Abstract
Individual subunits of protein phosphatase 2A (PP2A), protein phosphatase 4, and protein phosphatase 5 were knocked out in Drosophila Schneider 2 cells by using RNA interference. Ablation of either the scaffold (A) or catalytic (C) subunits of PP2A caused the disappearance of all PP2A subunits. Treating cells with double-stranded RNA targeting all four of the Drosophila PP2A regulatory subunits caused the disappearance of both the A and C subunits. The loss of PP2A subunits was associated with decreased protein stability indicating that only the heterotrimeric forms of PP2A are stable in intact cells. Ablation of total PP2A by using double-stranded RNA against either the A or C subunit, or specific ablation of the R2/B regulatory subunit, enhanced insulin-induced ERK activation. These results indicated that the R2/B subunit targets PP2A to the mitogen-activated protein (MAP) kinase cascade in Schneider 2 cells, where it acts as a negative regulator. A severe loss of viability occurred in cells in which total PP2A or both isoforms of the Drosophila R5/B56 subunit had been ablated. The reduced viability of these cells correlated with the induction of markers of apoptosis including membrane blebbing and stimulation of caspase-3-like activity. These observations indicated that PP2A has a powerful antiapoptotic activity that is specifically mediated by the R5/B56 regulatory subunits. In contrast to PP2A, ablation of protein phosphatase 4 caused only a slight reduction in cell growth but had no effect on MAP kinase signaling or apoptosis. Depletion of protein phosphatase 5 had no effects on MAP kinase, cell growth, or apoptosis.
The protein serine/threonine phosphatase 2A (PP2A) controls the phosphorylation of numerous proteins involved in cell signaling and is an important regulator of cell growth (1, 2). PP2A is the prototype of a subset of PP2A-like phosphatases that includes PP4, PP5, and PP6. The PP2A holoenzyme is a heterotrimer that consists of a core dimer, composed of a scaffold (A) and a catalytic subunit (C) that associates with a variety of regulatory subunits. Three families (R2/B, R3/PR72, and R5/B56) of PP2A regulatory subunits have been characterized (1, 2). The regulatory subunits have distinct properties and generate a diversity of PP2A holoenzymes. A current model for regulation of PP2A suggests that heterotrimers containing different regulatory subunits have distinct functions in vivo. Only limited support exists for this model. Genetic analysis has shown that the two regulatory subunits of Saccharomyces cerevisiae direct PP2A to distinct cellular functions (3). PP2A holoeznymes containing different regulatory subunits also have distinct properties in vitro (4). The functions of individual regulatory subunits in higher eukaryotes are poorly understood.
One characterized function of PP2A is the regulation of Ras-Raf-mitogen-activated protein (MAP) kinase signaling pathways. PP2A has both positive and negative effects on these pathways that depend on the cell type. PP2A can dephosphorylate and inactivate both MAP/ERK kinase (MEK) and extracellular signal-regulated kinase (ERK) family kinases in vitro (5–7). Treatment of cells with the PP2A-selective inhibitor, okadaic acid, causes activation of both MEK and ERK (8, 9). Incorporation of simian virus 40 small-tumor antigen into PP2A complexes inhibits PP2A-mediated dephosphorylation of MEK and ERK in vitro and causes activation of both kinases in intact cells (10). Activation of MEK and ERK by simian virus 40 small-tumor antigen correlates with loss of the R2/B subunit. These data all suggest that PP2A is a negative regulator of MAP kinase signaling. PP2A can associate with Raf, and PP2A-selective concentrations of okadaic acid suppress Raf activation in a mammalian macrophage cell line (11). Mutation of the R2/B subunit in Caenorhabditis elegans causes a decrease in Ras-mediated vulval induction (12). These later two observations suggest that PP2A can act as a positive regulator of Raf activation. Within the Ras-mediated photoreceptor development pathway in Drosophila, PP2A has a negative effect upstream of Raf, but a positive effect downstream of Raf (13). A strong possibility is that multiple actions of PP2A on MAP kinase signaling are mediated by distinct holoenzymes.
Indirect evidence supports a role for PP2A in promoting cell survival through inhibition of apoptosis. Treatment with okadaic acid activates apoptosis in many cell types (14–20). Although these observations suggest PP2A plays a role in preventing apoptosis, they are not conclusive because other phosphatases, including PP1, PP4, PP5, and PP6, are also inhibited by okadaic acid. PP2A associates with several proteins involved in apoptosis including Bcl-2 (21, 22), caspase-3 (23), and the adenovirus E4orf4 protein (24–27). Apoptosis induced by the adenovirus E4orf4 protein is associated with PP2A isoforms containing the R2/B- and R5/B56 regulatory subunits. It is not known whether PP2A is directly involved in other forms of apoptosis or if the antiapoptotic activity is mediated by a particular regulatory subunit.
Double-stranded RNA (dsRNA)-mediated RNA interference (RNAi) has proven to be a useful method for “knocking out” proteins expressed in C. elegans and Drosophila melanogaster (28–32). In contrast to mammals and yeast, Drosophila have a single gene encoding each of the PP2A A (33), C (34), and R2/B subunits (35), two genes encoding distinct isoforms of the R5/B56 subunit homolog (36), and a single gene for a R3/PR72 homolog. The limited number of phosphatase isoforms makes Drosophila an attractive organism for PP2A gene knockout studies. Drosophila also has a single gene encoding PP4 (37) and PP5 (38). We used RNAi to ablate PP2A, PP4, and PP5 from Drosophila Schneider 2 (S2) cells to examine the roles of these proteins in cellular signaling. We also used RNAi to ablate individual PP2A regulatory subunits to test whether they have unique functions. Ablation of individual PP2A subunits revealed a mechanism controlling the assembly of PP2A oligomers by means of protein stability. The data show that PP2A plays a negative role in regulation of a MAP kinase pathway in S2 cells. Loss of PP2A also caused apoptosis, demonstrating that this phosphatase is crucial for cell survival. Consistent with the model in which regulatory subunits target PP2A to distinct functions, we show that regulation of MAP kinase signaling and the antiapoptotic actions of PP2A are mediated by distinct regulatory subunits.
Materials and Methods
Production of Double-Stranded RNA.
Drosophila cDNA clones corresponding to PP2A subunits, PP4 and PP5, were purchased from Research Genetics (Birmingham, AL). The cDNA clones were used as templates in PCR reactions where both the sense and antisense primers contain a T7 polymerase binding site at the 5′ end. A 700-bp region of the Drosophila PP2A R3/PR72 regulatory subunit was amplified by PCR from a 48-h embryo cDNA library (kindly provided by Denis McKearin, University Texas Southwestern Medical Center). PCR products (≈500–700 bp) were cleaned in Microcon spin concentrators (Millipore) and resuspended at a final concentration of 200 ng/μl in sterile water. One microgram of each PCR product was used to synthesize dsRNA with a large-scale T7 transcription kit from Novagen. To anneal the single-stranded RNA, samples were incubated for 30 min at 65°C and cooled to room temperature. The pEGFP-C3 vector (CLONTECH) was used as a template to produce a control dsRNA corresponding to enhanced green fluorescent protein (EGFP).
Cell Culture and dsRNA Treatment.
Serum-free medium adapted Schneider S2 cells (D.Mel-2) and Drosophila serum-free medium were purchased from Life Technologies (Rockville, MD). S2 cells were maintained in Drosophila serum-free medium supplemented with 16.5 mM L-glutamine and 46 μg/ml gentamicin at 28°C in T-75 flasks. For dsRNA treatments, 1 ml of S2 cell suspension (1 × 106 cells/ml) in Drosophila expression system expression medium (Invitrogen) was mixed with a final concentration of 15 μg/ml of the specified dsRNA and plated in 35-mm culture dishes. For cells that were treated with more than one dsRNA, the final concentration of each dsRNA was 15 μg/ml. Cells were incubated for 3 h at 28°C before adding 2 ml of Drosophila serum-free medium. The dsRNA treatment was performed for 3 days or as indicated in the figure legends.
Cell Extraction and Western Blotting.
The medium was aspirated, and cells were lysed for 10 min at 4°C in RIPA buffer (20 mM Tris, pH 8.0/150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS/0.2 mM sodium vanadate/10 mM sodium fluoride/0.4 mM EDTA/10% glycerol). Lysates were clarified by centrifugation at 15,000 rpm for 10 min in a microcentrifuge, and samples were normalized according to protein concentrations determined by the bicinchoninic acid protein assay (Pierce). Total protein (30–60 μg) was resolved by SDS/PAGE, and individual proteins were detected by Western blotting. The immunoblots were probed with 0.1% F725 antiserum against the PP2A A subunit (4); 0.1% C-20 antiserum against the PP2A C subunit (Affinity Bioreagents, Neshanic Station, NJ); 0.1% M878 antiserum against the PP2A B56–1 subunit (4); 0.1% PP5 antiserum (kindly provided by Michael Chinkers, University of South Alabama, Mobile, AL) (39); 0.05% affinity-purified anti-PP4 c-int polyclonal antibody (kindly provided by Brian Wadzinski, Vanderbilt University, Nashville, TN) (40); 0.02% MAPK-YT monoclonal antibody against activated ERK-1&2 (Sigma); or 0.01% polyclonal anti-ERK 1&2 (Sigma). A peptide corresponding to amino acids 409–423 of the Drosophila R2/B subunit was sent to Capralogics (Hardwick, MA) for production of the R2/B (409–423) rabbit polyclonal antibody, which was used at 0.1% to probe immunoblots. Bound antibodies were detected with the appropriate horseradish peroxidase-conjugated secondary antibody and visualized by enhanced chemiluminescence.
Reverse Transcription (RT)-PCR.
The SUPERSCRIPT One-Step RT-PCR with PLATINUM Taq kit was purchased from Invitrogen. S2 cells in 35-mm dishes were treated with dsRNA for 72 h, and RT-PCR was performed as described by the manufacturer. Primers used for RT-PCR were identical to those used to make the PCR products used for dsRNA synthesis. Five microliters (1/10) of the reaction was resolved on a 1% agarose gel.
Caspase Assays.
The ApoAlert Caspase-3 and Caspase-8 assay kits were purchased from CLONTECH. S2 cells in 35-mm dishes were treated with dsRNA for 72 h, harvested, and assayed for caspase activity as described by the manufacturer. Caspase-3-like and caspase-8-like activity were determined by reading the absorbance at 405 nm in a 96-well plate reader and adjusted for the protein concentration of each sample. The results were normalized to the EGFP control sample. Values shown represent the mean ± SE from four experiments.
Microscopy.
Cells were photographed by using phase-contrast optics at ×32 magnification with a Zeiss Axiovert 35 microscope and ONCOR image software. Images were imported into ADOBE PHOTOSHOP, and cell number and the number of apoptotic (blebbing) cells were counted manually.
Results and Discussion
Selective Ablation of PP2A, PP4, and PP5 with RNAi.
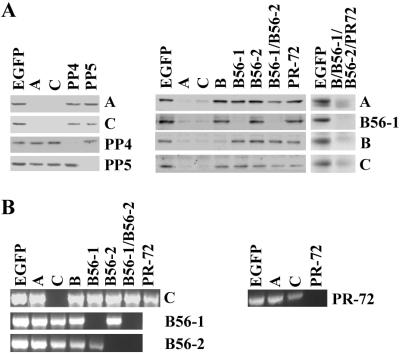
Although studies with okadaic acid and other inhibitors have implicated PP2A in multiple signaling pathways, it has been difficult to assign specific functions to this phosphatase. Okadaic acid also inhibits PP1 at higher concentrations, and PP4, PP5, and PP6 are inhibited by the same concentrations that block PP2A activity (41). To dissect the roles of individual phosphatases in cellular signaling, we used RNAi to delete selectively PP2A, PP4, and PP5 (42–44). Drosophila S2 cells were treated with dsRNA targeted to individual PP2A subunits, PP4, or PP5, and the effects on protein levels were determined by Western blotting (Fig. 1A). Treating cells with A- or C-subunit dsRNA for 72 h reduced the corresponding subunits to barely detectable levels. Treatment with either A- or C-subunit dsRNA alone caused a loss of all of the PP2A subunits. Treatment with PP4 or PP5 dsRNA reduced the level of the corresponding protein but had no effect on the levels of the other phosphatase, or on PP2A. When cells were treated with individual dsRNA targeting the R2/B, R3/PR72, R5/B56–1, or R5/B56–2 subunits, each of the targeted proteins was ablated, but little effect was seen on the other regulatory subunits. In general, ablation of individual regulatory subunits had little effect on the levels of the A and C subunits. The exception was the R5/B56–1 and -2 double knockout where a significant decrease in the A and C subunits was observed. Ablation of all four regulatory subunits resulted in loss of the A and C subunits. Treatment of S2 cells with a control dsRNA targeted to EGFP had no effect on the levels of any PP2A subunits, PP4, or PP5.
Figure 1.
RNAi knockout of PP2A, PP4, and PP5 in Drosophila S2 cells. (A) Drosophila S2 cells were incubated in the presence of dsRNA corresponding to EGFP or the phosphatase subunits indicated at the top. After 72 h, cells were lysed in RIPA buffer containing 0.2 mM Na2VO4 and 10 mM NaF2, and lysates were clarified by centrifugation. Equal amounts of protein (30–60 μg) were separated on SDS-polyacrylamide gels, and detected by Western blotting with antibodies against the proteins indicated on the right. (B) Drosophila S2 cells were incubated in the absence or presence of dsRNA corresponding to EGFP or the phosphatase subunits indicated at the top. After 72 h, RT-PCR was performed as described under Materials and Methods, with primers against the phosphatase subunits indicated at the right. Individual reactions (5 μl of the final reaction mixture) were resolved on a 1% agarose gel and detected by staining with ethidium bromide. Results shown are representative of three to seven experiments.
Because antibodies against the Drosophila R5/B56–2 and R3/PR72 subunits were not available, the effectiveness of dsRNAs targeting these subunits was assessed by RT-PCR. Fig. 1B shows that treating S2 cells with R5/B56–2 and R3/PR72 dsRNA caused a significant reduction in the mRNA for these subunits. Treatment of cells with C-subunit dsRNA caused a complete loss of detectable C-subunit mRNA but had no effect on other PP2A subunit mRNAs. The loss of C-subunit mRNA in dsRNA-treated cells was consistent with the significant reduction in C-subunit protein levels (Fig. 1A). Treating cells with EGFP dsRNA had no effect on the level of PP2A subunit mRNAs.
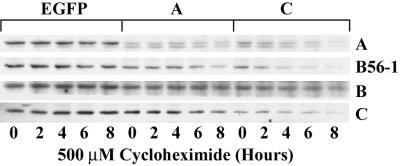
The data in Fig. 1A showed that knocking out either the A or C subunits of PP2A caused the loss of all of the other PP2A subunits. To determine whether the loss of subunits in dsRNA-treated cells was caused by decreased stability, cells were treated with cycloheximide to block new protein synthesis, and the decay in the levels of PP2A subunits was monitored by Western blotting. The loss of proteins in dsRNA-treated S2 cells is usually maximal after 3 days (28; our observations). To examine protein stability, cells were treated with dsRNA for 48 h before addition of cycloheximide to ensure that protein levels had not dropped below the limit of detection. In control cells treated with EGFP dsRNA, the levels of A- and C-subunit protein were stable for up to 8 h after addition of cycloheximide (Fig. 2, EGFP). When cells were treated with A-subunit dsRNA a time-dependent decrease occurred in the levels of C-subunit and R5/B56–1 protein after cycloheximide addition. A time-dependent decrease in A subunit and R5/B56–1 protein also occurred when cells were treated with C-subunit dsRNA. The R2/B-subunit protein level decreased only modestly in cells treated with A- or C-subunit dsRNA for 48 h, and the loss of the R2/B subunit in the presence of cycloheximide was much slower than the other subunits, which suggested that the R2/B subunit was more stable than the A, C, or R5/B56–1 subunits. In cells treated with C-subunit dsRNA, a marked decrease occurred in the stability of the C-subunit protein. The reason for the rapid loss of C-subunit protein under these conditions is not clear. The levels of C-subunit present before cycloheximide addition are considerably lower in cells treated with C-subunit dsRNA. The level of C subunit may be close to the limit of detection by the antibody, and any further decrease would be magnified.
Figure 2.
Loss of the PP2A holoenzyme destabilizes individual PP2A subunits. Drosophila S2 cells were incubated in the presence of EGFP, A- or C-subunit dsRNA for 48 h (Right). The cells were then treated with 500 μM cycloheximide for the times indicated and lysed in RIPA buffer containing 0.2 mM Na2VO4 and 10 mM NaF2. Lysates were clarified by centrifugation, and equal amounts of proteins (50 μg) were resolved by SDS-polyacrylamide gel electrophoresis and detected by Western blotting with antibodies against the A, C, R2/B, or R5/B56–1 subunits as indicated at the right of each row. Results shown are representative of three experiments.
The data in Figs. 1 and 2 show that RNAi is an effective method for knocking out protein phosphatases in Drosophila S2 cells. The data provide new insights into mechanisms controlling the distribution of PP2A holoenzymes. In addition to potential autoregulation of the C subunit at the level of translation (45), our results suggest that PP2A is autoregulated at the posttranslational level to control the amounts of free subunits. The results in Figs. 1A and 2 are most consistent with a model in which individual PP2A subunits have low intrinsic stability. Assembly of the subunits into heterotrimeric complexes increases the stability of the individual proteins. Rapid degradation of free subunits may be a mechanism to protect the cell from deleterious effects of unregulated forms of PP2A. Attempts to overexpress the PP2A catalytic subunit in mammalian cells have been largely unsuccessful. Although this failure may be partly due to translational repression (45), our data argue that a major factor in limiting expression is rapid degradation of the free subunit. Depleting all four of the PP2A regulatory subunits led to loss of both the A and C subunit (Fig. 1A), which suggests that the AC dimer is unstable when not associated with one of the regulatory subunits. The presence of a pool of free AC dimers has been suggested by analysis of cell extracts (46, 47). In contrast, our data imply that little free dimer is present in vivo. Although a transient population of free AC dimer may exist, it seems unlikely that this form could accumulate to significant levels. The substantial amounts of AC dimer present in vitro may be generated during cell lysis by dissociation of PP2A holoenzymes.
PP2A Is a Negative Regulator of MAP Kinase Signaling in S2 Cells.
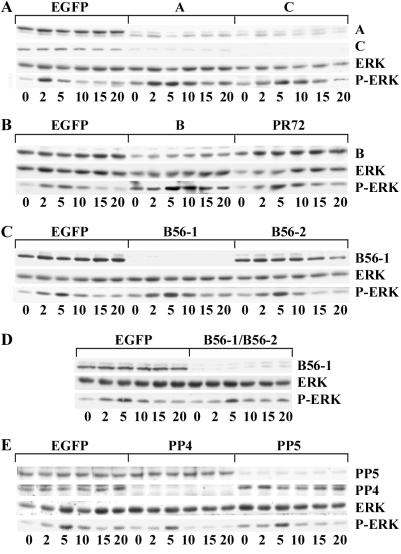
RNAi was used to examine the role of PP2A regulatory subunits in regulating MAP kinase activation in Drosophila S2 cells. Stimulation of the Drosophila insulin receptor activates MAP kinase in S2 cells in a Ras- and Raf-dependent manner (28, 48). Addition of insulin to untreated S2 cells resulted in a transient activation of MAP kinase activity that peaked at 5 min and returned to control values by 15 min (Fig. 3). Depletion of PP2A by using A- or C-subunit dsRNA caused enhanced activation of MAP kinase, especially at the 5- to 15-min time points (Fig. 3A). In contrast, depletion of PP4 or PP5 had little effect on insulin-stimulated MAP kinase activation (Fig. 3E). To test whether the actions of PP2A on MAP kinase signaling in S2 cells depended on a specific regulatory subunit, individual subunits were knocked out with RNAi. Loss of the R2/B subunit resulted in enhanced MAP kinase activation similar to that observed with the A- or C-subunit knockouts (Fig. 3B). In contrast, ablation of any of the other regulatory subunits had little effect on insulin-stimulated MAP kinase activation (Fig. 3 B–D).
Figure 3.
PP2A negatively regulates insulin-dependent ERK activation. Drosophila S2 cells were incubated in the presence of dsRNA targeted to EGFP or to the specific phosphatase subunits indicated across the tops of A–E. After 72 h, cells were treated with 10 μg/ml insulin for the minutes indicated at the bottoms of A–E and lysed in RIPA buffer containing 0.2 mM Na2VO4 and 10 mM NaF2. Lysates were clarified by centrifugation, and equal amounts of protein (50 μg) were resolved by SDS/PAGE. Proteins were transferred to nitrocellulose membranes and Western blotted with antibodies against the PP2A-A, -C, -R2/B, -R5/B56–1, PP4, or PP5 as indicated at the right of each row. MAP kinase activation was detected by blotting with antibodies specific for the phosphorylated forms of ERK-1 and -2 (P-ERK). The amount of MAP kinase in each sample was determined by blotting with an antibody that recognizes both the phosphorylated and nonphosphorylated forms of ERK-1 and ERK-2. Results shown are representative of three to five experiments for each dsRNA treatment.
Enhanced ERK activation in cells depleted of PP2A demonstrates that PP2A is a negative regulator of the MAP kinase pathway in Drosophila S2 cells. A role for PP2A in Drosophila Ras-mediated signaling pathways is consistent with previous studies on photoreceptor development (11). A predominant role of the Drosophila R2/B subunit in Ras-dependent signaling is supported by a study showing that decreased R2/B-subunit levels caused defects in photoreceptor development (49). PP2A can play both positive and negative roles in Ras-mediated signaling (11). Multiple actions of PP2A within the same pathway may be due to different PP2A holoenzymes acting at different steps. Although we cannot rule out a positive role for PP2A, our data indicate that the predominant action of PP2A in insulin-mediated MAP kinase activation in S2 cells is as a negative regulator. PP2A is also a negative regulator of MAP kinase signaling initiated by the epidermal growth factor receptor in mammalian CV-1 cells (8). Like S2 cells, the effects of PP2A in CV-1 cells seem to be mediated by the R2/B subunit.
Several potential targets for PP2A in insulin-stimulated pathways lead to ERK activation. Prolonged activation of ERK in PP2A- or R2/B-deficient cells could be caused by enhanced stimulation of upstream signals or a decreased rate of ERK dephosphorylation. PP2A dephosphorylates and inactivates ERK in vitro (50) and is responsible for the rapid phase of ERK inactivation (7). Insulin-dependent activation of ERK in S2 cells depends on the Drosophila MEK homolog DSOR1 (27), and PP2A also dephosphorylates and inactivates MEK in vitro (51). The effects of depleting PP2A are consistent with a role in the direct dephosphorylation of either MEK or ERK. The R2/B subunit acts as a positive regulator of Ras-mediated signal transduction in C. elegans. Genetic analysis suggests that R2/B acts downstream of Ras but upstream of Raf in the vulval induction pathway (12). PP2A can associate with Raf, and PP2A-selective concentrations of okadaic acid suppress Raf activation in a mammalian macrophage cell line (9). In contrast, PP2A acts as a negative regulator of Raf in Drosophila photoreceptor development (11). If Drosophila Raf is a target of PP2A, our data would indicate that it negatively regulates Raf activity. Finally, the effects of PP2A on ERK activation could be mediated by an alternative pathway. Simian virus 40 small-tumor antigen activates MEK in mammalian cells by pathways that use phosphatidylinositol-3-kinase and atypical isoforms of protein kinase C (52). A similar pathway may exist in S2 cells because insulin also stimulates phosphatidylinositol-3-kinase in S2 cells (27).
Loss of the R5/B56 Subunits of PP2A Induces Apoptosis.
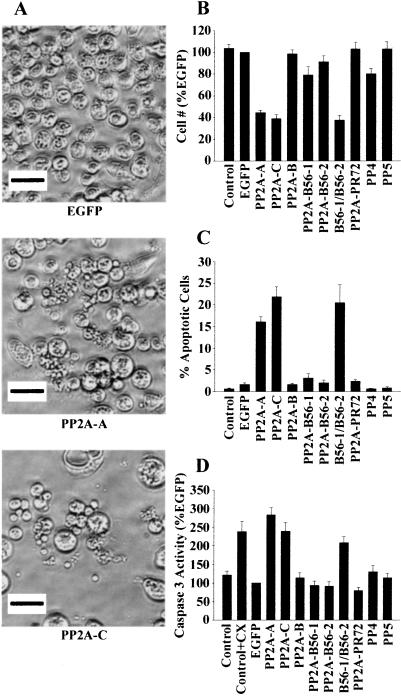
We noted a striking decrease in S2 cell viability in some RNAi-treated cells. Treating cells for 3 days with A-, C-, or both R5/B56-subunit dsRNAs caused a 65–70% decrease in the number of cells (Fig. 4 A and B). RNAi depletion of PP5 had no effect on cell number, whereas depletion of PP4 reduced cell growth by 20%. One explanation for the dramatic decrease in the viability of dsRNA-treated cells was the induction of apoptosis. A common method to identify apoptotic S2 cells is the characteristic membrane blebbing that occurs in the later stages of apoptosis (53). In asynchronous cells undergoing active apoptosis, the characteristic features of membrane blebbing, DNA fragmentation, and chromosome condensation occur in about 15% of the population at any given time (54). Treating S2 cells with A- or C-subunit dsRNA caused membrane blebbing in 15–20% of the remaining cells (Fig. 4 A and C). No significant increases in blebbing cells were observed in S2 cells treated with the EGFP control dsRNA, or dsRNA corresponding to PP4 or PP5. The increase in the number of blebbing cells in PP2A-depleted cells was highly significant when compared with untreated or EGFP dsRNA-treated cells.
Figure 4.
Depleting the PP2A R2/B56 regulatory subunit reduces Drosophila S2 cell number and activates apoptosis. (A–C) Drosophila S2 cells were incubated in the absence (Control) or presence of dsRNA targeted to EGFP or to the specific phosphatase subunits indicated at the bottom of A–C. After 72 h, cells were photographed with phase-contrast optics. (Magnification ×32.) (A) Depleting the PP2A-A or -C subunit reduces cell number and increases membrane blebbing. Representative photographs are shown for cells treated with dsRNAs targeting EGFP or the PP2A A or C subunits. (Bar = 10 μm.) (B) Depleting the PP2A-A , -C subunit, or both R2/B56 regulatory subunits reduces Drosophila S2 cell number. dsRNA-treated cells from ×32 magnification fields were counted, and values represent the mean ± SE from four experiments treated with two different batches of dsRNA where at least three fields from each treatment were scored. (C) Depleting the PP2A-A, -C subunit, or both R2/B56 regulatory subunits induces membrane blebbing in Drosophila S2 cells. dsRNA-treated cells from magnification fields ×32 were scored for total cells and apoptotic (membrane blebbing) cells, and % apoptotic values represent the mean ± SE from three experiments treated with two different batches of dsRNA where at least three fields from each treatment were scored. (D) Depleting the PP2A-A, -C subunit, or both R2/B56 regulatory subunits stimulates caspase-3-like activity. Drosophila S2 cells were incubated in the absence or presence of 500 μM cycloheximide (CX) for 3 h, or dsRNA targeted to EGFP or to the specific phosphatase subunits indicated at the bottom for 3 days. Caspase assays were performed as described under Materials and Methods. Values represent the mean caspase-3 activity ± SE from four experiments. Caspase activity was normalized for protein concentration and expressed as a percent of EGFP dsRNA-treated cells.
To provide further evidence that depletion of PP2A caused apoptosis, caspase activity was measured. S2 cells were treated with dsRNA targeting individual phosphatases and harvested 3 days later. Cell lysates were used to assay caspase activity by using a colorimetric assay that measures cleavage of p-nitroanaline from a peptide substrate specific for caspase-3. Control cells were also treated for 4 h with cycloheximide, a well characterized inducer of apoptosis. Depletion of PP2A with A- or C-subunit dsRNA caused a 2- to 3-fold increase in caspase-3-like activity (Fig. 4D). The level of caspase-3-like activity in PP2A-depleted cells was equivalent to that seen with cycloheximide treatment. In contrast, depletion of PP4 or PP5 did not cause a significant increase in caspase-3-like activity. When cell lysates were assayed with a caspase-8-specific substrate, any of the dsRNAs or cycloheximide had little effect on activity (data not shown).
Individual PP2A regulatory subunits were knocked out with RNAi to determine which of the Drosophila subunits were involved in the apoptotic response. Treating S2 cells for 3 days with dsRNA targeting the R2/Bα, R5/B56–1, R5/B56–2, or R3/PR72 subunits had no effect on viability, membrane blebbing, or caspase activity (Fig. 4 B–D). When both the R5/B56–1 and -2 subunits were knocked out with RNAi, cell number was decreased and the percentage of blebbed cells increased to the same levels seen in the A- and C-subunit knockouts (Fig. 4 B and C). Caspase-3-like activity was increased to levels comparable with PP2A-depleted or cyclocheximide-treated cells in R5/B56 double-knockout cells (Fig. 4D). These results show that the R5/B56 subunits, and not other PP2A regulatory subunits, have a specific function in preventing apoptosis. The results also show that the antiapoptotic function of R5/B56–1 and R5/B56–2 are redundant, because the presence of either subunit alone is sufficient to prevent apoptosis.
The activity of serine/threonine phosphatase inhibitors as potent inducers of apoptosis has been documented in a variety of cell types (20, 55, 56). Because the serine/threonine phosphatase inhibitors that cause apoptosis act on both PP1- and PP2A-like enzymes, it has been difficult to determine which phosphatase is involved. Although other phosphatases may play a role, our data show that depletion of a single family of PP2A regulatory subunits in S2 cells is sufficient to induce apoptosis. This result implies that PP2A activity is critical for cell survival and suggests that the apoptotic actions of serine/threonine phosphatase inhibitors are due to inhibition of PP2A. The rapid induction of apoptosis in mammalian cells by serine/threonine phosphatase inhibitors is associated with increased protein phosphorylation and depends on activation of caspase-3 (55). Apoptosis induced by dsRNA knockout of PP2A correlated with activation of protease activity toward a caspase-3 substrate peptide. Drosophila contains three caspases (DRICE, DCP-1, and DECAY) that are highly homologous to mammalian caspase-3 (57). Each of these fly caspases cleaves caspase-3 substrates. Although our assays cannot distinguish between these enzymes, the essential role of DRICE in S2 cell apoptosis (58) suggests that loss of PP2A, or of its R5/B56 subunits, leads to activation of DRICE. These results establish a critical role for PP2A in cell survival. Additional studies are needed to determine the signaling pathways and components of the apoptotic machinery that are targeted by R5/B56-containing forms of PP2A.
Acknowledgments
We thank Michael White and John Abrams for helpful discussions and critical review of this manuscript. This work was supported by Grant GM49505 from the National Institutes of Health (to M.C.M.). A.M.S. is a recipient of National Research Service Award GM20530 from the National Institutes of Health.
Abbreviations
- PP2A
protein phosphatase 2A
- dsRNA
double-stranded RNA
- RNAi
RNA-mediated interference
- S2
Schneider 2
- EGFP
enhanced green fluorescent protein
- MAP
mitogen-activated protein
- ERK
extracellular signal-regulated kinase
- MEK
MAP/ERK kinase
- RT
reverse transcription
References
- 1.Virshup D M. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 2.Janssens V, Goris J. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Boguslawski G, Zitomer R S, DePaoli-Roach A A. J Biol Chem. 1997;272:8256–8262. doi: 10.1074/jbc.272.13.8256. [DOI] [PubMed] [Google Scholar]
- 4.Kamibayashi C, Estes R, Lickteig R L, Yang S-I, Craft C, Mumby M C. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 5.Haccard O, Jessus C, Cayla X, Goris J, Merlevede W, Ozon R. Eur J Biochem. 1990;192:633–642. doi: 10.1111/j.1432-1033.1990.tb19270.x. [DOI] [PubMed] [Google Scholar]
- 6.Moran M F, Koch C A, Anderson D, Ellis C, England L, Martin G S, Pawson T. Proc Natl Acad Sci USA. 1990;87:8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi D R, Gomez N, Moorhead G, Lewis T, Keyse S M, Cohen P. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- 8.Gause K C, Homma M K, Licciardi K A, Seger R, Ahn N G, Peterson M J, Krebs E G, Meier K E. J Biol Chem. 1993;268:16124–16129. [PubMed] [Google Scholar]
- 9.Sonoda Y, Kasahara T, Yamaguchi Y, Kuno K, Matsushima K, Mukaida N. J Biol Chem. 1997;272:15366–15372. doi: 10.1074/jbc.272.24.15366. [DOI] [PubMed] [Google Scholar]
- 10.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 11.Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings B A, Dilworth S M, Mischak H, Kolch W, Baccarini M. J Biol Chem. 2000;275:22300–22304. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- 12.Sieburth D S, Sundaram M, Howard R M, Han M. Genes Dev. 1999;13:2562–2569. doi: 10.1101/gad.13.19.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassarman D A, Solomon N M, Chang H C, Karim F D, Therrien M, Rubin G M. Genes Dev. 1996;10:272–278. doi: 10.1101/gad.10.3.272. [DOI] [PubMed] [Google Scholar]
- 14.Jensen P H, Fladmark K E, Gjertsen B T, Vintermyr O K. Br J Cancer. 1999;79:1685–1691. doi: 10.1038/sj.bjc.6690269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajesh D, Schell K, Verma A K. Mol Pharmacol. 1999;56:515–525. doi: 10.1124/mol.56.3.515. [DOI] [PubMed] [Google Scholar]
- 16.von Zezschwitz C, Vorwerk H, Tergau F, Steinfelder H J. FEBS Lett. 1997;413:147–151. doi: 10.1016/s0014-5793(97)00896-x. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Shay J W, Wright W E, Mumby M C. J Biol Chem. 1997;272:15220–15226. doi: 10.1074/jbc.272.24.15220. [DOI] [PubMed] [Google Scholar]
- 18.Thiery J P, Blazsek I, Legras S, Marion S, Reynes M, Anjo A, Adam R, Misset J L. Hepatology. 1999;29:1406–1417. doi: 10.1002/hep.510290534. [DOI] [PubMed] [Google Scholar]
- 19.Lim I K, Park T J, Park S C, Yoon G, Kwak C S, Le M S, Song K Y, Choi Y K, Hyun B H. Int J Cancer. 2001;91:32–40. doi: 10.1002/1097-0215(20010101)91:1<32::aid-ijc1004>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Gjertsen B T, Doskeland S O. Biochim Biophys Acta. 1995;1269:187–199. doi: 10.1016/0167-4889(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 21.Ruvolo P P, Deng X, Ito T, Carr B K, May W S. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 22.Deng X M, Ito T, Carr B, Mumby M, May W S. J Biol Chem. 1998;273:34157–34163. doi: 10.1074/jbc.273.51.34157. [DOI] [PubMed] [Google Scholar]
- 23.Santoro M F, Annand R R, Robertson M M, Peng Y W, Brady M J, Mankovich J A, Hackett M C, Ghayur T, Walter G, Wong W W, et al. J Biol Chem. 1998;273:13119–13128. doi: 10.1074/jbc.273.21.13119. [DOI] [PubMed] [Google Scholar]
- 24.Kleinberger T, Shenk T. J Virol. 1993;67:7556–7560. doi: 10.1128/jvi.67.12.7556-7560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shtrichman R, Sharf R, Barr H, Dobner T, Kleinberger T. Proc Natl Acad Sci USA. 1999;96:10080–10085. doi: 10.1073/pnas.96.18.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shtrichman R, Sharf R, Kleinberger T. Oncogene. 2000;19:3757–3765. doi: 10.1038/sj.onc.1203705. [DOI] [PubMed] [Google Scholar]
- 27.Marcellus R C, Chan H, Paquette D, Thirlwell S, Boivin D, Branton P E. J Virol. 2000;74:7869–7877. doi: 10.1128/jvi.74.17.7869-7877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemens J C, Worby C A, Simonson-Leff N, Muda M, Maehama T, Hemmings B A, Dixon J E. Proc Natl Acad Sci USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplen N J, Fleenor J, Fire A, Morgan R A. Gene. 2000;252:95–105. doi: 10.1016/s0378-1119(00)00224-9. [DOI] [PubMed] [Google Scholar]
- 30.Fire A. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 31.Hunter C P. Curr Biol. 1999;9:R440–R442. doi: 10.1016/s0960-9822(99)80276-0. [DOI] [PubMed] [Google Scholar]
- 32.Carthew R W. Curr Opin Cell Biol. 2001;13:244–248. doi: 10.1016/s0955-0674(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 33.Mayer-Jaekel R E, Baumgartner S, Bilbe G, Ohkura H, Glover D M, Hemmings B A. Mol Biol Cell. 1992;3:287–298. doi: 10.1091/mbc.3.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orgad S, Brewis N D, Alphey L, Axton J M, Dudai Y, Cohen P T. FEBS Lett. 1990;275:44–48. doi: 10.1016/0014-5793(90)81435-q. [DOI] [PubMed] [Google Scholar]
- 35.Mayer-Jaekel R E, Ohkura H, Gomes R, Sunkel C E, Baumgartner S, Hemmings B A, Glover D M. Cell. 1993;72:621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- 36.Berry M, Gehring W. EMBO J. 2000;19:2946–2957. doi: 10.1093/emboj/19.12.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helps N R, Brewis N D, Lineruth K, Davis T, Kaiser K, Cohen P T. J Cell Sci. 1998;111, Part 10:1331–1340. doi: 10.1242/jcs.111.10.1331. [DOI] [PubMed] [Google Scholar]
- 38.Brown L, Borthwick E B, Cohen P T. Biochim Biophys Acta. 2000;1492:470–476. doi: 10.1016/s0167-4781(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 39.Chen M S, Silverstein A M, Pratt W B, Chinkers M. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 40.Kloeker S, Bryant J C, Strack S, Colbran R J, Wadzinski B E. Biochem J. 1997;327:481–486. doi: 10.1042/bj3270481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen P, Holmes C F, Tsukitani Y. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 42.Brewis N D, Street A J, Prescott A R, Cohen P T W. EMBO J. 1993;12:987–996. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastie C J, Cohen P T W. FEBS Lett. 1998;431:357–361. doi: 10.1016/s0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- 44.Chen M X, McPartlin A E, Brown L, Chen Y H, Barker H M, Cohen P T. EMBO J. 1994;13:4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baharians Z, Schönthal A H. J Biol Chem. 1998;273:19019–19024. doi: 10.1074/jbc.273.30.19019. [DOI] [PubMed] [Google Scholar]
- 46.Umi H, Imazu M, Maeta K, Tsukamoto H, Azuma K, Takeda M. J Biol Chem. 1988;263:3752–3761. [PubMed] [Google Scholar]
- 47.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Mol Cell Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biggs W H, III, Zipursky S L. Proc Natl Acad Sci USA. 1992;89:6295–6299. doi: 10.1073/pnas.89.14.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiomi K, Takeichi M, Nishida Y, Nishi Y, Uemura T. Development (Cambridge, UK) 1994;120:1591–1599. doi: 10.1242/dev.120.6.1591. [DOI] [PubMed] [Google Scholar]
- 50.Anderson N G, Maller J L, Tonks N K, Sturgill T W. Nature (London) 1990;343:651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 51.Gomez N, Cohen P. Nature (London) 1991;353:170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- 52.Sontag E, Sontag J M, Garcia A. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen P, Lee P, Otto L, Abrams J. J Biol Chem. 1996;271:25735–25737. doi: 10.1074/jbc.271.42.25735. [DOI] [PubMed] [Google Scholar]
- 54.Mills J C, Lee V M, Pittman R N. J Cell Sci. 1998;111:625–636. doi: 10.1242/jcs.111.5.625. [DOI] [PubMed] [Google Scholar]
- 55.Fladmark K E, Brustugun O T, Hovland R, Boe R, Gjertsen B T, Zhivotovsky B, Doskeland S O. Cell Death Differ. 1999;6:1099–1108. doi: 10.1038/sj.cdd.4400590. [DOI] [PubMed] [Google Scholar]
- 56.Yan Y, Mumby M C. J Biol Chem. 1999;274:31917–31924. doi: 10.1074/jbc.274.45.31917. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, Doumanis J. Cell Death Differ. 2000;7:1039–1044. doi: 10.1038/sj.cdd.4400756. [DOI] [PubMed] [Google Scholar]
- 58.Fraser A G, McCarthy N J, Evan G I. EMBO J. 1997;16:6192–6199. doi: 10.1093/emboj/16.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]