Abstract
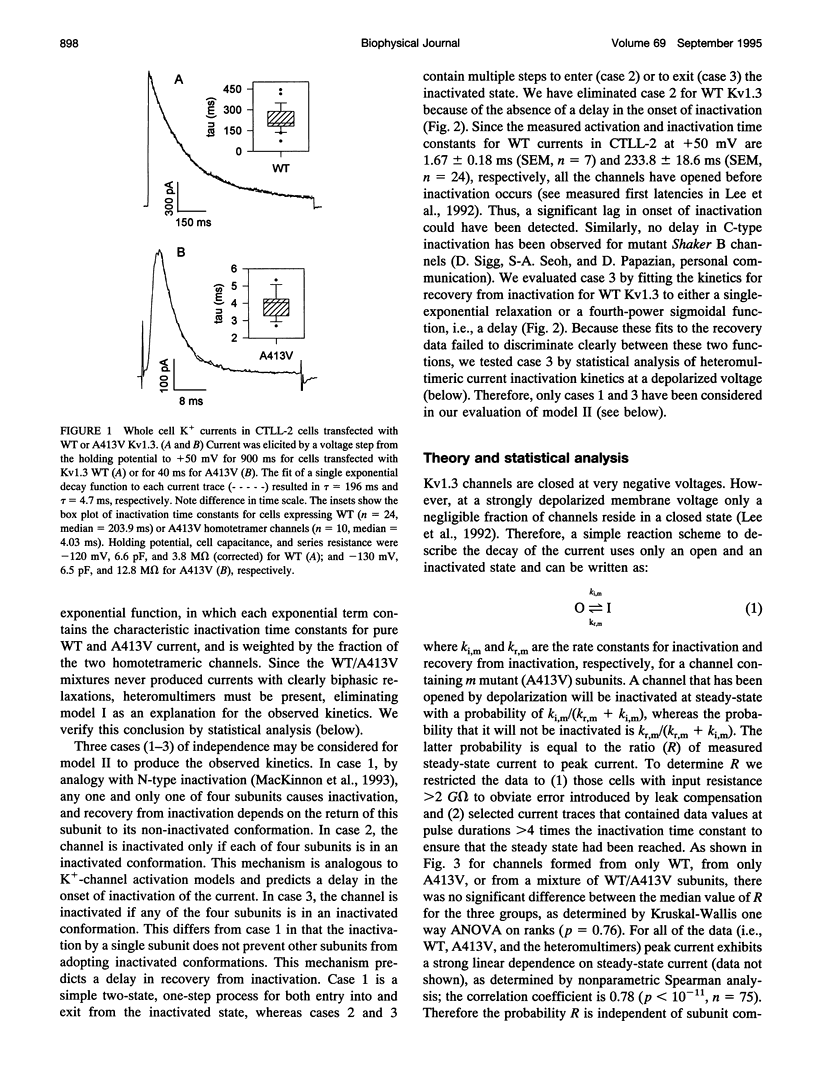
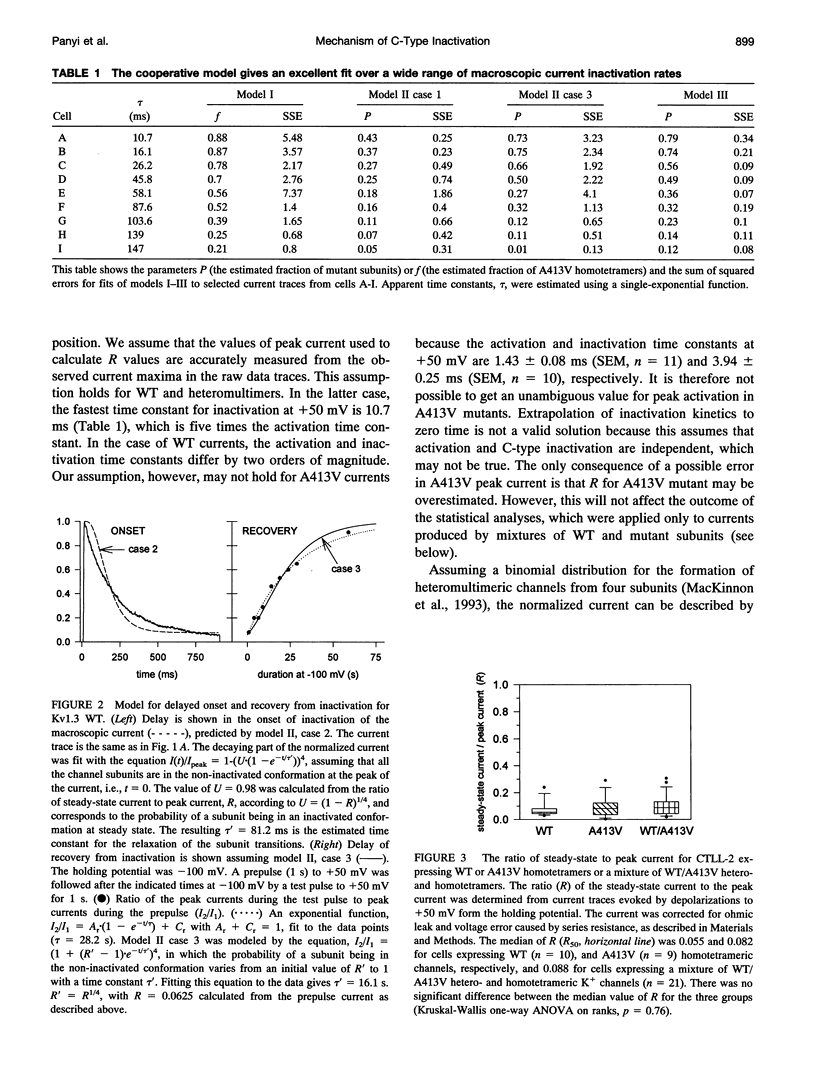
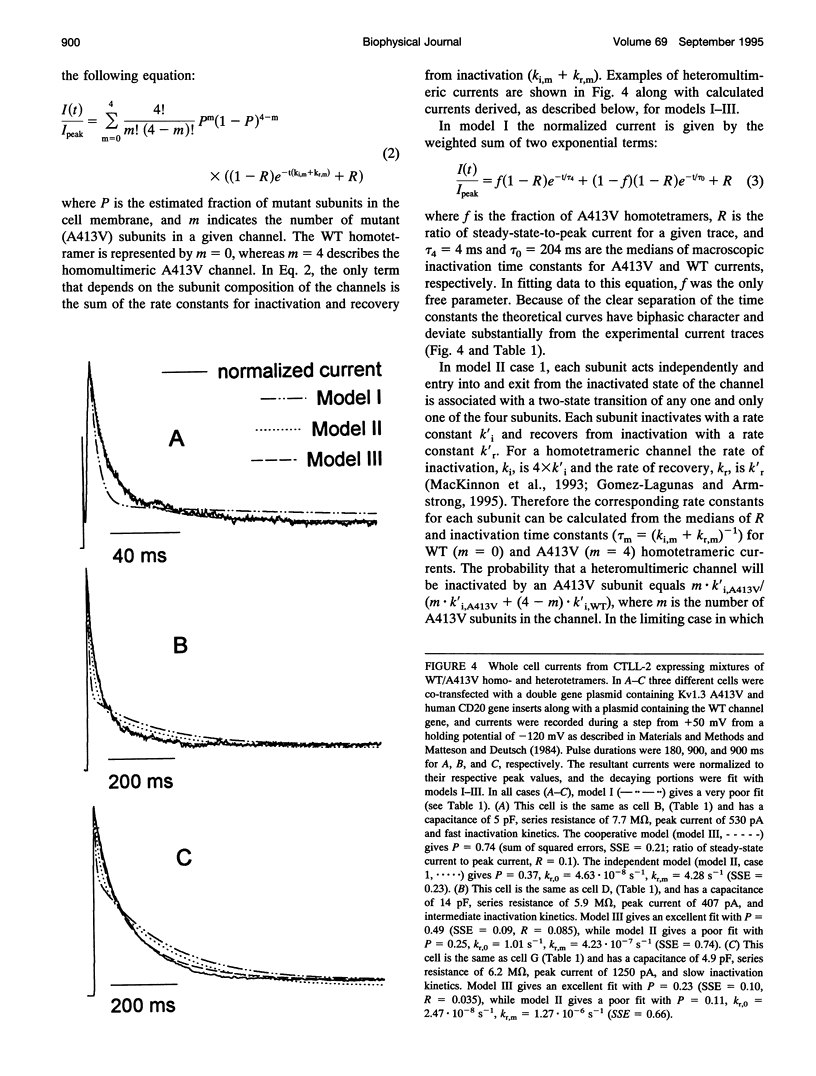
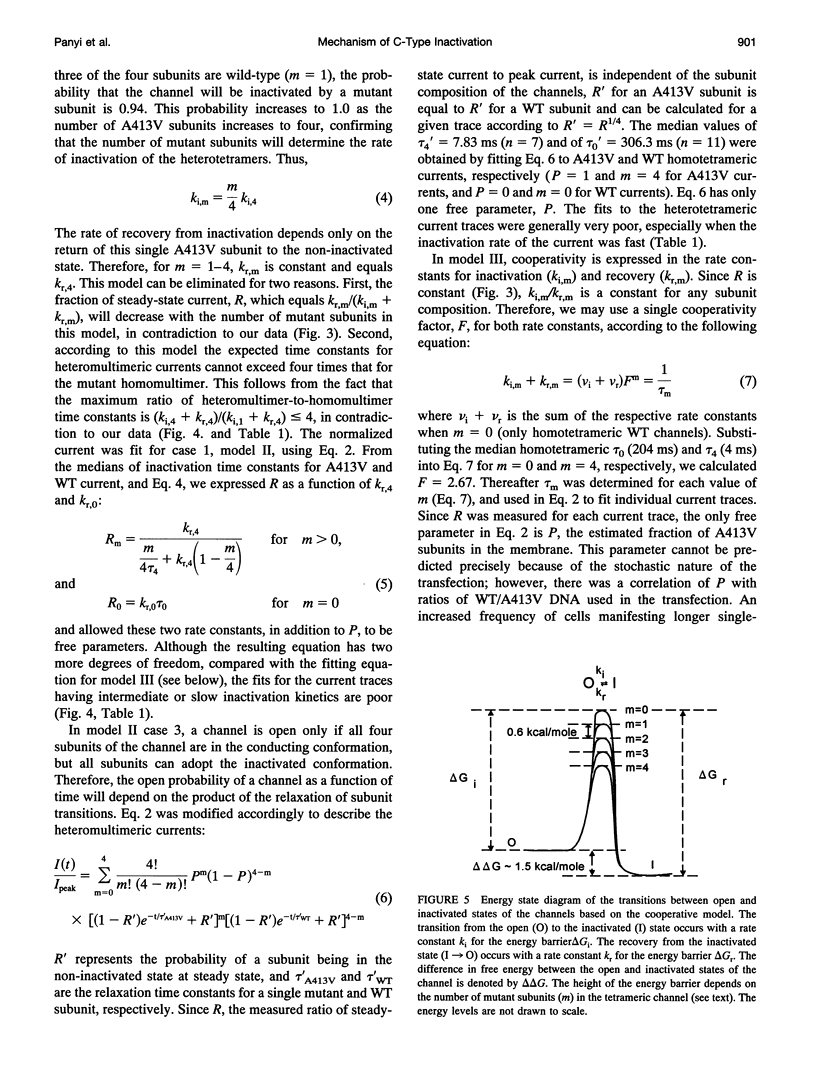
The lymphocyte voltage-gated K+ channel, Kv1.3, inactivates by a C-type process. We have elucidated the molecular basis for this process using a kinetic analysis of wild-type and mutant (A413V) Kv1.3 homo- and heteromultimeric currents in a mammalian lymphoid expression system. The medians of the measured inactivation time constants for wild-type and A413V homotetrameric currents are 204 and 4 ms, respectively. Co-expression of these subunits produces heteromultimeric channels manifesting inactivation kinetics intermediate between those of wild-type and A413V homomultimers. We have considered several models in which each subunit acts either independently or cooperatively to produce the observed inactivation kinetics. The cooperative model gives excellent fits to the data for any heteromultimeric composition of subunits, clearly distinguishing it from the independent models. In the cooperative model, the difference in free energy between the open and inactivated states of the channel is invariant with subunit composition and equals approximately 1.5 kcal/mol. Each subunit contributes equally to the activation free energy for transitions between open and inactivated states, with an A413V subunit decreasing the free energy barrier for inactivation (and for recovery from inactivation) by approximately 0.6 kcal/mol. Our results are consistent with a physical model in which the outer mouth of the channel constricts during C-type inactivation (G. Yellen, D. Sodickson, T. Chen, and M.E. Jurman, 1994, Biophys. J. 66:1068-1075).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991 Nov;7(5):743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Chen L. Q. Heterologous expression of specific K+ channels in T lymphocytes: functional consequences for volume regulation. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10036–10040. doi: 10.1073/pnas.90.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B. D., Price M. A., Deutsch C. J. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J Immunol. 1992 Dec 15;149(12):3784–3794. [PubMed] [Google Scholar]
- Gomez-Lagunas F., Armstrong C. M. Inactivation in ShakerB K+ channels: a test for the number of inactivating particles on each channel. Biophys J. 1995 Jan;68(1):89–95. doi: 10.1016/S0006-3495(95)80162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989 Jan;55(1):203–206. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Hurst R. S., Kavanaugh M. P., Yakel J., Adelman J. P., North R. A. Cooperative interactions among subunits of a voltage-dependent potassium channel. Evidence from expression of concatenated cDNAs. J Biol Chem. 1992 Nov 25;267(33):23742–23745. [PubMed] [Google Scholar]
- Kavanaugh M. P., Hurst R. S., Yakel J., Varnum M. D., Adelman J. P., North R. A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992 Mar;8(3):493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Levy D. I., Deutsch C. Diverse K+ channels in primary human T lymphocytes. J Gen Physiol. 1992 May;99(5):771–793. doi: 10.1085/jgp.99.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- MacKinnon R., Aldrich R. W., Lee A. W. Functional stoichiometry of Shaker potassium channel inactivation. Science. 1993 Oct 29;262(5134):757–759. doi: 10.1126/science.7694359. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Tu L., Santarelli V., Deutsch C. Truncated K+ channel DNA sequences specifically suppress lymphocyte K+ channel gene expression. Biophys J. 1995 Jan;68(1):147–156. doi: 10.1016/S0006-3495(95)80169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994 Apr;66(4):1068–1075. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]


