Abstract
Background
Evidence revealed that fasting intervention may have favorable effects on metabolic and inflammation profile. However, the results are controversial. This study attempted to investigate and assess the effects of fasting on cardiometabolic risk factors in adults.
Methods
We searched PubMed, Embase, Cochrane, Scopus, and Web of Science databases until March 2025 for randomized controlled trials (RCTs) which have evaluated the effect of fasting intervention on metabolic and inflammation profile. The GRADE approach was applied to assess the quality of evidence, and the Cochrane risk-of-bias (RoB) 2 tool was used to evaluate study quality.
Results
Eight RCTs with 573 participants were included in the meta-analysis. Findings revealed that fasting could significantly decreased fasting blood glucose (FBS) (WMD = -3.34; 95% CI: -6.24, -0.45, P = 0.024), HbA1c (WMD = -0.08; 95% CI: -0.13, -0.02; P = 0.005), and HOMA-IR (WMD= -0.60; 95% CI: -0.91, -0.28; P < 0.001) in adults. Similarly, fasting intervention exerted its favorable effect by reducing low-density lipoprotein cholesterol (LDL-c) (WMD = -6.42; 95% CI: -12.26, -0.58; P = 0.031), and IL-6 (WMD = -0.58; 95% CI: -1.08, -0.08; P = 0.022), significantly. The sensitivity analysis, indicated that excluding any single study has no effect on the overall effect size. No evidence of publication bias was observed using Begg’s test (P > 0.05).
Conclusion
Overall this systematic review and meta-analysis demonstrated that fasting state contributed to improved glycemic control including FBS, HbA1c, and HOMA-IR levels. In addition, fasting intervention caused to an improvement in lipid metabolism (LDL-c) and inflammatory state indicated by IL-6.
Keywords: Fasting, Cardiometabolic factors, Inflammation biomarkers, Insulin-resistance, Systematic review, Meta-analysis
Introduction
Metabolic disorders such as type 2 diabetes mellitus, cardiovascular disease, and metabolic syndrome are considered as a major contributor to global morbidity and mortality [1, 2]. These conditions are characterized by glucose intolerance, insulin resistance, dyslipidemia, and obesity [3, 4]. In addition, low-grade inflammation may trigger these metabolic abnormalities [5, 6]. The growing prevalence of these disorders underscores the urgent need to address this health challenge. In this context, several non-pharmacological strategies have been developed and diet as a modifiable factor has emerged its positive properties.
Fasting interventions have emerged promising results to modify metabolic and inflammatory state [7, 8]. Intermittent fasting is characterized by repeated intentional interruptions in energy expenditure or a very low calorie diet (500–700 kcal) for 2–4 days a week [9, 10]. Various fasting regimens such as intermittent fasting, alternate-day fasting, and time-restricted feeding, demonstrated beneficial effects which are thought to be mediated through decreased level of glucose production, insulin sensitivity, lipid metabolism, and inflammatory state properly [7, 11, 12]. In this regard, Cramer et al. indicated that 300–350 kcal/day contribute to a significant improvement in the blood sugar level [13]. However, other studies found that, intermittent energy restriction does not affect the blood sugar level in patients with metabolic syndrome [14, 15]. Similarly, there were conflicting results in term of HOMA-IR level too. Patients with type 2 diabetes and metabolic syndrome undergone various fasting protocols which have shown both protective [13, 14] and unfavorable [16, 17] effects on HOMA-IR levels. Also, findings related to lipid profiles have been inconsistent. Most of studies investigating the effect of fasting program, reported no significant impact on high-density lipoprotein cholesterol (HDL-c). In contrast, Kunduraci et al. demonstrated that fasting program may have beneficial effect on HDL-c level [15].
Despite growing interest in fasting dietary approach, there are limited studies with variability across the results of studies, population, and the type of fasting. Therefore, this systematic review and meta-analysis of randomized controlled trials aimed to examine the effects of fasting interventions on metabolic and inflammatory profile. Specifically, it assessed key cardiometabolic risk factors, including fasting blood glucose (FBS), insulin resistance, and lipid parameters, as well as inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) to provide a more conclusive understanding of the impact of fasting state on metabolic health.
Method
The current systematic review and meta-analysis was conducted according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [18], and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [19]. In addition, the protocol of the current research has been approved by International Prospective Register of Systematic Reviews.
Search strategy
A comprehensive literature search has been done in PubMed, Scopus, Embase, Web of Science, and Cochrane Library up to March 2025. In addition, the reference list of relevant studies has been screened manually to avoid missing any relevant studies. The language was restricted to English. The search strategy was completed using relevant Medical Subject Heading (MeSH) terms and keywords.
Inclusion and exclusion criteria
The included studies were included if they were aligned with our specified PICOS criteria; Population/Patients (P: adults aged > 18), Intervention (I: fasting diet), Comparison (C: placebo or control group), Outcome (O: glycemic control (fasting plasma glucose (FPG), HbA1c, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), insulin), lipid profile (total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglyceride (TG)), and inflammatory markers (CRP, IL-6, and TNF-α) and Study (S: randomized controlled trial (RCT) design (Parallel and Crossover). Nevertheless, studies were excluded if: (1) Lacked a control group. (2) Observational studies (case-control or cross-sectional), animal or review studies. (3) Trials lacking adequate data and research containing duplicated data.
Data screening procedures and data extraction
The references were managed using EndNote (version 9) to screen the articles. Two independent reviewers screened the titles and abstracts of the included studies based on predefined eligibility criteria. Subsequently, full-text of articles were retrieved and evaluated for inclusion. Any discrepancies between the reviewers were resolved through discussion, and any disagreements were resolved by third investigator.
Two authors completed data extraction process from each included studies including the name of first author, publication year, study design, location, mean age, mean body mass index (BMI), duration, health status. Additionally, the sample size and the mean ± standard deviation (SD) changes of predefined outcomes before and after intervention in both groups.
Quality assessment and quality of evidence
The quality assessments were done to determine the potential bias for each study. The selected RCTs underwent a risk of bias assessment utilizing the RoB 2 [20]. This assessment contains five specific domains: concealment of allocation, generation of random sequences, selective reporting, blinding of outcome assessment, and incomplete outcome data. All these domains were rated as low, unclear, or high risk.
However, the quality of evidence was assessed using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [21]. This approach considers several factors: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Accordingly, the quality of evidence was categorized as high, moderate, and low.
Statistical analysis
The analyses were performed using Stata Statistical Software version 14 (Stata Corp, College Station, TX, United States), and a random-effects model was applied to conduct the analyses [22]. The overall effect size was determined using the MD and SDs of changes in outcome levels. The mean difference (MD) along with the 95% confidence intervals (CIs) were computed to calculate the overall estimates [23]. The heterogeneity of included RCTs was assessed by the I2 index, and I2 > 50% and P < 0.05 was considered as significant and high heterogeneity between studies [24]. The sensitivity analysis using the leave-one-out method was utilized to identify the effect of each RCT on the overall effect size [25]. Funnel plots, as well as Begg’s and Egger’s tests were used to evaluate the publication bias [26, 27].
Results
Study selection
Our comprehensive and systematic search identified 1268 results, and 627 of them were duplicates. Title and abstract screening process ended in the exclusion of 628 irrelevant studies. Subsequently, 13 studies were evaluated for eligibility to be included in the present study. Likewise, five studies were excluded. At last, eight studies were included according to abovementioned inclusion criteria. The flowchart of the study selection procedure is provided in the Fig. 1.
Fig. 1.
PRISMA flow diagram of selection studies
Study characteristics
The study characteristics of the eight eligible publications in relation to the effect of fasting regimen on metabolic and inflammation profile are summarized in Table 1. The total sample size of all included studies was 573 and ranged from 32 to 145. All included studies were published from 2017 up to 2024. Also, the mean age of included study participants ranged from 25 to 58 years. The geographical location of included studies were in USA [16], Germany [28], Turkey [15], China [14], Iran [17, 29], and Thailand [30]. The duration of fasting regimen treatment was 1 to 16 weeks. The health condition in most of the included studies was metabolic syndrome and the others focused on type 2 diabetes and impaired glucose tolerance patients.
Table 1.
Characteristics of included studies
| Author, Year | Country | Study, Design | Sex | Duration (week) | Sample Size (IN, CON) | Mean age (IN, CON) | BMI | Intervention type | Control group | Health status | Main outcome (IN) | Main outcome (CON) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cramer H et al., 2022 |
Germany | SB, multicenter, parallel, RCT | M/F | 0.71 | 145 (73, 72) |
(58.6 ± 10.8) (60.8 ± 10.8) |
33.7 ± 4.5 | 300–350 kcal/day, obtained from vegetable juices and vegetable broth | Modified DASH diet, exercise, mindfulness | MetS |
BS: -6.30 ± 11.77 HbA1c: -0.1 ± 0.31 HOMA-IR: -1.50 ± 1.55 Ins: -4.4 ± 4.4 TC: 26.9 ± 30.94 LDL: -6.1 ± 24.70 HDL: -4.2 ± 9.63 TG: -71.6 ± 170.57 CRP: 0.1 ± 0.25 IL-6: -0.3 ± 1.62 |
BS: 4.30 ± 16.44 HbA1c: 0.1 ± 0.44 HOMA-IR: -0.3 ± 1.48 Ins: -0.6 ± 4.6 TC: 15.9 ± 30.33 LDL: 2.7 ± 27.24 HDL: -2.5 ± 12.01 TG: -5.6 ± 66.81 CRP: 0.1 ± 0.18 IL-6: 0.3 ± 1.68 |
| Guo Y et al., 2021 | China | RCT | M/F | 8 | 39 (21, 18) |
(40.2 ± 5.7) (42.7 ± 4.1) |
28 | Intermittent fasting (“two-day”; a 75% of energy restriction for two nonconsecutive days a week and an ad libitum diet the other five days) | Routine diet without dietary instructions | MetS |
BS: -2.26 ± 5.40 HOMA-IR: -0.63 ± 0.93 Ins: -1.3 ± 5.0 TC: -1.6 ± 21.15 LDL: 0.7 ± 16.90 HDL: -0.4 ± 5.82 TG: -35.4 ± 67.14 |
BS: 0.72 ± 12.31 HOMA-IR:0.12 ± 0.52 Ins: -0.07 ± 1.98 TC: -10.8 ± 20.35 LDL: 20.9 ± 20.69 HDL: 8.1 ± 6.83 TG: 10.6 ± 80.70 |
| Kunduraci YE et al., 2020 | Turkey | RCT | M/F | 12 | 65 (32, 33) |
(47.44 ± 2.17) (48.76 ± 2.13) |
36.58 ± 0.93 | Intermittent Energy Restriction | Continuous Energy Restriction (CER) | MetS |
BS: -15.47 ± 5.70 HbA1c: -0.32 ± 0.18 HOMA-IR: -1.29 ± 0.45 Ins: -2.23 ± 1.64 TC: -29.32 ± 4.88 LDL: -17.0 ± 3.57 HDL: 0.53 ± 1.12 TG: -41.84 ± 15.42 |
BS: -13.12 ± 4.29 HbA1c: -0.31 ± 0.15 HOMA-IR: -0.94 ± 0.49 Ins: -2.39 ± 1.31 TC: -29.36 ± 5.25 LDL: -15.97 ± 3.49 HDL: -0.38 ± 1.37 TG: -40.0 ± 20.77 |
|
Li et al., 2017 |
Germany | RCT | M/F | 1 | 32 (16, 16) | 25–75 | Fasting | T2DM with MetS |
BS: -10.70 ± 17.55 HbA1c: -2.2 ± 12.0 HOMA-IR: -1.50 ± 4.6 Ins: -3.4 ± 6.78 TC: -0.5 ± 27.1 LDL: -2.6 ± 26.9 HDL: 6.5 ± 23.3 TG: -26.6 ± 88.5 |
BS: -38.5 ± 27.18 HbA1c: -2.2 ± 8.7 HOMA-IR: -1.50 ± 2.1 Ins: -0.2 ± 7.22 TC: -15.5 ± 27.4 LDL: -7.8 ± 17.3 HDL: -2.3 ± 6.9 TG: -2.5 ± 81.9 |
||
| Manoogian E.N.C et al., 2024 | USA | RCT | M/F | 12 | 108 (54, 54) |
56.6 60.6 |
31.5 | Time-Restricted Eating | MetS |
FBS: -4.84 ± 6.22 HbA1c: -0.12 ± 0.20 HOMA-IR: -0.89 ± 2.18 Ins: -2.87 ± 8.72 LDL: -10.48 ± 25.78 HDL: -1.37 ± 9.34 TG: -7.77 ± 41.47 CRP: -0.19 ± 1.64 |
FBS: -1.5 ± 7.39 HbA1c: -0.02 ± 0.17 HOMA-IR: -0.38 ± 1.63 Ins: -1.2 ± 5.97 LDL: -1.37 ± 24.98 HDL: -0.37 ± 9.82 TG: -13.94 ± 46.24 CRP: -0.09 ± 1.89 |
|
|
Parvaresh A et al., 2019 |
Iran | RCT | M/F | 8 | 69 (35, 34) |
44.6 46.4 |
31.1 ± 3.35 | Modified Alternate-Day Fasting | Calorie Restriction | MetS |
FBS: -6.00 ± 5.78 HOMA-IR: -2.42 ± 3.82 TC: -11.0 ± 21.99 LDL: -6.0 ± 17.83 HDL: -1.0 ± 5.56 TG: -52.0 ± 67.25 |
FBS: 0.0 ± 5.34 HOMA-IR: -1.57 ± 4.09 TC: -9.0 ± 22.57 LDL: 0.0 ± 17.89 HDL: -1.0 ± 5.97 TG: -40.0 ± 69.85 |
| Razavi R et al., 2021 | Iran | single-center, RCT | M/F | 16 | 69 (35, 34) |
41.3 43.1 |
31.3 | Alternate-day fasting diet | Calorie restriction diet | MetS |
CRP: -2.06 ± 1.18 IL-6: -1.08 ± 2.7 TNF-α: -3.47 ± 5.77 |
CRP: -0.97 ± 0.82 IL-6: -0.61 ± 2.82 TNF-α: -2.21 ± 5.04 |
|
Suthutvoravut U et al., 2023 |
Thailand | RCT | M/F | 4 | 46 (24, 22) |
55.5 55.2 |
- | Time-Restricted Eating | Usual care for impaired glucose | Impaired Fasting Glucose |
FBS: -3.03 ± 1.94 HbA1c: 0.09 ± 0.08 |
FBS: -1.79 ± 1.75 HbA1c: 0.15 ± 0.08 |
SB; single blind, RCT; randomized clinical trial, M; male, F; female, MetS; metabolic syndrome, T2DM; type 2 diabetes mellitus, BS; blood sugar, FBS; fasting blood sugar, HOMA-IR;, Ins; insulin, TC; total cholesterol, LDL; low-density lipoprotein, HDL; high-density lipoprotein, TG; triglyceride, CRP; C-reactive protein, IL-6; interleukin-6, TNF-α; tumor necrosis factor-α
Quality assessment and meta-evidence
According to quality assessment of included studies, randomization process was conducted in all included studies properly and rated as lower risk of bias. Moreover, all included studies for both domains including “selection of reported results” and “missing outcome data” were scored as lower risk of bias. Detailed information regarding the quality of the included RCTs are available in Table 2. The GRADE quality of evidence for the effect of fasting on HOMA-IR level was assessed as high quality of evidence. However, the effect of fasting was considered as moderate for insulin, TC, LDL-c, HDL-c, TG, CRP, IL-6, and TNF-α values (Table 3).
Table 2.
Results of risk of bias assessment for randomized clinical trials included in the present study
| Author, Year | Randomization Process | Deviation from Intended Interventions | Selection of the Reported Result | Measurement of the Outcome | Missing Outcome Data | General risk of bias |
|---|---|---|---|---|---|---|
| Cramer H et al., 2022 | Low | Unclear | Low | Low | Low | Low |
| Guo Y et al., 2021 | Low | High | Low | High | Low | Moderate |
| Kunduraci YE et al., 2020 | Low | Unclear | Low | Unclear | Low | Low |
| Li et al., 2017 | Low | High | Low | Low | Low | Low |
| Manoogian E.N.C et al., 2024 | Low | Unclear | Low | Unclear | Low | Low |
| Parvaresh A et al., 2019 | Low | Low | Low | Low | Low | Low |
| Razavi R et al., 2021 | Low | Low | Low | Low | Low | Low |
| Suthutvoravut U et al., 2023 | Low | Low | Low | Low | Low | Low |
Note: Each study was assessed for risk of bias using the ROB2 tool. Domains of assessment included were Randomization Process, Deviation from Intended Interventions, Selection of the Reported Result, Measurement of the Outcome, Missing Outcome Data, and General risk of bias. Each domain was scored as “high risk” if it contained methodological flaws that may have affected the results, “low risk” if the flaw was deemed inconsequential, and “some concern” if information was insufficient to determine
Table 3.
Summary of findings and quality of evidence assessment using the GRADE approach
| No of patients (meta-analysis) | WMD (95% CI) | Risk of bias1 | Inconsistency2 | Indirectness3 | Imprecision4 | Publication bias5 | Quality of evidence6 | |
|---|---|---|---|---|---|---|---|---|
| FBS (mg/dl) | 223 (3) | -3.34 (-6.24, -0.45) | Not Serious | Serious | Not Serious | Serious | Not Serious | Low |
| BS (mg/dl) | 281 (4) | -0.79 (-8.50, 6.92) | Not Serious | Serious | Not Serious | Serious | Not Serious | Low |
| HbA1c | 396 (5) | -0.08 (-0.13, -0.02) | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Moderate |
| HOMA-IR | 458 (6) | -0.60 (-0.91, -0.28) | Not Serious | Not Serious | Not Serious | Not Serious | Not Serious | High |
| Insulin (mU/L) | 458 (6) | -1.60 (-3.26, 0.06) | Not Serious | Serious | Not Serious | Not Serious | Not Serious | Moderate |
| TC (mg/dL) | 350 (5) | 4.39 (-1.68, 10.47) | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Moderate |
| LDL-c (mg/dL) | 458 (6) | -6.42 (-12.26, -0.58) | Not Serious | Serious | Not Serious | Not Serious | Not Serious | Moderate |
| HDL-c (mg/dL) | 458 (6) | -1.11 (-3.67, 1.45) | Not Serious | Serious | Not Serious | Not Serious | Not Serious | Moderate |
| TG (mg/dL) | 458 (6) | -1.85 (-10.63, 6.94) | Not Serious | Serious | Not Serious | Not Serious | Not Serious | Moderate |
| CRP | 322 (3) | -0.39 (-1.11, 0.34) | Not Serious | Serious | Not Serious | Serious | Not Serious | Moderate |
| IL-6 | 214 (2) | -0.58 (-1.08, -0.08) | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Moderate |
| TNF-α | 69 (3) | -1.26 (-3.81, 1.29) | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Moderate |
FBS; Fasting blood sugar, BS; Blood sugar, HOMAIR; Homeostatic Model Assessment for Insulin Resistance, TC; Total cholesterol, LDL-c; Low density lipoprotein-cholesterol, HDL-c; High density lipoprotein- cholesterol, TG; Triglyceride, CRP; C-reactive protein, IL-6; Interleuki-6, TNF-α; Tumor necrosis factor-α
1 Risk of bias based on the Cochrane risk of bias tool
2 Downgraded if there was a substantial unexplained heterogeneity (I2 > 50%, P < 0.10) that was not explained by subgroup analyses
3 Downgraded if there were factors present relating to the participants, interventions, or outcomes which restrict the overall generalizability of the results
4 Downgraded if the 95% confidence interval (95% CI) crossed the minimally important difference (MID)
5 Downgraded if there was an evidence of publication bias using funnel plot
6 Since all included studies were randomized controlled trials, the certainty of the evidence was graded as high for all outcomes by default and then downgraded based on prespecified criteria
The impact of fasting on FBS and BS level in adults
The effect of fasting regimen on FBS level was reported in three RCTs including 223 individuals. This intervention caused a significant reduction in patients with metabolic syndrome and glucose intolerance (WMD = -3.34; 95% CI: -6.24, -0.45, P = 0.024; I2 = 83.1%, P = 0.003) (Fig. 2).
Fig. 2.
Forest plot of impacts of fasting state on FBS level
However, the effect of fasting intervention on BS level was evaluated in four studies involving 281 participants. This intervention showed a non-significant effect on BS level in patients with metabolic syndrome and type 2 diabetes (WMD = -0.79; 95% CI: -8.50, 6.92, P = 0.841; I2 = 88.0%, P < 0.001) (Fig. 3). Sensitivity analyses indicated that removing each study could not alter the overall effect of fasting intervention on FBS and BS levels. Since the number of included studies for FBS and BS value was less than ten studies, the Begg’s test was performed for publication bias and no publication bias was observed accordingly (Begg’s test > 0.05).
Fig. 3.
Forest plot of impacts of fasting state on BS level
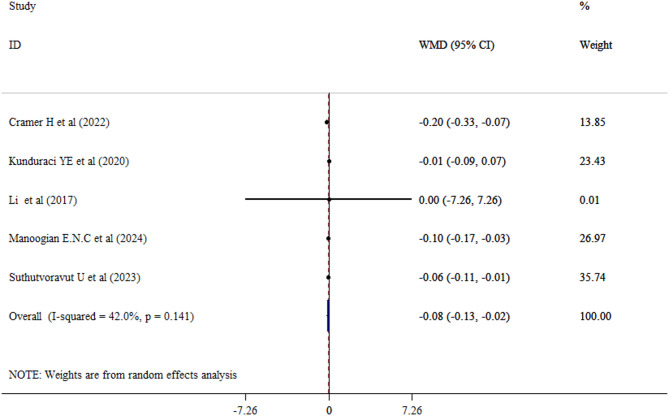
The impact of fasting on HbA1c level in adults
Overall, five studies with a total of 396 adults (intervention group = 199) examined the effect of fasting on HbA1c levels. Our results revealed that fasting intervention statically decreased HbA1c level (WMD = -0.08; 95% CI: -0.13, -0.02; P = 0.005) with a moderate between-study heterogeneity (I2 = 42.0%, P = 0.141) (Fig. 4). Sensitivity analyses for the HbA1c value was conducted and the overall effect size remained unchanged. No publication bias was found using Begg’s test (Begg’s test > 0.05).
Fig. 4.
Forest plot of impacts of fasting state on HbA1c level
The impact of fasting on HOMA-IR level in adults
The pooled effect of six studies evaluating the effect of fasting regimen on HOMA-IR level encompassing 458 participants (intervention group = 231, control group = 227) revealed a significant reduction on HOMA-IR level (WMD = -0.60; 95% CI: -0.91, -0.28, P < 0.001; I2 = 54.4.0%, P = 0.052) (Fig. 5). A leave-one-out sensitivity analysis evaluated the effect of individual studies and the overall effect size remained consistent. The Begg’s test was performed and no publication bias was found accordingly (Begg’s test > 0.05).
Fig. 5.
Forest plot of impacts of fasting state on HOMA-IR level
The impact of fasting on insulin level in adults
The combined effect of six studies (458 participants) which have assessed the effect of fasting intervention on insulin level demonstrated a non-significant effect (WMD = -1.60; 95% CI: -3.26, 0.06, P = 0.059; I2 = 79.4.0%, P < 0.001) (Fig. 6). Sensitivity analyses reported that removing one by one study could not affect the overall result of fasting on insulin level. The Begg’s test was performed and no publication bias was found accordingly (Begg’s test > 0.05).
Fig. 6.
Forest plot of impacts of fasting state on insulin level
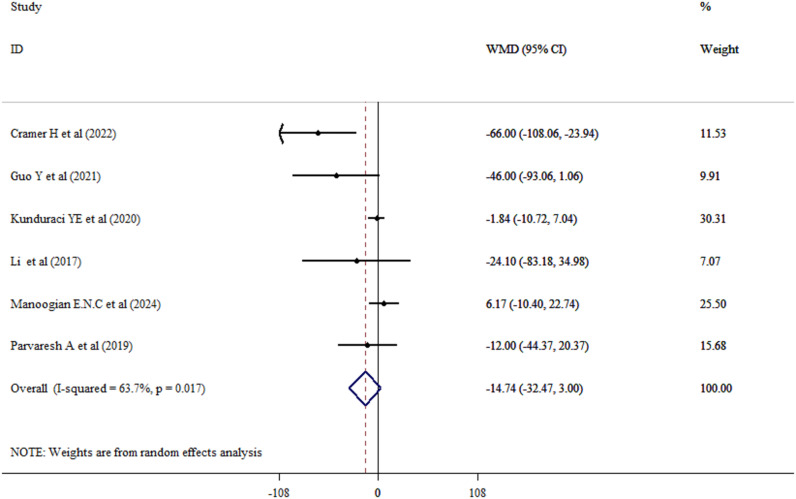
The impact of fasting on lipid profile in adults
Effect of fasting intervention on LDL-c levels was explored from six studies among adults (Intervention group = 231, control group = 227). Combined results through the random-effects model revealed that fasting significantly reduced LDL-c levels in adults (WMD = -6.42, 95% CI: -12.26, -0.58; P = 0.031; I2 = 69.4%, P = 0.006) (Fig. 7). While, fasting could not succeed to improve TC (WMD = 4.39, 95% CI: -1.68, 10.47; P = 0.156; I2 = 53.0%, P = 0.075) (Fig. 8), HDL-c (WMD = -1.11, 95% CI: -3.67, 1.45; P = 0.396; I2 = 77.0%, P = 0.001) (Fig. 9), and TG (WMD = -14.74, 95% CI: -32.47, 3.00; P = 0.103; I2 = 63.7%, P = 0.017) (Fig. 10) levels significantly.
Fig. 7.
Forest plot of impacts of fasting state on LDL-c level
Fig. 8.
Forest plot of impacts of fasting state on TC level
Fig. 9.
Forest plot of impacts of fasting state on HDL-c level
Fig. 10.
Forest plot of impacts of fasting state on TG level
However, a leave-one-out sensitivity analysis evaluated the effect of individual studies on TC, LDL-c, HDL-c, and TG outcomes and the overall effect size remained consistent across the studies. Since the number of included studies were less than ten, the Begg’s test was used to evaluate the publication bias which indicated no evidence of publication bias for all lipid profile outcomes (P > 0.05).
The impact of fasting inflammatory markers in adults
Two studies including 214 participants investigated the impact of fasting on IL-6 levels. A significant decrease in IL-6 was observed in adult patients who experienced the fasting regimen (WMD = -0.58, 95% CI: -1.08, -0.08; P = 0.022; I2 = 0.0%, P = 0.857). Whereas, random effect model showed non-significant changes on CRP (WMD = -0.39, 95% CI: -1.11, 0.34; P = 0.294; I2 = 89.8%, P < 0.001) and TNF-α (WMD = -1.26, 95% CI: -3.81, 1.29; P = 0.334) (Fig. 11) following the fasting intervention. In addition, sensitivity analyses indicated that excluding any single study did not influence on the overall effect size. Begg’s test reported no evidence of publication bias (P > 0.05).
Fig. 11.
Forest plot of impacts of exercise program on inflammatory markers
Discussion
In the present systematic review and meta-analysis of available RCTs investigating the efficacy of fasting intervention in adults, we summarized the available evidence on the fasting intervention on metabolic risk factors and inflammatory markers to provide a firm conclusion. The previous meta-analyses [31–33] focused on either glycemic control or lipid profile parameters in isolation. While, our study offers a more comprehensive, multidimensional assessment of cardiometabolic factors and inflammatory markers. This integrated approach helps to have more clinically relevant interpretation of fasting effects on cardiometabolic profile. Accordingly, it has been shown that fasting intervention caused a significant decrease in FBS, HbA1c, and HOMA-IR levels. These findings indicate that fasting can be accompanied with glycemic control and improved insulin sensitivity. Subgroup analysis revealed that the duration of the fasting intervention significantly influenced its effect on insulin resistance. Interventions lasting ≥ 12 weeks demonstrated a greater effect size, with a standardized mean difference (SMD) of -0.55, indicating a more pronounced improvement in insulin sensitivity. In comparison, shorter interventions (< 12 weeks) showed a smaller yet statistically significant effect (SMD = -0.30). These findings suggest that longer fasting interventions may confer greater metabolic benefits with respect to insulin resistance.
Several probable mechanisms can be considered to exert these favorable effects. Fasting state can promote weight loss and reduce the glucose production in the liver and insulin resistance subsequently [34–36]. Likewise, this process may contribute to an improvement in HOMA-IR level specifically in patients with metabolic syndrome. Nonetheless, no significant changes were observed in blood glucose and insulin level. This discrepancy may be explained by the fact that blood glucose ad the insulin levels are representative of short-term metabolic responses, while FBS and HbA1c reflect the long-term effect glycemic state. Cramer et al. investigated the effect of fasting regimen congaing 300–350 kcal/day, obtained from vegetable juices and vegetable broth on BS level in patients with metabolic syndrome and found a significant reduction in BS level [13]. In contrast, seven day fasting program based on Buchinger approach (300 kcal/day by liquids only and then start of solid foods) did not show such effect in type 2 diabetes patients [28].
In addition, the fasting contributed to significant reduction in LDL-c level. This finding is clinically valuable and can be considered as cardioprotective strategy to improve atherosclerosis and cardiovascular disease. However, this finding may be bold in patients with metabolic syndrome. The probable mechanism indicating that lipolysis process may be activated following to fasting state. Accordingly, fasting state stimulate the body to utilize the fats as a source of energy which contributes to decreased atherogenic lipoproteins [37, 38]. All these are consistent with the previous studies which suggest that intermittent fasting and caloric restriction can favorably influence lipid profiles. Although the baseline lipid levels, type of fasting and the degree of compliance with intervention may affect the degree of LDL-c reduction. However, no significant changes were observed in term of TC, HDL-c, and TG levels following fasting. It may be attributed to the high sensitivity of HDL-c and TG level to lifestyle modifications. The variability in fasting protocols (e.g., time-restricted feeding, alternate-day fasting) and the duration of interventions across studies may have influenced the results. Guo et al. investigated the impact of intermittent fasting (“two-day” fasting; a 75% of energy restriction for two nonconsecutive days a week and an ad libitum diet the other five days) in patients with metabolic syndrome [14]. While modified alternate-day fasting could not affect the metabolic syndrome population in Iran [17].
Furthermore, our findings indicated that fasting state could improve IL-6 level significantly. This suggests an anti-inflammatory effect for fasting state [39]. IL-6 is a proinflammatory cytokine which is involved in the low-grade-inflammation and its increased level is more probable in the obesity or insulin resistance [40]. In addition, fasting state may contribute to reduced adiposity, improved gut microbiota composition, and decreased oxidative stress which are all interrelated with improved inflammation [41–43]. However, it failed to exert its favorable properties on CRP and TNF-α levels. The limited number of included studies and small sample sizes limits, the statistical power to detect meaningful differences. Overall reduced level of IL-6 supports the anti-inflammatory potential of fasting state.
This meta-analysis has several strengths. This study was performed based on current methodological guidelines. In addition, this study employed sensitivity analysis and investigated the risk of bias using an updated assessment tool. Moreover, this study presented an integrated interpretation of the results which is more realistic and holistic view of biological processes. Since most disorders arise from the interaction of multiple pathways, studies that focus on a single parameter in isolation may not provide the full picture. Our multipolar approach offers a more comprehensive understanding in relation to fasting effect on cardiometabolic health. Our study has some limitations needed to be mentioned too. First, the number of included studies were limited and the sample size was small which lower the statistical power of the study. Second, the type of fasting varied and it was not possible to group studies accordingly.
Conclusion
In conclusion, the current systematic review and meta0analysis demonstrated that fasting state may have favorable effects on glycemic control (FBS, HbA1c, and HOMA-IR levels). In addition, improvement in LDL-c and IL-6 levels revealed its cardioprotective properties.
Acknowledgements
None.
Author contributions
Designing this study: Ling Lu, Xiuping Weng. Performed this study: Ling Lu, Xi Chen. Drafted the article, Revised the article critically for important intellectual content, and approved the version to be published: Ling Lu, Xi Chen, Sho Liou, Xiuping Weng.
Funding
Zhejiang Provincial Health and Wellness Science and Technology Plan 2022 (2022KY039), Zhejiang Provincial Medical and Health Science and Technology Plan 2024 (2024KY026), Science and Technology Foundation Project of Health Commission of Guizhou Province (Project No.: gzwkj2024-539).
Data availability
The original data used during the current study can be obtained by contacting the corresponding author.
Declarations
Ethical approval
Ethical approval was not required for this study as it is a meta-analysis based on previously published data.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Z, Chen J, Zhu L, Jiao S, Chen Y, Sun Y. Metabolic disorders and risk of cardiovascular diseases: a two-sample Mendelian randomization study. BMC Cardiovasc Disord. 2023;23(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed MS, Shalaby MA, Riham A, El-Shiekh HA, El-Banna, Emam SR, et al. Metabolic syndrome: risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem Adv. 2023;3:100335. [Google Scholar]
- 3.Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahdkaran M, Sistanizad M. From lipids to glucose: investigating the role of dyslipidemia in the risk of insulin resistance. J Steroid Biochem Mol Biol. 2025;250:106744. [DOI] [PubMed] [Google Scholar]
- 5.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasim I, Majeed CN, DeBoer MD. Intermittent fasting metabolic health. Nutrients. 2022;14(3). [DOI] [PMC free article] [PubMed]
- 8.Hansen B, Sánchez-Castro M, Schintgen L, Khakdan A, Schneider JG, Wilmes P. The impact of fasting and caloric restriction on rheumatoid arthritis in humans: a narrative review. Clin Nutr. 2025;49:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37(1):371–93. [DOI] [PubMed] [Google Scholar]
- 10.Vasim I, Majeed CN, DeBoer MD. Intermittent fasting and metabolic health. Nutrients. 2022;14(3):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song DK, Kim YW. Beneficial effects of intermittent fasting: a narrative review. J Yeungnam Med Sci. 2023;40(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E. Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity. 2020;28:S29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer H, Hohmann C, Lauche R, Choi KA, Schneider N, Steckhan N, et al. Effects of fasting and lifestyle modification in patients with metabolic syndrome: a randomized controlled trial. J Clin Med. 2022;11(16):4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106(1):64–79. [DOI] [PubMed] [Google Scholar]
- 15.Kunduraci YE, Ozbek H. Does the energy restriction intermittent fasting diet alleviate metabolic syndrome biomarkers? A randomized controlled trial. Nutrients. 2020;12(10):3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manoogian ENC, Wilkinson MJ, O’Neal M, Laing K, Nguyen J, Van D, et al. Time-restricted eating in adults with metabolic syndrome: a randomized controlled trial. Ann Intern Med. 2024;177(11):1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvaresh A, Razavi R, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complement Ther Med. 2019;47:102187. [DOI] [PubMed] [Google Scholar]
- 18.Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. 2010, Version.
- 19.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, et al. RoB 2. BMJ: Br Med J. 2019;366:1–8. [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 23.Feingold A. Confidence interval estimation for standardized effect sizes in multilevel and latent growth modeling. J Consult Clin Psychol. 2015;83(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods. 2006;11(2):193. [DOI] [PubMed] [Google Scholar]
- 25.Okumura S, Suzuki Y, Takeuchi I. Quick sensitivity analysis for incremental data modification and its application to leave-one-out CV in linear classification problems. in Proceedings of the 21th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2015.
- 26.Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, Chen Y. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med. 2018;33(8):1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Berdine G. Publication bias in meta-analysis. Southwest Respiratory Crit Care Chronicles. 2021;9(41):67–70. [Google Scholar]
- 28.Li C, Sadraie B, Steckhan N, Kessler C, Stange R, Jeitler M, Michalsen A. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome–a randomized controlled explorative study. Exp Clin Endocrinol Diabetes. 2017;125(09):618–24. [DOI] [PubMed] [Google Scholar]
- 29.Razavi R, Parvaresh A, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. 2020. [DOI] [PubMed]
- 30.Suthutvoravut U, Anothaisintawee T, Boonmanunt S, Pramyothin S, Siriyothin S, Attia J, et al. Efficacy of time-restricted eating and behavioral economic intervention in reducing fasting plasma glucose, hba1c, and cardiometabolic risk factors in patients with impaired fasting glucose: a randomized controlled trial. Nutrients. 2023;15(19):4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of intermittent fasting diet on glucose and lipid metabolism and insulin resistance in patients with impaired glucose and lipid metabolism: a systematic review and meta-analysis. Int J Endocrinol. 2022;2022(1):6999907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;179:109003. [DOI] [PubMed] [Google Scholar]
- 33.Meng H, Zhu L, Kord-Varkaneh H, O Santos H, Tinsley GM, Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020;77:110801. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic Sirtuin 1. Proc Natl Acad Sci U S A. 2007;104(31):12861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med. 2015;46:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Mei H, Xue L, Cheng C, Wu Y, Zou C, et al. Testing the carbohydrate-insulin model: short-term metabolic responses to consumption of meals with varying glycemic index in healthy adults. Cell Metab. 2025;37(3):606–e6153. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wu R. The effect of fasting on human metabolism and psychological health. Dis Markers. 2022;2022:p5653739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersten S. The impact of fasting on adipose tissue metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868(3):159262. [DOI] [PubMed] [Google Scholar]
- 39.Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, et al. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obes (Silver Spring). 2011;19(8):1586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 41.Yuliyanasari N, Rejeki PS, Hidayati HB, Subsomwong P, Miftahussurur M. The effect of intermittent fasting on preventing obesity-related early aging from a molecular and cellular perspective. J Med Life. 2024;17(3):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato K, Nakashima A, Fukuda S, Inoue J, Kim YG. Fasting builds a favorable environment for effective gut microbiota modulation by microbiota-accessible carbohydrates. BMC Microbiol. 2025;25(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bu T, Xu L, Zhu X, Cheng J, Li Y, et al. Influence of short-term fasting on oxidative stress, antioxidant-related signaling molecules and autophagy in the intestine of adult Siniperca chuatsi. Aquac Rep. 2021;21:100933. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data used during the current study can be obtained by contacting the corresponding author.