Abstract
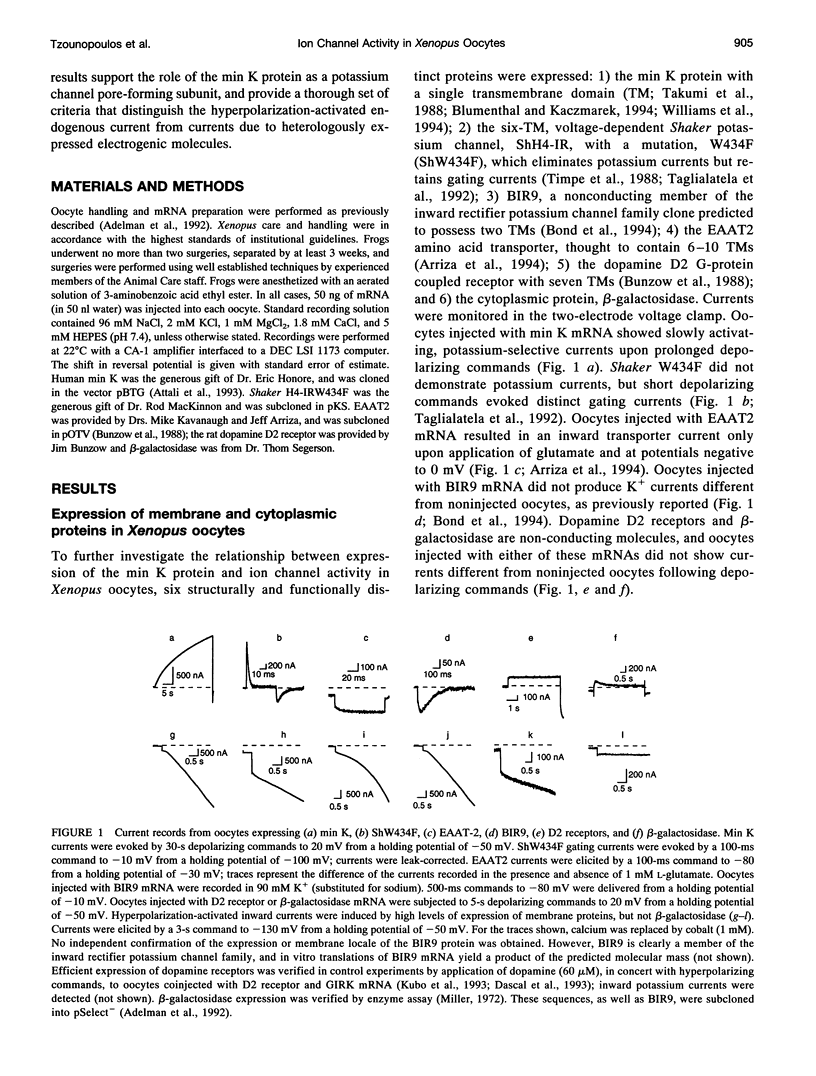
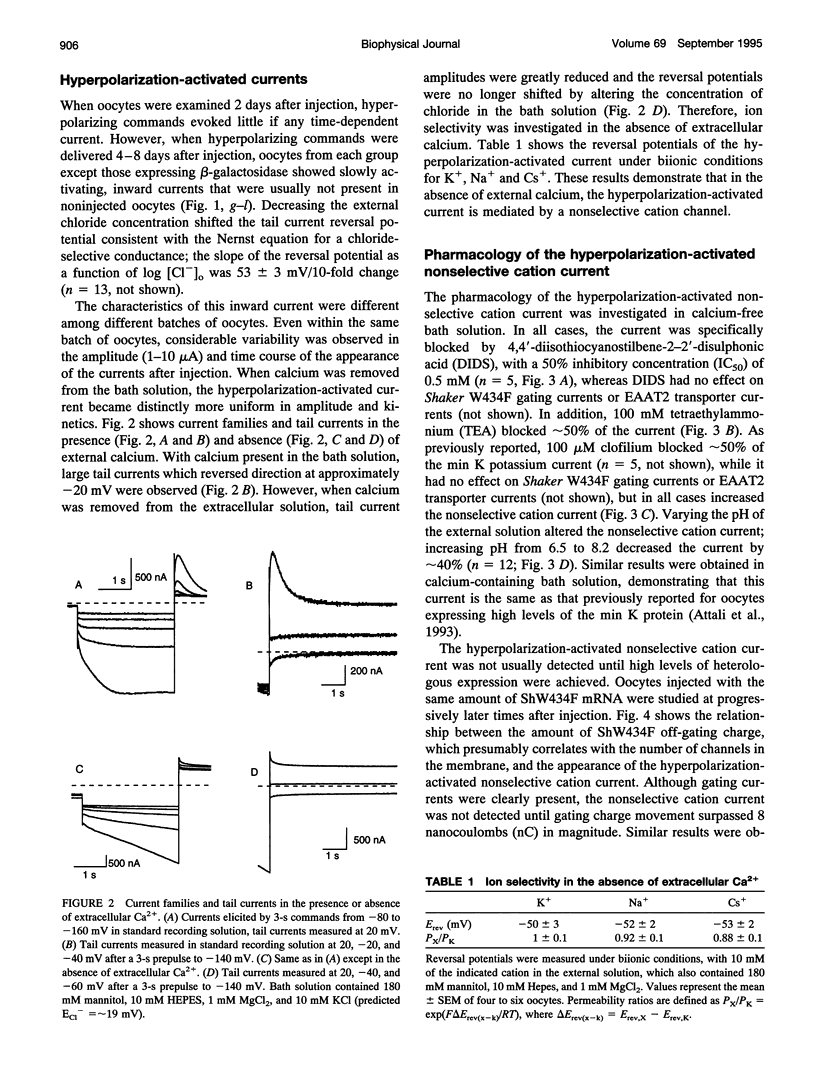
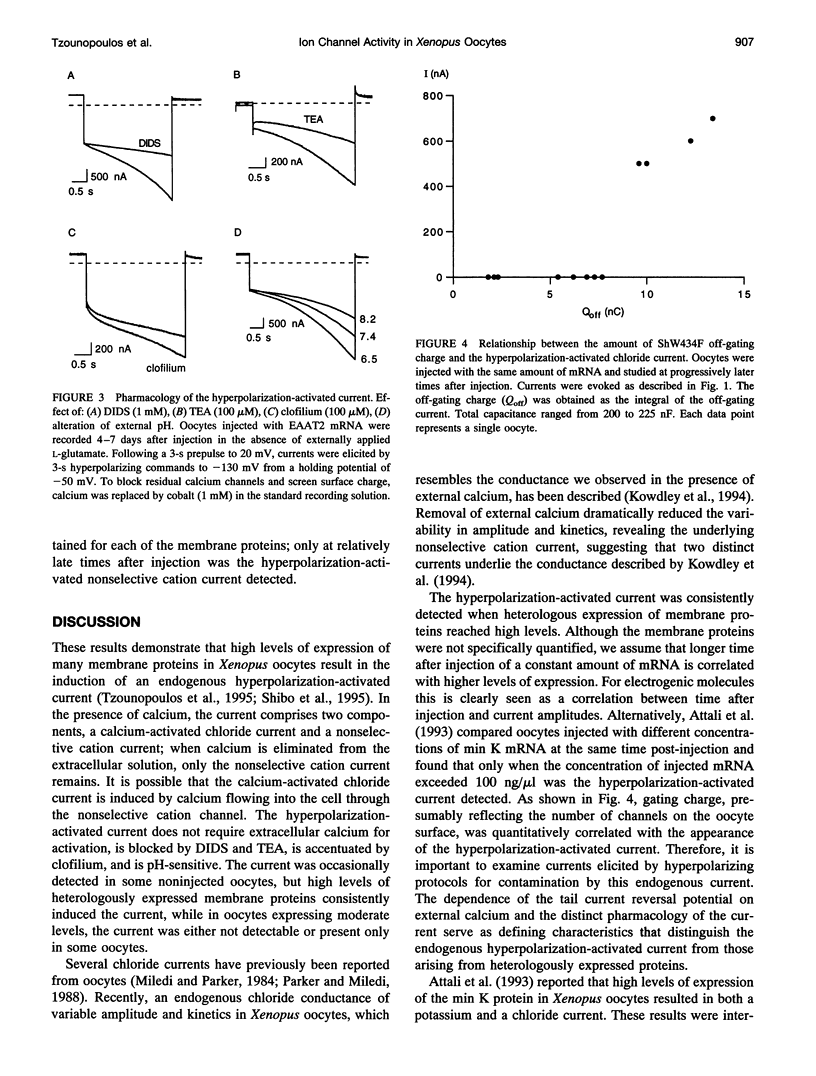
Xenopus oocytes are widely employed for heterologous expression of cloned proteins, particularly electrogenic molecules such as ion channels and transporters. The high levels of expression readily obtained permit detailed investigations without interference from endogenous conductances. Injection of min K mRNA into Xenopus oocytes results in expression of voltage-dependent potassium-selective channels. Recent data show that injections of high concentrations of min K mRNA also induce a chloride current with very different biophysical, pharmacological, and regulatory properties from the min K potassium current. This led to the suggestion that the min K protein acts as an inducer of endogenous, normally silent oocyte ion channels. We now report that high levels of heterologous expression of many membrane proteins in Xenopus oocytes specifically induce this chloride current and a hyperpolarization-activated cation-selective current. The current is blocked by 4,4'-diisothiocyanostilbene-2-2'-disulphonic acid and tetraethylammonium, enhanced by clofilium, and is pH-sensitive. Criteria are presented that distinguish this endogenous current from those due to heterologous expression of electrogenic proteins in Xenopus oocytes. Together with structure-function studies, these results support the hypothesis that the min K protein comprises a potassium-selective channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Shen K. Z., Kavanaugh M. P., Warren R. A., Wu Y. N., Lagrutta A., Bond C. T., North R. A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992 Aug;9(2):209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Fairman W. A., Wadiche J. I., Murdoch G. H., Kavanaugh M. P., Amara S. G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994 Sep;14(9):5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Blumenthal E. M., Kaczmarek L. K. The minK potassium channel exists in functional and nonfunctional forms when expressed in the plasma membrane of Xenopus oocytes. J Neurosci. 1994 May;14(5 Pt 2):3097–3105. doi: 10.1523/JNEUROSCI.14-05-03097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. T., Pessia M., Xia X. M., Lagrutta A., Kavanaugh M. P., Adelman J. P. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2(3):183–191. [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Dascal N., Schreibmayer W., Lim N. F., Wang W., Chavkin C., DiMagno L., Labarca C., Kieffer B. L., Gaveriaux-Ruff C., Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991 Sep;7(3):403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Kowdley G. C., Ackerman S. J., John J. E., 3rd, Jones L. R., Moorman J. R. Hyperpolarization-activated chloride currents in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):217–230. doi: 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. A calcium-independent chloride current activated by hyperpolarization in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):191–199. doi: 10.1098/rspb.1988.0018. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular basis of potassium channel diversity. Pflugers Arch. 1989;414 (Suppl 1):S71–S75. doi: 10.1007/BF00582251. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Toro L., Stefani E. Novel voltage clamp to record small, fast currents from ion channels expressed in Xenopus oocytes. Biophys J. 1992 Jan;61(1):78–82. doi: 10.1016/S0006-3495(92)81817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T., Moriyoshi K., Aramori I., Ishii T., Oiki S., Okada Y., Ohkubo H., Nakanishi S. Alteration of channel activities and gating by mutations of slow ISK potassium channel. J Biol Chem. 1991 Nov 25;266(33):22192–22198. [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]


