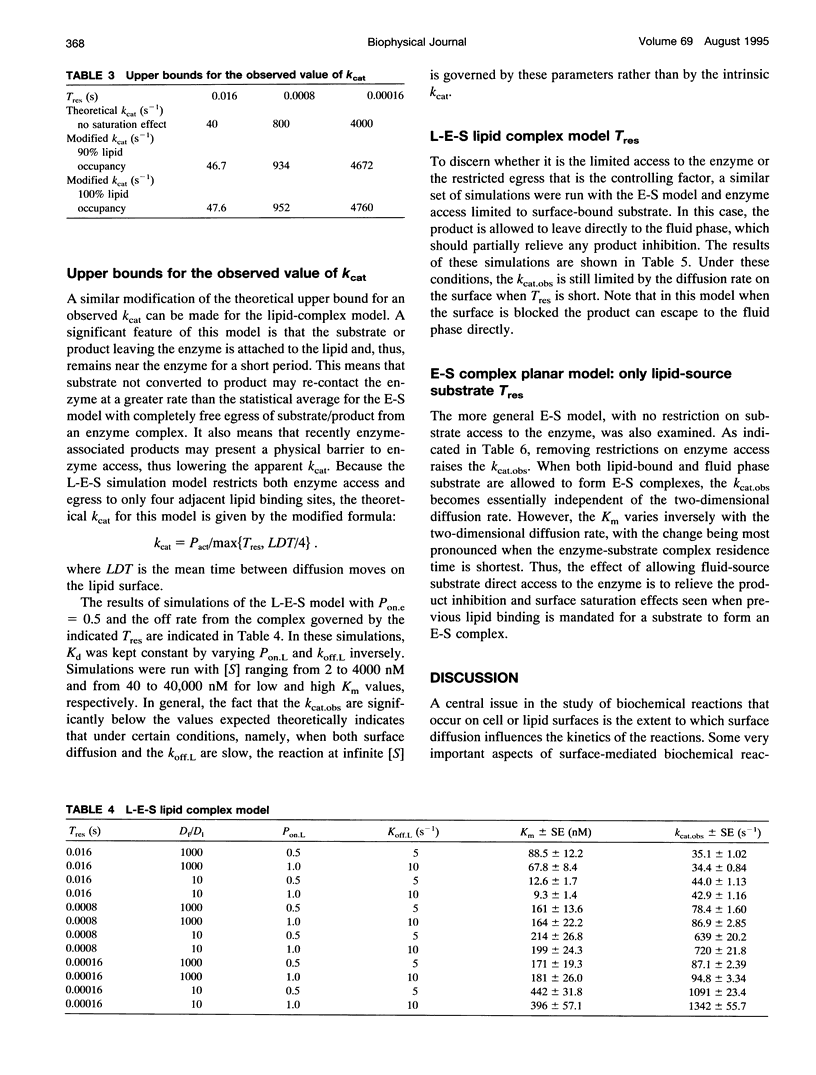
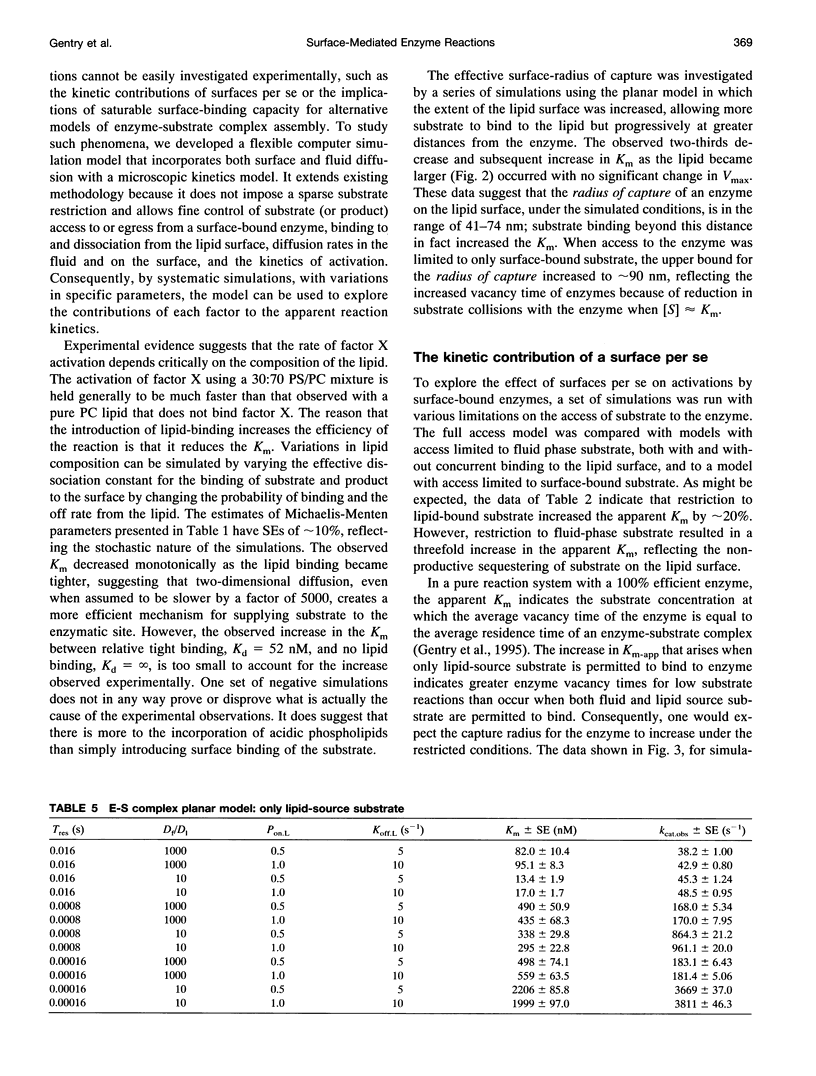
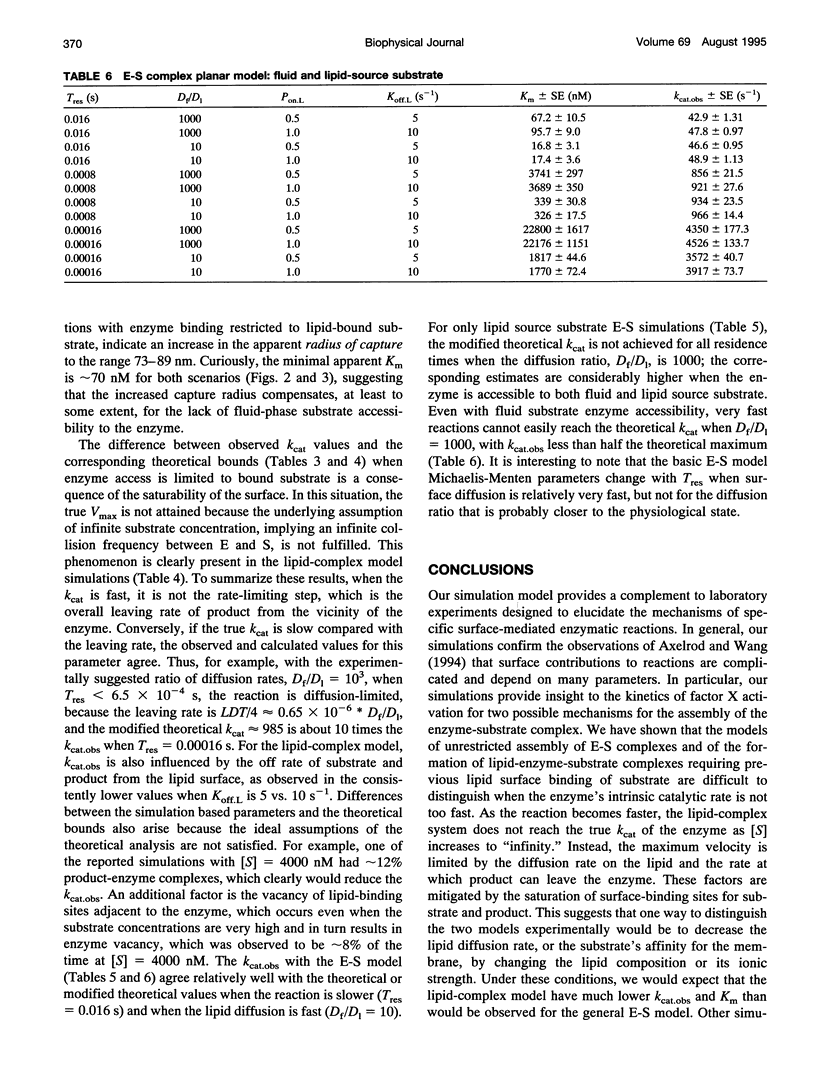
Abstract
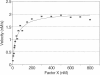
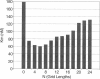
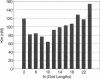
Blood coagulation proceeds via reactions in which zymogen coagulation factors are activated to proteases. An essential step is the activation of factor X by a complex of tissue factor and factor VIIa. This complex usually is studied using phospholipid vesicles into which tissue factor is inserted. Because factor X exists free in solution and bound to the lipid-surface, it is difficult to establish experimentally the kinetic contribution of surfaces. We therefore developed a stochastic model to simulate such reactions and generate initial velocity data from which Michaelis-Menten parameters are estimated. Simulated Km values decrease slightly when substrate binding to lipid is increased and by a factor of four when the rates of surface diffusion are increased to that of fluid phase-diffusion. Simulations with various size planar surfaces established an enzyme capture radius of 32-64 nm. Simulations with different modes of enzyme-substrate complex assembly show that if the true substrate is lipid-bound, under certain conditions, the true Kcat is not measured; rather, the product "leaving rate" from the complex is the rate-limiting step that is measured as substrate is taken to infinity. This model is applicable to any surface-bound enzyme reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Wang M. D. Reduction-of-dimensionality kinetics at reaction-limited cell surface receptors. Biophys J. 1994 Mar;66(3 Pt 1):588–600. doi: 10.1016/s0006-3495(94)80834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg O. G., von Hippel P. H. Diffusion-controlled macromolecular interactions. Annu Rev Biophys Biophys Chem. 1985;14:131–160. doi: 10.1146/annurev.bb.14.060185.001023. [DOI] [PubMed] [Google Scholar]
- Forman S. D., Nemerson Y. Membrane-dependent coagulation reaction is independent of the concentration of phospholipid-bound substrate: fluid phase factor X regulates the extrinsic system. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4675–4679. doi: 10.1073/pnas.83.13.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen P. L., Willems G. M., Hermens W. T. Production of thrombin by the prothrombinase complex is regulated by membrane-mediated transport of prothrombin. J Biol Chem. 1991 Jan 25;266(3):1379–1382. [PubMed] [Google Scholar]
- Hardt S. L. Rates of diffusion controlled reactions in one, two and three dimensions. Biophys Chem. 1979 Nov;10(3-4):239–243. doi: 10.1016/0301-4622(79)85012-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. L., Callahan P. J., Prendergast F. G., Nesheim M. E., Mann K. G. Lipid mobility in the assembly and expression of the activity of the prothrombinase complex. J Biol Chem. 1985 Mar 25;260(6):3604–3612. [PubMed] [Google Scholar]
- Krieg U. C., Isaacs B. S., Yemul S. S., Esmon C. T., Bayley H., Johnson A. E. Interaction of blood coagulation factor Va with phospholipid vesicles examined by using lipophilic photoreagents. Biochemistry. 1987 Jan 13;26(1):103–109. doi: 10.1021/bi00375a015. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Jones K. C., Mann K. G. Prothrombinase complex assembly. Kinetic mechanism of enzyme assembly on phospholipid vesicles. J Biol Chem. 1988 Mar 15;263(8):3823–3834. [PubMed] [Google Scholar]
- Mann K. G., Jenny R. J., Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Gentry R. An ordered addition, essential activation model of the tissue factor pathway of coagulation: evidence for a conformational cage. Biochemistry. 1986 Jul 15;25(14):4020–4033. doi: 10.1021/bi00362a006. [DOI] [PubMed] [Google Scholar]
- Nemerson Y. Tissue factor and hemostasis. Blood. 1988 Jan;71(1):1–8. [PubMed] [Google Scholar]
- Nesheim M. E., Tracy R. P., Mann K. G. "Clotspeed," a mathematical simulation of the functional properties of prothrombinase. J Biol Chem. 1984 Feb 10;259(3):1447–1453. [PubMed] [Google Scholar]
- Somogyi B., Damjanovich S. Relationship between the lifetime of an enzyme-substrate complex and the properties of the molecular environment. J Theor Biol. 1975 Jun;51(2):393–401. doi: 10.1016/0022-5193(75)90068-5. [DOI] [PubMed] [Google Scholar]
- Somogyi B., Karasz F. E., Trón L., Couchman P. R. The effect of viscosity on the apparent decomposition rate on enzyme--ligand complexes. J Theor Biol. 1978 Sep 21;74(2):209–216. doi: 10.1016/0022-5193(78)90072-3. [DOI] [PubMed] [Google Scholar]
- Wang D., Gou S. Y., Axelrod D. Reaction rate enhancement by surface diffusion of adsorbates. Biophys Chem. 1992 Jun;43(2):117–137. doi: 10.1016/0301-4622(92)80027-3. [DOI] [PubMed] [Google Scholar]
- Waxman E., Ross J. B., Laue T. M., Guha A., Thiruvikraman S. V., Lin T. C., Konigsberg W. H., Nemerson Y. Tissue factor and its extracellular soluble domain: the relationship between intermolecular association with factor VIIa and enzymatic activity of the complex. Biochemistry. 1992 Apr 28;31(16):3998–4003. doi: 10.1021/bi00131a015. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F. Membrane and lipid involvement in blood coagulation. Biochim Biophys Acta. 1978 Jul 31;515(2):163–205. doi: 10.1016/0304-4157(78)90003-5. [DOI] [PubMed] [Google Scholar]