Abstract
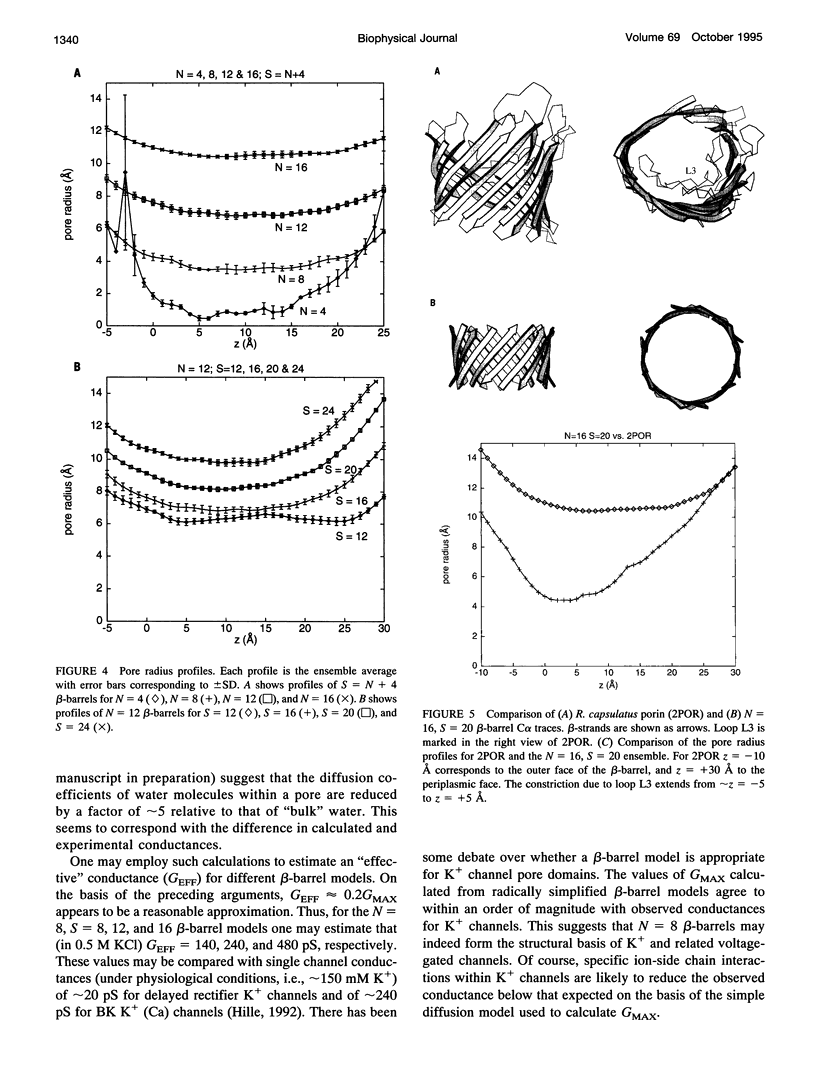
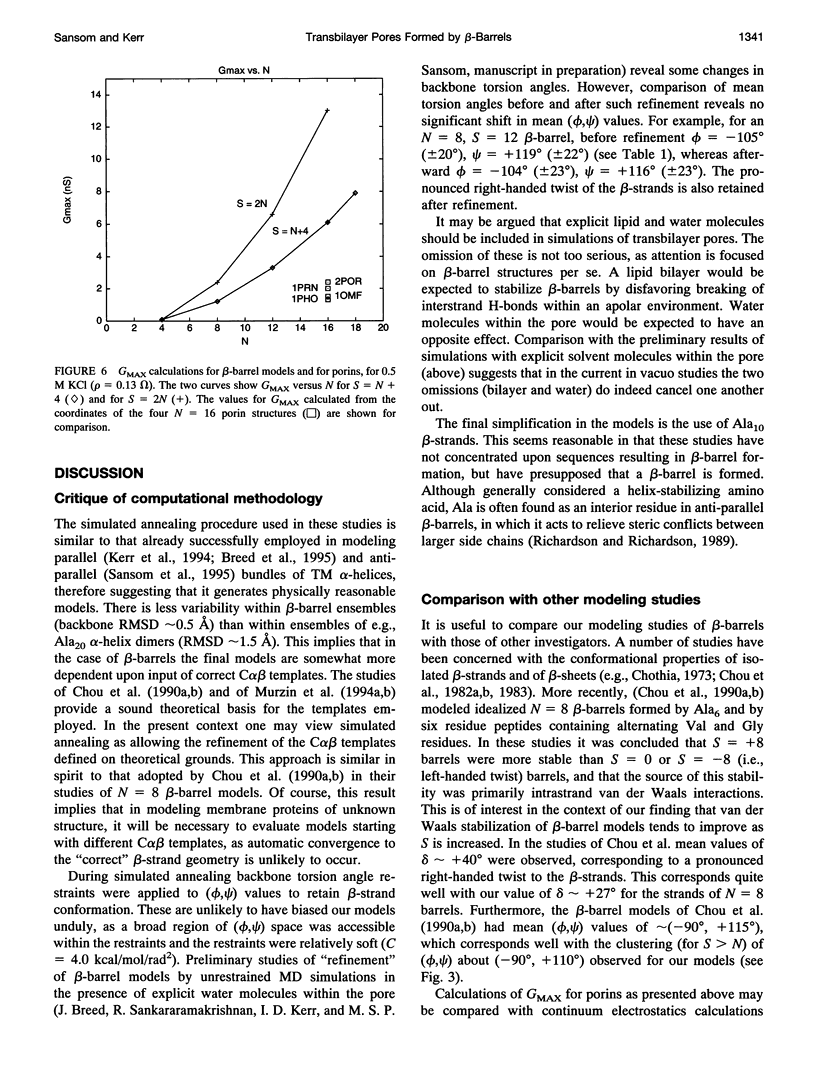
Transmembrane beta-barrels, first observed in bacterial porins, are possible models for a number of membrane channels. Restrained molecular dynamics simulations based on idealized C alpha beta templates have been used to generate models of such beta-barrels. Model beta-barrels have been analyzed in terms of their conformational, energetic, and pore properties. Model beta-barrels formed by N = 4, 8, 12 and 16 anti-parallel Ala10 strands have been developed. For each N, beta-barrels with shear numbers S = N to 2N have been modeled. In all beta-barrel models the constituent beta-strands adopt a pronounced right-handed twist. Interstrand interactions are of approximately equal stability for all models with N > or = 8, whereas such interactions are weaker for the N = 4 beta-barrels. In N = 4 beta-barrels the pore is too narrow (minimum radius approximately 0.6 A) to allow ion permeation. For N > or = 8, the pore radius depends on both N and S; for a given value of N an increase in S from N to 2N is predicted to result in an approximately threefold increase in pore conductance. Calculated maximal conductances for the beta-barrel models are compared with experimental values for porins and for K+ channels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994 Jun 29;1197(2):167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Bogusz S., Boxer A., Busath D. D. An SS1-SS2 beta-barrel structure for the voltage-activated potassium channel. Protein Eng. 1992 Jun;5(4):285–293. doi: 10.1093/protein/5.4.285. [DOI] [PubMed] [Google Scholar]
- Bradley J. C., Richards W. G. Potassium channels: a computer prediction of structure and selectivity. Protein Eng. 1994 Jul;7(7):859–862. doi: 10.1093/protein/7.7.859. [DOI] [PubMed] [Google Scholar]
- Breed J., Kerr I. D., Sankararamakrishnan R., Sansom M. S. Packing interactions of Aib-containing helices: molecular modeling of parallel dimers of simple hydrophobic helices and of alamethicin. Biopolymers. 1995 Jun;35(6):639–655. doi: 10.1002/bip.360350610. [DOI] [PubMed] [Google Scholar]
- Chiu S. W., Jakobsson E., Subramaniam S., McCammon J. A. Time-correlation analysis of simulated water motion in flexible and rigid gramicidin channels. Biophys J. 1991 Jul;60(1):273–285. doi: 10.1016/S0006-3495(91)82049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. W., Novotny J. A., Jakobsson E. The nature of ion and water barrier crossings in a simulated ion channel. Biophys J. 1993 Jan;64(1):98–109. doi: 10.1016/S0006-3495(93)81344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. Conformation of twisted beta-pleated sheets in proteins. J Mol Biol. 1973 Apr 5;75(2):295–302. doi: 10.1016/0022-2836(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Helix to helix packing in proteins. J Mol Biol. 1981 Jan 5;145(1):215–250. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- Chothia C., Murzin A. G. New folds for all-beta proteins. Structure. 1993 Dec 15;1(4):217–222. doi: 10.1016/0969-2126(93)90010-e. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Carlacci L., Maggiora G. M., Maggiora G. G. Conformational and geometrical properties of idealized beta-barrels in proteins. J Mol Biol. 1990 May 20;213(2):315–326. doi: 10.1016/s0022-2836(05)80193-7. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Heckel A., Némethy G., Rumsey S., Carlacci L., Scheraga H. A. Energetics of the structure and chain tilting of antiparallel beta-barrels in proteins. Proteins. 1990;8(1):14–22. doi: 10.1002/prot.340080105. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Némethy G., Scheraga H. A. Role of interchain interactions in the stabilization of the right-handed twist of beta-sheets. J Mol Biol. 1983 Aug 5;168(2):389–407. doi: 10.1016/s0022-2836(83)80025-4. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Pottle M., Némethy G., Ueda Y., Scheraga H. A. Structure of beta-sheets. Origin of the right-handed twist and of the increased stability of antiparallel over parallel sheets. J Mol Biol. 1982 Nov 25;162(1):89–112. doi: 10.1016/0022-2836(82)90163-2. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Scheraga H. A. Origin of the right-handed twist of beta-sheets of poly(LVal) chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7047–7051. doi: 10.1073/pnas.79.22.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R., Arispe N., Rojas E., Pollard H. B. Theoretical models of the ion channel structure of amyloid beta-protein. Biophys J. 1994 Dec;67(6):2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Cheung M., Czegledy F., Li J., Iserovich P., Kuang K., Hubbard J., Garner M., Rosen O. M., Golde D. W. Evidence that facilitative glucose transporters may fold as beta-barrels. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11658–11662. doi: 10.1073/pnas.90.24.11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Li J., Cheung M., Czegledy F., Iserovich P., Kuang K. Predictive evidence for a porin-type beta-barrel fold in CHIP28 and other members of the MIP family. A restricted-pore model common to water channels and facilitators. J Membr Biol. 1995 Feb;143(3):177–188. doi: 10.1007/BF00233446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Weber A., Brink S., Arbinger B., Schünemann D., Borchert S., Heldt H. W., Popp B., Benz R., Link T. A. Porins from plants. Molecular cloning and functional characterization of two new members of the porin family. J Biol Chem. 1994 Oct 14;269(41):25754–25760. [PubMed] [Google Scholar]
- Jap B. K., Walian P. J. Biophysics of the structure and function of porins. Q Rev Biophys. 1990 Nov;23(4):367–403. doi: 10.1017/s003358350000559x. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Karshikoff A., Spassov V., Cowan S. W., Ladenstein R., Schirmer T. Electrostatic properties of two porin channels from Escherichia coli. J Mol Biol. 1994 Jul 22;240(4):372–384. doi: 10.1006/jmbi.1994.1451. [DOI] [PubMed] [Google Scholar]
- Kerr I. D., Sankararamakrishnan R., Smart O. S., Sansom M. S. Parallel helix bundles and ion channels: molecular modeling via simulated annealing and restrained molecular dynamics. Biophys J. 1994 Oct;67(4):1501–1515. doi: 10.1016/S0006-3495(94)80624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch A., Schulz G. E. Refined structure of the porin from Rhodopseudomonas blastica. Comparison with the porin from Rhodobacter capsulatus. J Mol Biol. 1994 Nov 11;243(5):891–905. doi: 10.1006/jmbi.1994.1690. [DOI] [PubMed] [Google Scholar]
- Kuyucak S., Chung S. H. Temperature dependence of conductivity in electrolyte solutions and ionic channels of biological membranes. Biophys Chem. 1994 Sep;52(1):15–24. doi: 10.1016/0301-4622(94)00034-4. [DOI] [PubMed] [Google Scholar]
- Lasters I., Wodak S. J., Alard P., van Cutsem E. Structural principles of parallel beta-barrels in proteins. Proc Natl Acad Sci U S A. 1988 May;85(10):3338–3342. doi: 10.1073/pnas.85.10.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin A. G., Lesk A. M., Chothia C. Principles determining the structure of beta-sheet barrels in proteins. I. A theoretical analysis. J Mol Biol. 1994 Mar 11;236(5):1369–1381. doi: 10.1016/0022-2836(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Murzin A. G., Lesk A. M., Chothia C. Principles determining the structure of beta-sheet barrels in proteins. II. The observed structures. J Mol Biol. 1994 Mar 11;236(5):1382–1400. doi: 10.1016/0022-2836(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Buckley J. T., Postma J. P., Tucker A. D., Leonard K., Pattus F., Tsernoglou D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature. 1994 Jan 20;367(6460):292–295. doi: 10.1038/367292a0. [DOI] [PubMed] [Google Scholar]
- Sansom M. S., Son H. S., Sankararamakrishnan R., Kerr I. D., Breed J. Seven-helix bundles: molecular modeling via restrained molecular dynamics. Biophys J. 1995 Apr;68(4):1295–1310. doi: 10.1016/S0006-3495(95)80303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T., Keller T. A., Wang Y. F., Rosenbusch J. P. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science. 1995 Jan 27;267(5197):512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- Smart O. S., Goodfellow J. M., Wallace B. A. The pore dimensions of gramicidin A. Biophys J. 1993 Dec;65(6):2455–2460. doi: 10.1016/S0006-3495(93)81293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Schulz G. E. Structure of porin refined at 1.8 A resolution. J Mol Biol. 1992 Sep 20;227(2):493–509. doi: 10.1016/0022-2836(92)90903-w. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Beta-turns and their distortions: a proposed new nomenclature. Protein Eng. 1990 May;3(6):479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]