Abstract
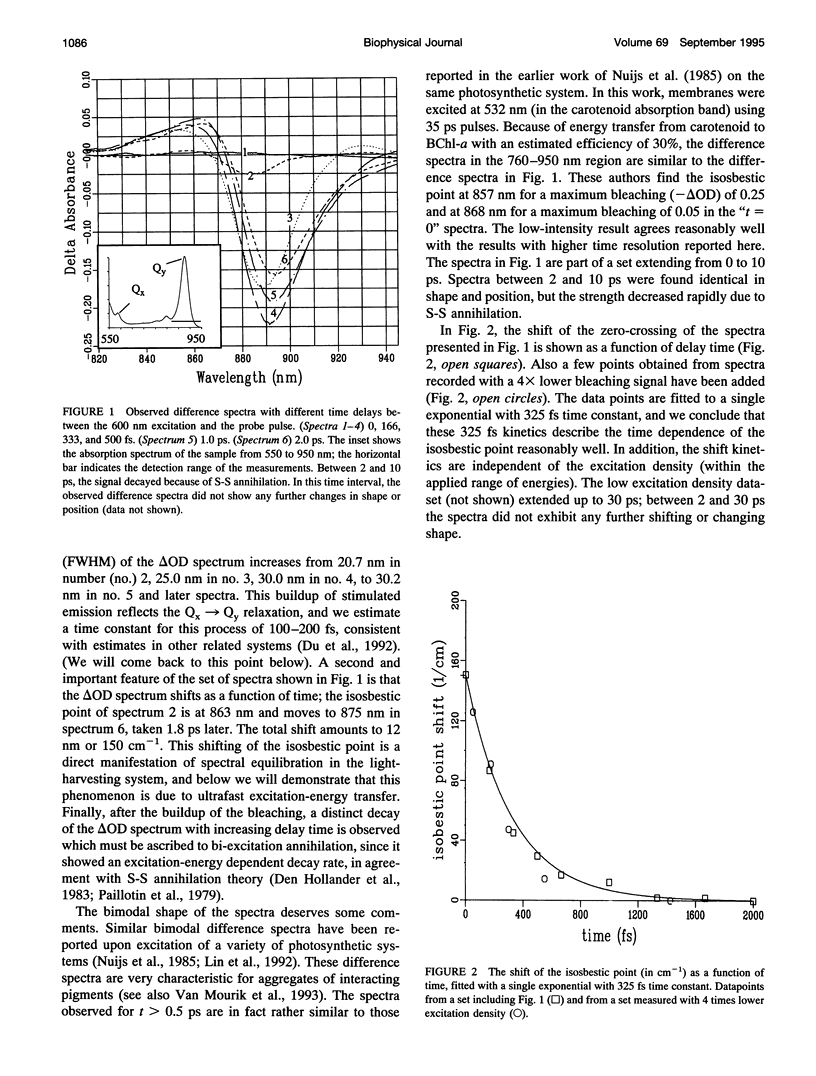
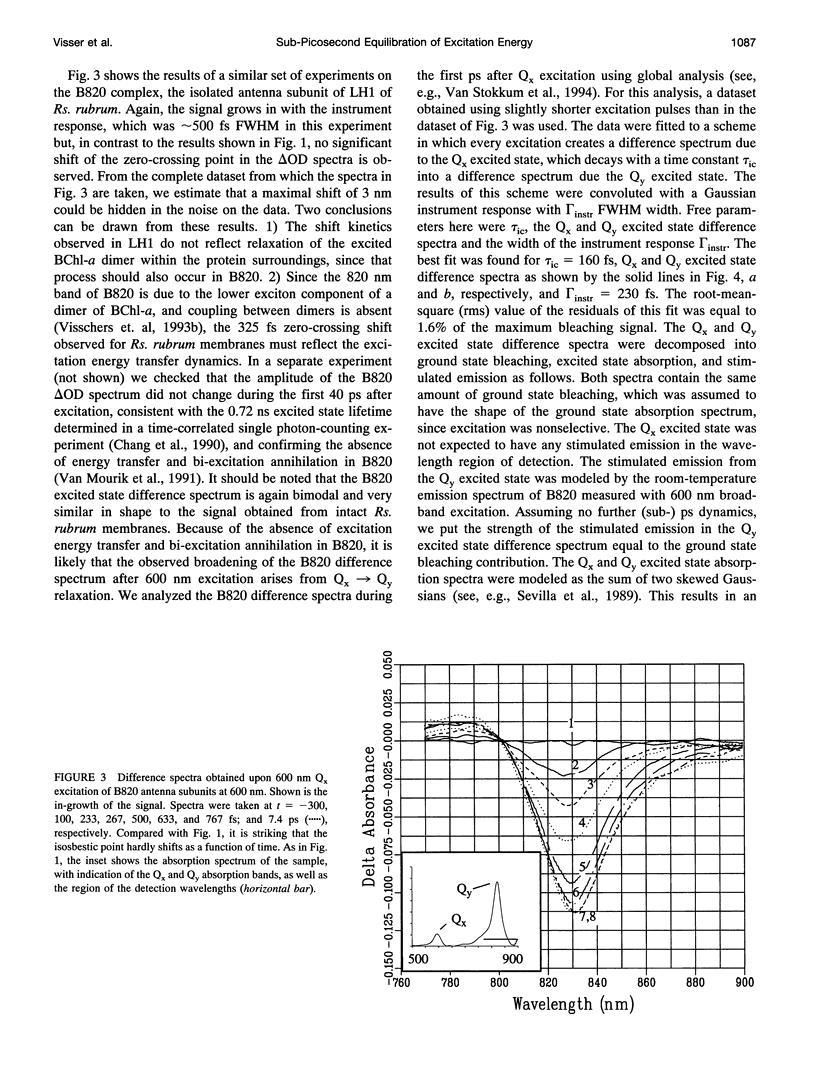
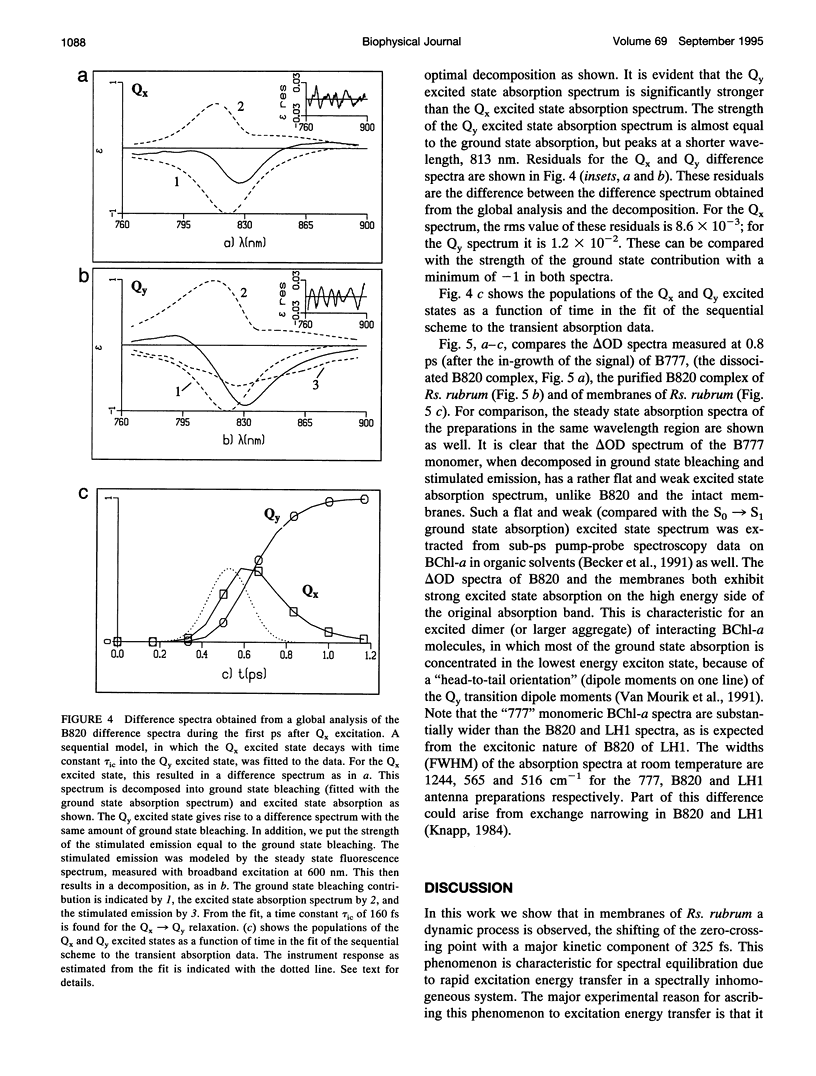
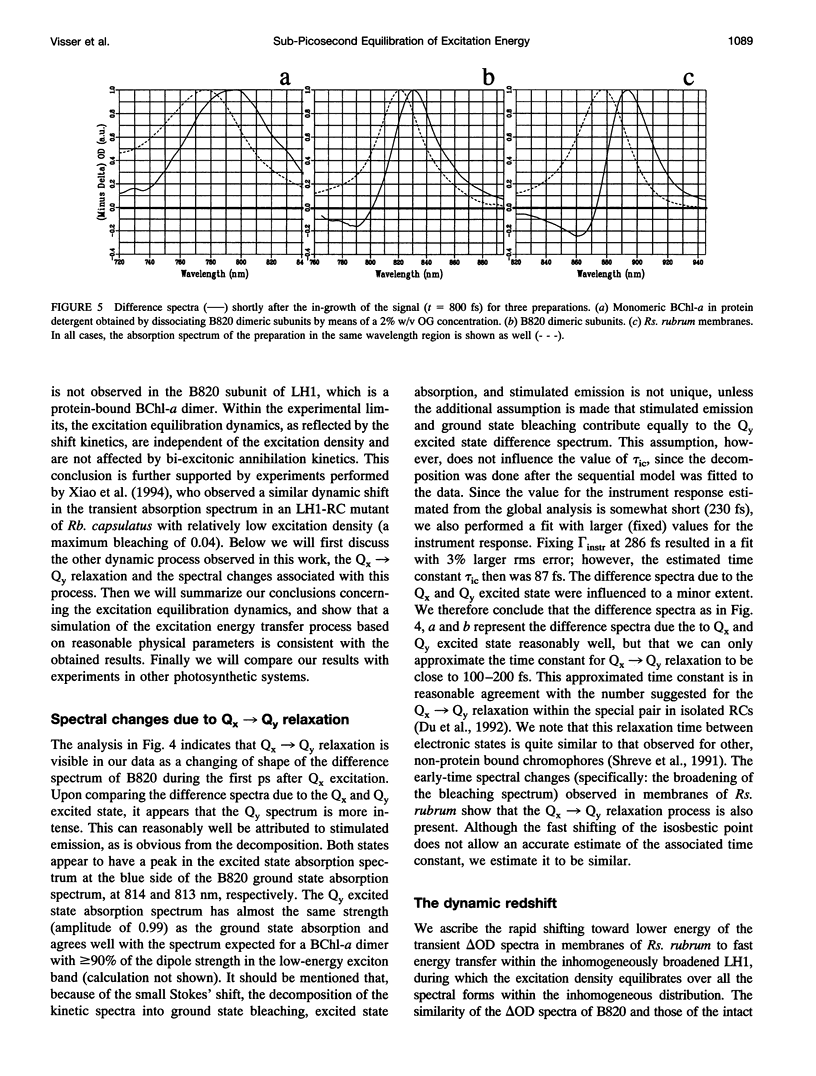
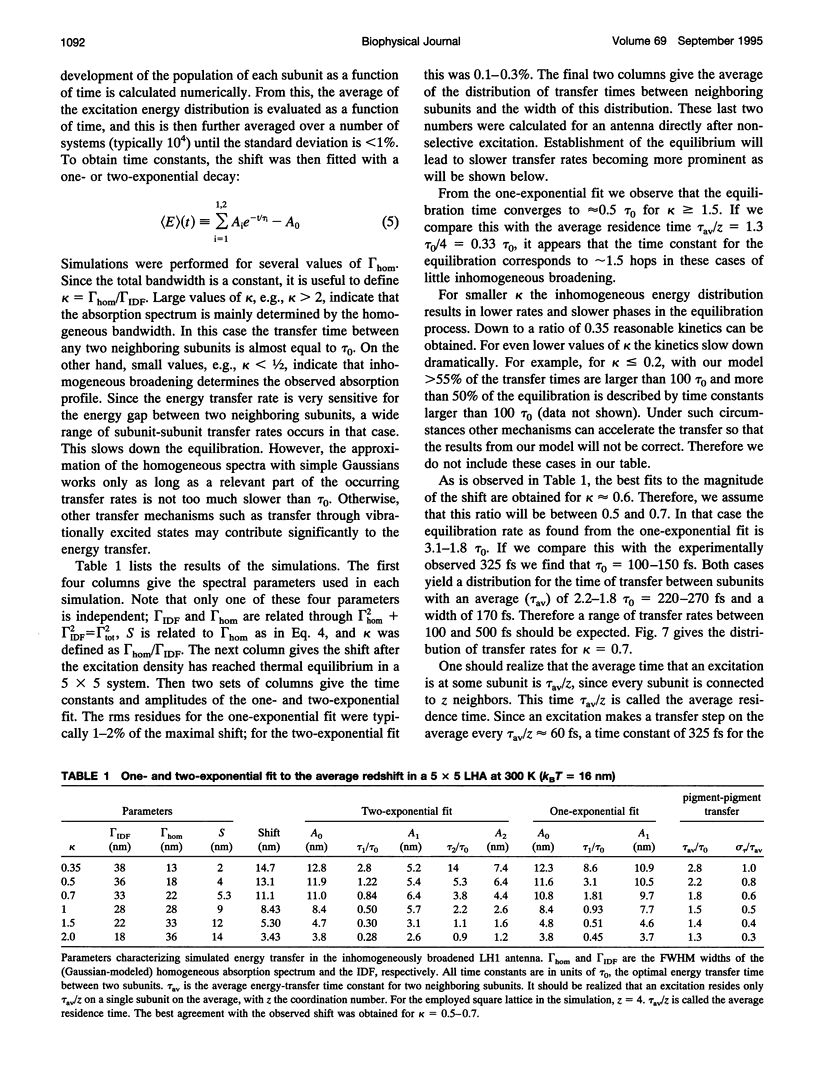
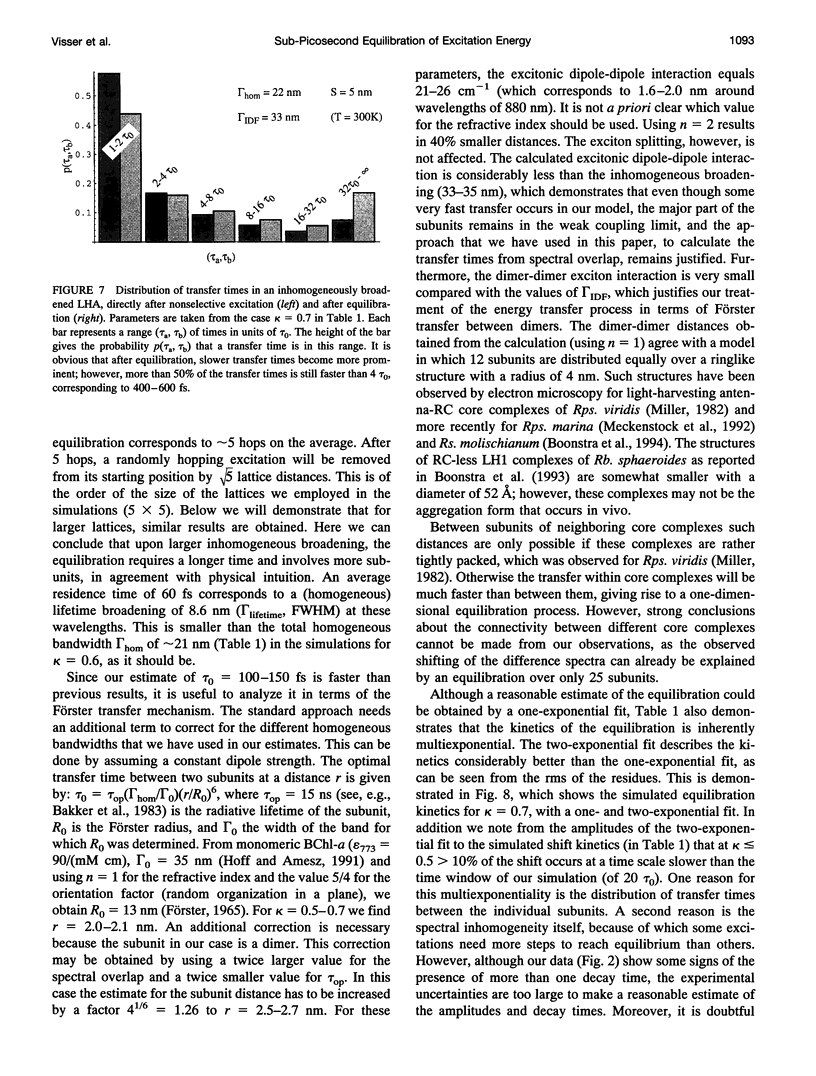
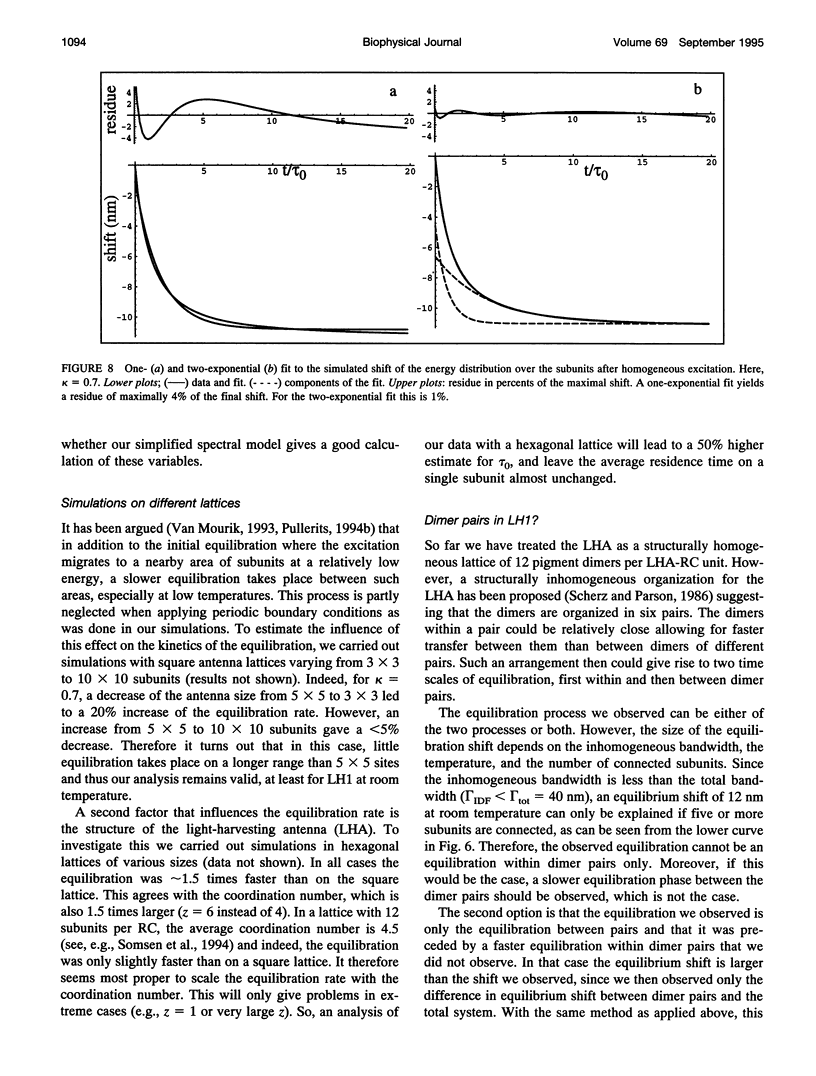
Excitation energy transfer in the light-harvesting antenna of Rhodospirillum rubrum was studied at room temperature using sub-picosecond transient absorption measurements. Upon excitation of Rs. rubrum membranes with a 200 fs, 600 nm laser flash in the Qx transition of the bacteriochlorophyll-a (BChl-a) absorption, the induced transient absorption changes in the Qy region were monitored. In Rs. rubrum membranes the observed delta OD spectrum exhibits ground state bleaching, excited state absorption and stimulated emission. Fast Qx --> Qy relaxation occurs in approximately 100-200 fs as reflected by the building up of stimulated emission. An important observation is that the zero-crossing of the transient difference absorption (delta OD) spectrum exhibits a dynamic redshift from 863 to 875 nm that can be described with by a single exponential with 325 fs time constant. The shape of the transient difference spectrum observed in a purified subunit of the core light-harvesting antenna, B820, consisting of only a single interacting pair of BChl-as, is similar to the spectrum observed in Rs. rubrum membranes and clearly different from the spectrum of BChl-a in a protein/detergent mixture. In the B820 and monomeric BChl-a preparations the 100-200 fs Qx --> Qy relaxation is still observed, but the dynamic redshift of the delta OD spectrum is absent. The spectral kinetics observed in the Rs. rubrum membranes are interpreted in terms of the dynamics of excitation equilibration among the antenna subunits that constitute the inhomogeneously broadened antenna. A simulation of this process using a set of reasonable physical parameters is consistent with an average hopping time in the core light harvesting of 220-270 fs, resulting in an average single-site excitation lifetime of 50-70 fs. The observed rate of this equilibration process is in reasonable agreement with earlier estimations for the hopping time from more indirect measurements. The implications of the findings for the process of excitation trapping by reaction centers will be discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman L. M., van Mourik F., Jones M. R., Visser H. M., Hunter C. N., van Grondelle R. Trapping kinetics in mutants of the photosynthetic purple bacterium Rhodobacter sphaeroides: influence of the charge separation rate and consequences for the rate-limiting step in the light-harvesting process. Biochemistry. 1994 Mar 22;33(11):3143–3147. doi: 10.1021/bi00177a001. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Callahan P. M., Parkes-Loach P. S., Cotton T. M., Loach P. A. Spectroscopic characterization of the light-harvesting complex of Rhodospirillum rubrum and its structural subunit. Biochemistry. 1990 Jan 16;29(2):421–429. doi: 10.1021/bi00454a017. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Du M., Rosenthal S. J., Xie X., DiMagno T. J., Schmidt M., Hanson D. K., Schiffer M., Norris J. R., Fleming G. R. Femtosecond spontaneous-emission studies of reaction centers from photosynthetic bacteria. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8517–8521. doi: 10.1073/pnas.89.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S., Visscher K. J., Pullerits T., Sundström V., Fowler G. J., Hunter C. N. Enhanced rates of subpicosecond energy transfer in blue-shifted light harvesting LH2 mutants of Rhodobacter sphaeroides. Biochemistry. 1994 Jul 12;33(27):8300–8305. doi: 10.1021/bi00193a017. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R., Schatz G., Brock H., Bittersmann E. Energy transfer and charge separation kinetics in photosystem I: Part 1: Picosecond transient absorption and fluorescence study of cyanobacterial photosystem I particles. Biophys J. 1993 Jun;64(6):1813–1826. doi: 10.1016/S0006-3495(93)81552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. N., van Grondelle R., Olsen J. D. Photosynthetic antenna proteins: 100 ps before photochemistry starts. Trends Biochem Sci. 1989 Feb;14(2):72–76. doi: 10.1016/0968-0004(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Jean J. M., Chan C. K., Fleming G. R., Owens T. G. Excitation transport and trapping on spectrally disordered lattices. Biophys J. 1989 Dec;56(6):1203–1215. doi: 10.1016/S0006-3495(89)82767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Lin S., Chiou H. C., Kleinherenbrink F. A., Blankenship R. E. Time-resolved spectroscopy of energy and electron transfer processes in the photosynthetic bacterium Heliobacillus mobilis. Biophys J. 1994 Feb;66(2 Pt 1):437–445. doi: 10.1016/s0006-3495(94)80794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckenstock R. U., Krusche K., Brunisholz R. A., Zuber H. The light-harvesting core-complex and the B820-subunit from Rhodopseudomonas marina. Part II. Electron microscopic characterisation. FEBS Lett. 1992 Oct 19;311(2):135–138. doi: 10.1016/0014-5793(92)81384-x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Hinchigeri S. B., Parkes-Loach P. S., Callahan P. M., Sprinkle J. R., Riccobono J. R., Loach P. A. Isolation and characterization of a subunit form of the light-harvesting complex of Rhodospirillum rubrum. Biochemistry. 1987 Aug 11;26(16):5055–5062. doi: 10.1021/bi00390a026. [DOI] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna size dependence of fluorescence decay in the core antenna of photosystem I: estimates of charge separation and energy transfer rates. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1532–1536. doi: 10.1073/pnas.84.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Swenberg C. E., Breton J., Geacintov N. E. Analysis of picosecond laser induced fluorescence phenomena in photosynthetic membranes utilizing a master equation approach. Biophys J. 1979 Mar;25(3):513–533. doi: 10.1016/S0006-3495(79)85320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits T., Freiberg A. Kinetic model of primary energy transfer and trapping in photosynthetic membranes. Biophys J. 1992 Oct;63(4):879–896. doi: 10.1016/S0006-3495(92)81688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits T., Visscher K. J., Hess S., Sundström V., Freiberg A., Timpmann K., van Grondelle R. Energy transfer in the inhomogeneously broadened core antenna of purple bacteria: a simultaneous fit of low-intensity picosecond absorption and fluorescence kinetics. Biophys J. 1994 Jan;66(1):236–248. doi: 10.1016/S0006-3495(94)80770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijgersberg C. P., van Grondelle R., Amesz J. Energy transfer and bacteriochlorophyll fluorescence in purple bacteria at low temperature. Biochim Biophys Acta. 1980 Aug 5;592(1):53–64. doi: 10.1016/0005-2728(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Savikhin S., Zhou W., Blankenship R. E., Struve W. S. Femtosecond energy transfer and spectral equilibration in bacteriochlorophyll a--protein antenna trimers from the green bacterium Chlorobium tepidum. Biophys J. 1994 Jan;66(1):110–113. doi: 10.1016/S0006-3495(94)80769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen O. J., van Mourik F., van Grondelle R., Valkunas L. Energy migration and trapping in a spectrally and spatially inhomogeneous light-harvesting antenna. Biophys J. 1994 May;66(5):1580–1596. doi: 10.1016/S0006-3495(94)80950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A. K., Stocker J. W., Alden R. G., Causgrove T. P., Peloquin J. M., Boxer S. G., Woodbury N. W. Biochemical characterization and electron-transfer reactions of sym1, a Rhodobacter capsulatus reaction center symmetry mutant which affects the initial electron donor. Biochemistry. 1992 Oct 27;31(42):10345–10355. doi: 10.1021/bi00157a024. [DOI] [PubMed] [Google Scholar]
- Trautman J. K., Shreve A. P., Violette C. A., Frank H. A., Owens T. G., Albrecht A. C. Femtosecond dynamics of energy transfer in B800-850 light-harvesting complexes of Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jan;87(1):215–219. doi: 10.1073/pnas.87.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkunas G., Holzwarth A. R. Kinetic modeling of exciton migration in photosynthetic systems. 2. Simulations of excitation dynamics in two-dimensional photosystem I core antenna/reaction center complexes. Biophys J. 1994 Feb;66(2 Pt 1):415–429. doi: 10.1016/s0006-3495(94)80792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Lin S., Taguchi A. K., Woodbury N. W. Femtosecond pump-probe analysis of energy and electron transfer in photosynthetic membranes of Rhodobacter capsulatus. Biochemistry. 1994 Jul 12;33(27):8313–8322. doi: 10.1021/bi00193a019. [DOI] [PubMed] [Google Scholar]