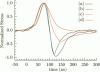
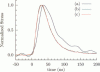
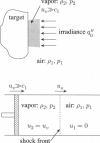
Abstract
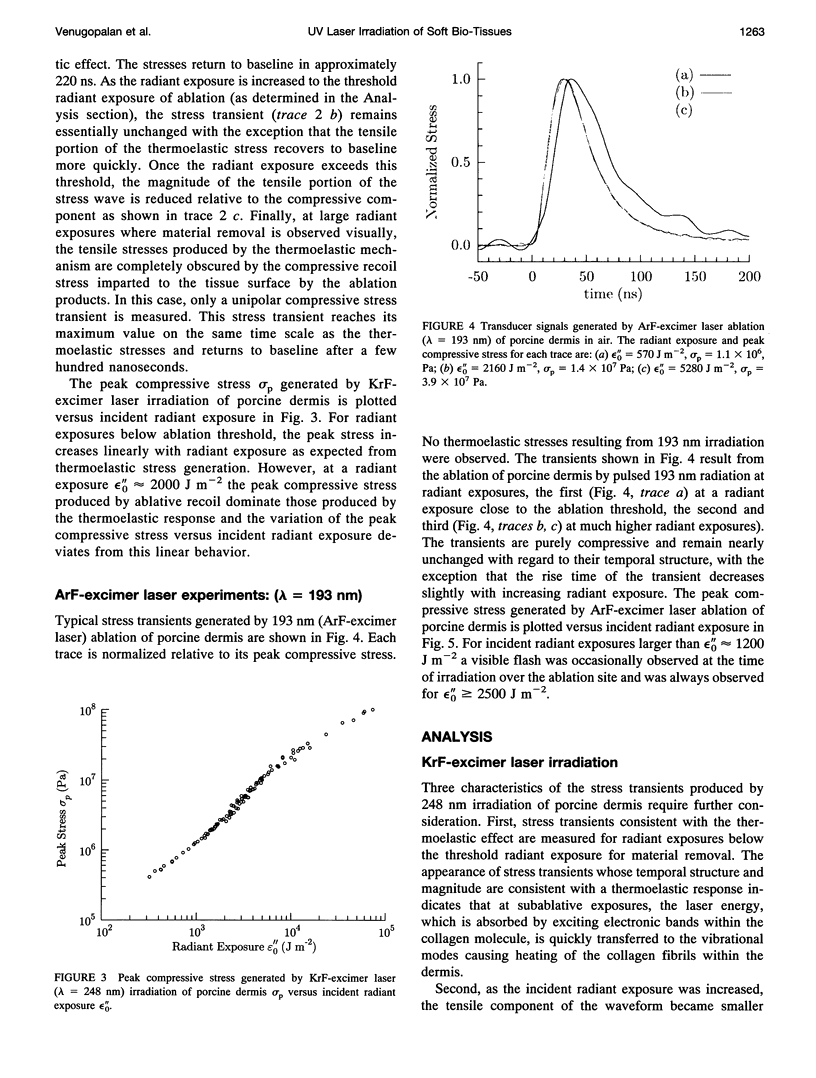
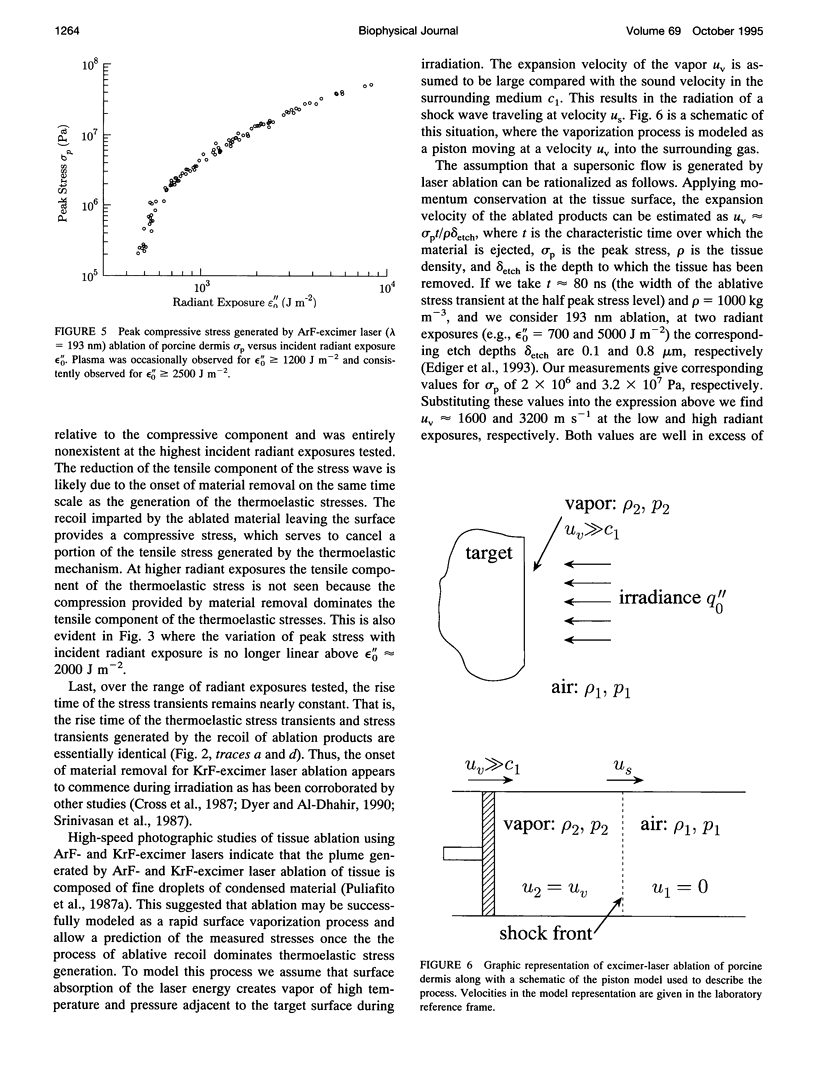
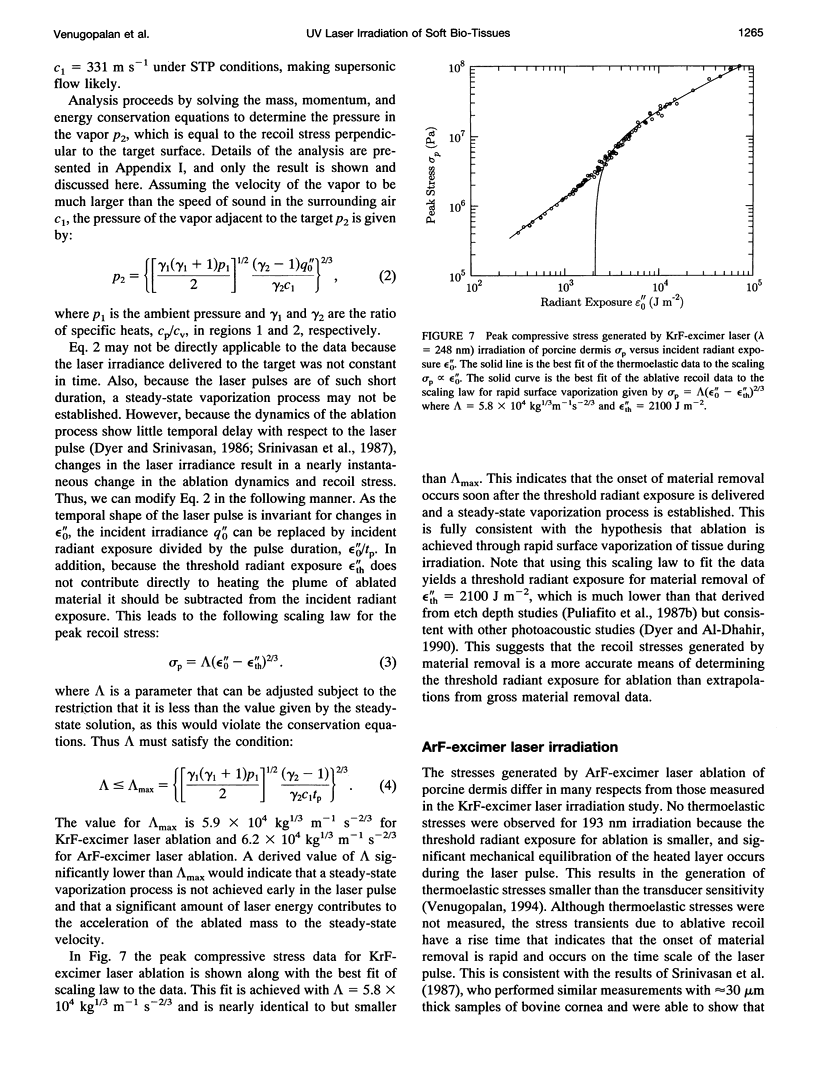
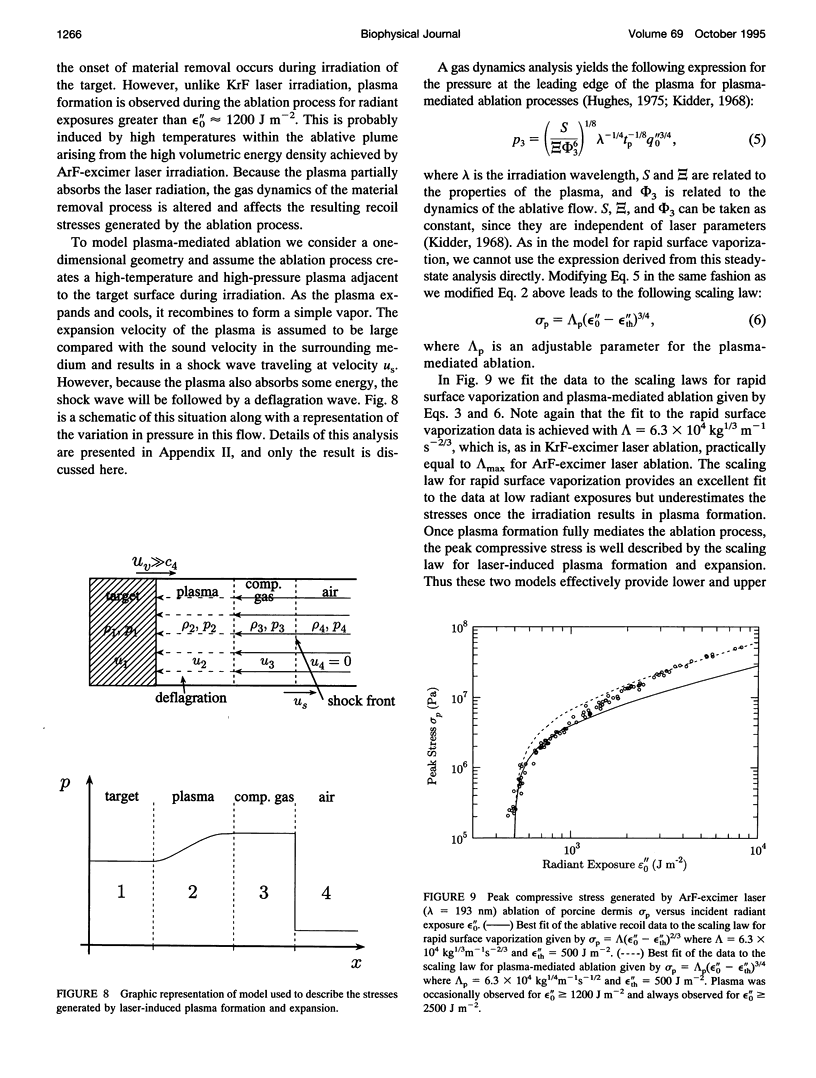
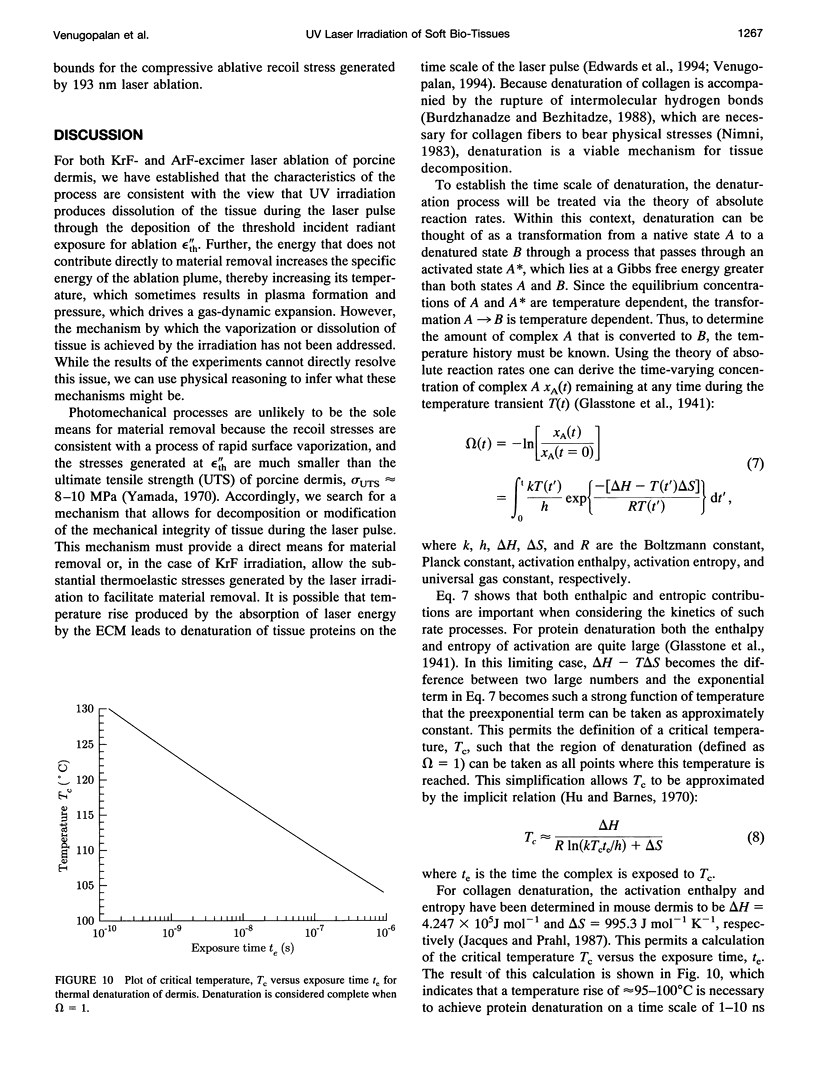
The physical mechanisms that enable short pulses of high-intensity ultraviolet laser radiation to remove tissue, in a process known as laser ablation, remain obscure. The thermodynamic response of biological tissue to pulsed laser irradiation was investigated by measuring and subsequently analyzing the stress transients generated by pulsed argon fluorine (ArF, lambda = 193 nm) and krypton fluorine (KrF, lambda = 248 nm) excimer laser irradiation of porcine dermis using thin-film piezoelectric transducers. For radiant exposures that do not cause material removal, the stress transients are consistent with rapid thermal expansion of the tissue. At the threshold radiant exposure for ablation, the peak stress amplitude generated by 248 nm irradiation is more than an order of magnitude larger than that produced by 193 nm irradiation. For radiant exposures where material removal is achieved, the temporal structure of the stress transient indicates that the onset of material removal occurs during irradiation. In this regime, the variation of the peak compressive stress with radiant exposure is consistent with laser-induced rapid surface vaporization. For 193 nm irradiation, ionization of the ablated material occurs at even greater radiant exposures and is accompanied by a change in the variation of peak stress with radiant exposure consistent with a plasma-mediated ablation process. These results suggest that absorption of ultraviolet laser radiation by the extracellular matrix of tissue leads to decomposition of tissue on the time scale of the laser pulse. The difference in volumetric energy density at ablation threshold between the two wavelengths indicates that the larger stresses generated by 248 nm irradiation may facilitate the onset of material removal. However, once material removal is achieved, the stress measurements demonstrate that energy not directly responsible for target decomposition contributes to increasing the specific energy of the plume (and plasma, when present), which drives the gas dynamic expansion of ablated material. This provides direct evidence that ultraviolet laser ablation of soft biological tissues is a surface-mediated process and not explosive in nature.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albagli D., Banish B., Dark M., Janes G. S., von Rosenberg C., Perelman L., Itzkan I., Feld M. S. Interferometric surface monitoring of biological tissue to study inertially confined ablation. Lasers Surg Med. 1994;14(4):374–385. doi: 10.1002/lsm.1900140410. [DOI] [PubMed] [Google Scholar]
- Anderson R. R., Parrish J. A. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983 Apr 29;220(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- Burdzhanadze T. V., Bezhitadze M. O. Termodinamicheskaia i strukturnaia kharakteristika chastichno denaturirovannogo kollagena. Biofizika. 1988 Mar-Apr;33(2):220–225. [PubMed] [Google Scholar]
- Ediger M. N., Pettit G. H., Weiblinger R. P., Chen C. H. Transmission of corneal collagen during ArF excimer laser ablation. Lasers Surg Med. 1993;13(2):204–210. doi: 10.1002/lsm.1900130208. [DOI] [PubMed] [Google Scholar]
- Edwards G., Logan R., Copeland M., Reinisch L., Davidson J., Johnson B., Maciunas R., Mendenhall M., Ossoff R., Tribble J. Tissue ablation by a free-electron laser tuned to the amide II band. Nature. 1994 Sep 29;371(6496):416–419. doi: 10.1038/371416a0. [DOI] [PubMed] [Google Scholar]
- Hu C. L., Barnes F. S. The thermal-chemical damage in biological material under laser irradiation. IEEE Trans Biomed Eng. 1970 Jul;17(3):220–229. doi: 10.1109/tbme.1970.4502736. [DOI] [PubMed] [Google Scholar]
- Jacques S. L., Prahl S. A. Modeling optical and thermal distributions in tissue during laser irradiation. Lasers Surg Med. 1987;6(6):494–503. doi: 10.1002/lsm.1900060604. [DOI] [PubMed] [Google Scholar]
- Lane R. J., Linsker R., Wynne J. J., Torres A., Geronemus R. G. Ultraviolet-laser ablation of skin. Arch Dermatol. 1985 May;121(5):609–617. [PubMed] [Google Scholar]
- Long C. G., Braswell E., Zhu D., Apigo J., Baum J., Brodsky B. Characterization of collagen-like peptides containing interruptions in the repeating Gly-X-Y sequence. Biochemistry. 1993 Nov 2;32(43):11688–11695. doi: 10.1021/bi00094a027. [DOI] [PubMed] [Google Scholar]
- Lustmann J., Ulmansky M., Fuxbrunner A., Lewis A. Photoacoustic injury and bone healing following 193nm excimer laser ablation. Lasers Surg Med. 1992;12(4):390–396. doi: 10.1002/lsm.1900120407. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrevlishvili G. M. Termodinamicheskie svoistva biopolimerov v spiral'nom i klubkovom sostoianiiakh v intervale temperature 4--400 gradusov K. Biofizika. 1977 Jan-Feb;22(1):180–191. [PubMed] [Google Scholar]
- Nimni M. E. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum. 1983 Aug;13(1):1–86. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Nomura S., Hiltner A., Lando J. B., Baer E. Interaction of water with native collagen. Biopolymers. 1977 Feb;16(2):231–246. doi: 10.1002/bip.1977.360160202. [DOI] [PubMed] [Google Scholar]
- Puliafito C. A., Steinert R. F., Deutsch T. F., Hillenkamp F., Dehm E. J., Adler C. M. Excimer laser ablation of the cornea and lens. Experimental studies. Ophthalmology. 1985 Jun;92(6):741–748. doi: 10.1016/s0161-6420(85)33962-3. [DOI] [PubMed] [Google Scholar]
- Puliafito C. A., Stern D., Krueger R. R., Mandel E. R. High-speed photography of excimer laser ablation of the cornea. Arch Ophthalmol. 1987 Sep;105(9):1255–1259. doi: 10.1001/archopht.1987.01060090113039. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Holbrook K. A., Byers P. H. Structure of the dermal matrix during development and in the adult. J Invest Dermatol. 1982 Jul;79 (Suppl 1):93s–104s. doi: 10.1111/1523-1747.ep12545877. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Dyer P. E., Braren B. Far-ultraviolet laser ablation of the cornea: photoacoustic studies. Lasers Surg Med. 1987;6(6):514–519. doi: 10.1002/lsm.1900060606. [DOI] [PubMed] [Google Scholar]
- Walsh J. T., Jr, Flotte T. J., Anderson R. R., Deutsch T. F. Pulsed CO2 laser tissue ablation: effect of tissue type and pulse duration on thermal damage. Lasers Surg Med. 1988;8(2):108–118. doi: 10.1002/lsm.1900080204. [DOI] [PubMed] [Google Scholar]
- Walsh J. T., Jr, Flotte T. J., Deutsch T. F. Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9(4):314–326. doi: 10.1002/lsm.1900090403. [DOI] [PubMed] [Google Scholar]
- Yashima Y., McAuliffe D. J., Jacques S. L., Flotte T. J. Laser-induced photoacoustic injury of skin: effect of inertial confinement. Lasers Surg Med. 1991;11(1):62–68. doi: 10.1002/lsm.1900110113. [DOI] [PubMed] [Google Scholar]