Abstract
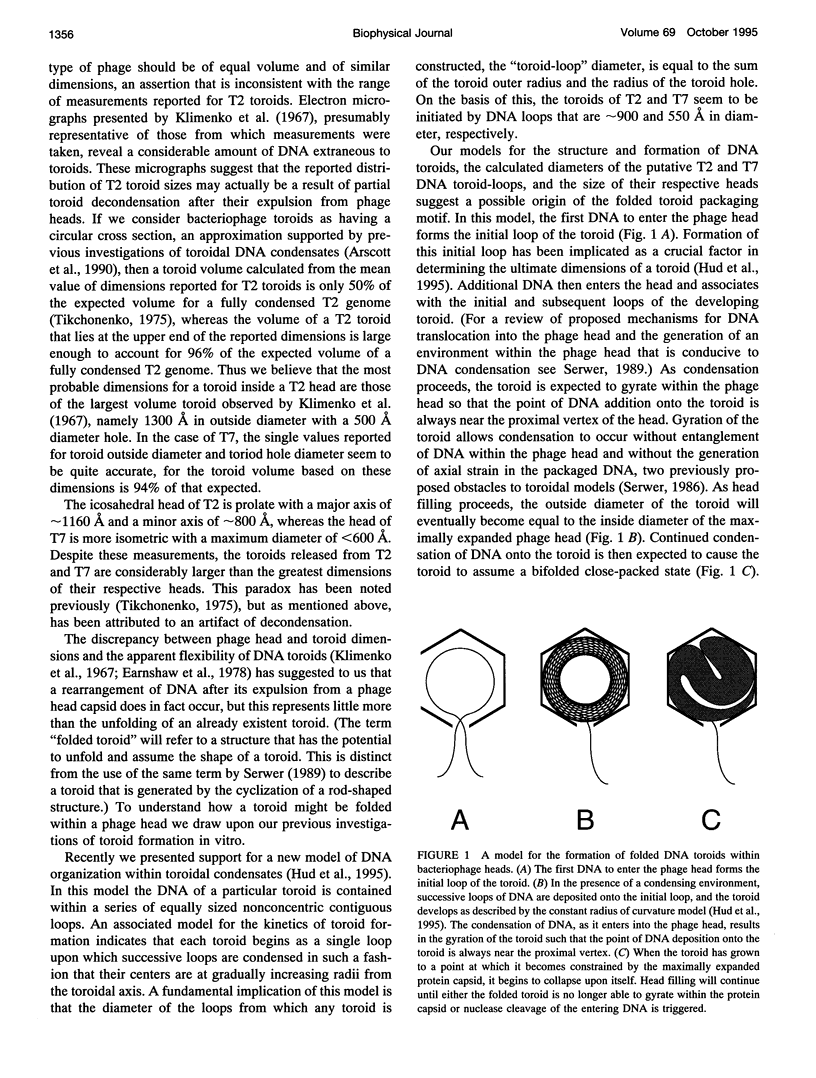
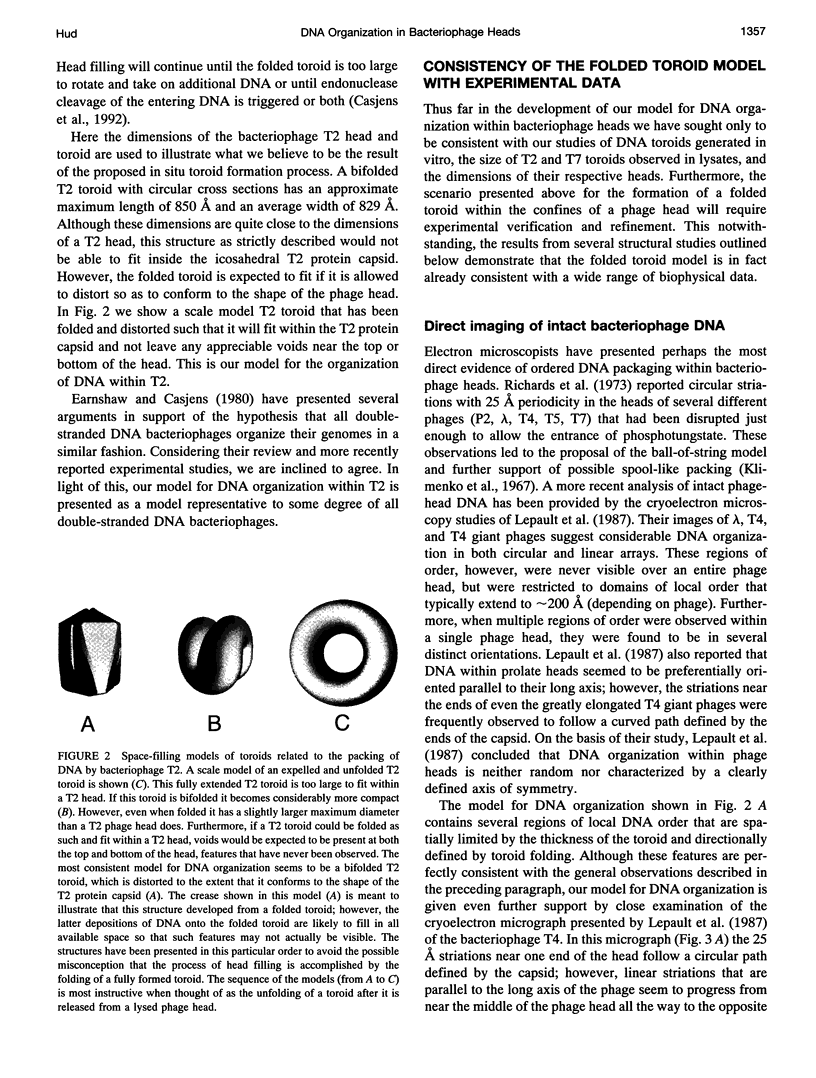
Studies of the organization of double-stranded DNA within bacteriophage heads during the past four decades have produced a wealth of data. However, despite the presentation of numerous models, the true organization of DNA within phage heads remains unresolved. The observations of toroidal DNA structures in electron micrographs of phage lysates have long been cited as support for the organization of DNA in a spool-like fashion. This particular model, like all other models, has not been found to be consistent will all available data. Recently we proposed that DNA within toroidal condensates produced in vitro is organized in a manner significantly different from that suggested by the spool model. This new toroid model has allowed the development of an alternative model for DNA organization within bacteriophage heads that is consistent with a wide range of biophysical data. Here we propose that bacteriophage DNA is packaged in a toroid that is folded into a highly compact structure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arscott P. G., Li A. Z., Bloomfield V. A. Condensation of DNA by trivalent cations. 1. Effects of DNA length and topology on the size and shape of condensed particles. Biopolymers. 1990;30(5-6):619–630. doi: 10.1002/bip.360300514. [DOI] [PubMed] [Google Scholar]
- Aubrey K. L., Casjens S. R., Thomas G. J., Jr Secondary structure and interactions of the packaged dsDNA genome of bacteriophage P22 investigated by Raman difference spectroscopy. Biochemistry. 1992 Dec 1;31(47):11835–11842. doi: 10.1021/bi00162a023. [DOI] [PubMed] [Google Scholar]
- BENDET I. J., GOLDSTEIN D. A., LAUFFER M. A. Evidence for internal organization of nucleic acid in T2 bacteriophage. Nature. 1960 Aug 27;187:781–782. doi: 10.1038/187781a0. [DOI] [PubMed] [Google Scholar]
- Basu S. Molecular arrangement of DNA in bacteriophage T4. Biopolymers. 1977 Oct;16(10):2299–2314. doi: 10.1002/bip.1977.360161015. [DOI] [PubMed] [Google Scholar]
- Black L. W., Newcomb W. W., Boring J. W., Brown J. C. Ion etching bacteriophage T4: support for a spiral-fold model of packaged DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7960–7964. doi: 10.1073/pnas.82.23.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Newcomb W. W. Ion etching of bacteriophage lambda: evidence that the right end of the DNA is located at the outside of the phage DNA mass. J Virol. 1986 Nov;60(2):564–568. doi: 10.1128/jvi.60.2.564-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Wyckoff E., Hayden M., Sampson L., Eppler K., Randall S., Moreno E. T., Serwer P. Bacteriophage P22 portal protein is part of the gauge that regulates packing density of intravirion DNA. J Mol Biol. 1992 Apr 20;224(4):1055–1074. doi: 10.1016/0022-2836(92)90469-z. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., King J., Harrison S. C., Eiserling F. A. The structural organization of DNA packaged within the heads of T4 wild-type, isometric and giant bacteriophages. Cell. 1978 Jul;14(3):559–568. doi: 10.1016/0092-8674(78)90242-8. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- GELLERT M., DAVIES D. R. ORGANIZATION OF DNA IN BACTERIOPHAGE T4. J Mol Biol. 1964 Mar;8:341–347. doi: 10.1016/s0022-2836(64)80197-2. [DOI] [PubMed] [Google Scholar]
- Ghosh A. N., Sen A., Das Gupta N. N. Packing pattern of DNA in bacteriophage T2. Z Naturforsch C. 1984 Jun;39(6):692–694. doi: 10.1515/znc-1984-0630. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Greiss G. A., Serwer P., Horowitz P. M. Binding of ethidium to bacteriophages t7 and p22. Biophys J. 1986 Jan;49(1):19–21. doi: 10.1016/S0006-3495(86)83576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griess G. A., Serwer P., Horowitz P. M. Binding of ethidium to bacteriophage T7 and T7 deletion mutants. Biopolymers. 1985 Aug;24(8):1635–1646. doi: 10.1002/bip.360240816. [DOI] [PubMed] [Google Scholar]
- Haas R., Murphy R. F., Cantor C. R. Testing models of the arrangement of DNA inside bacteriophage lambda by crosslinking the packaged DNA. J Mol Biol. 1982 Jul 25;159(1):71–92. doi: 10.1016/0022-2836(82)90032-8. [DOI] [PubMed] [Google Scholar]
- Hall S. B., Schellman J. A. Flow dichroism of capsid DNA phages. I. Fast and slow T4B. Biopolymers. 1982 Oct;21(10):1991–2010. doi: 10.1002/bip.360211006. [DOI] [PubMed] [Google Scholar]
- Hall S. B., Schellman J. A. Flow dichroism of capsid DNA phages. II. Effect of DNA deletions and intercalating dyes. Biopolymers. 1982 Oct;21(10):2011–2031. doi: 10.1002/bip.360211007. [DOI] [PubMed] [Google Scholar]
- Harrison S. C. Packaging of DNA into bacteriophage heads: a model. J Mol Biol. 1983 Dec 25;171(4):577–580. doi: 10.1016/0022-2836(83)90045-1. [DOI] [PubMed] [Google Scholar]
- Hud N. V., Downing K. H., Balhorn R. A constant radius of curvature model for the organization of DNA in toroidal condensates. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3581–3585. doi: 10.1073/pnas.92.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimenko S. M., Tikchonenko T. I., Andreev V. M. Packing of DNA in the head of bacteriophage T2. J Mol Biol. 1967 Feb 14;23(3):523–533. doi: 10.1016/s0022-2836(67)80122-0. [DOI] [PubMed] [Google Scholar]
- Kosturko L. D., Hogan M., Dattagupta N. Structure of DNA within three isometric bacteriophages. Cell. 1979 Mar;16(3):515–522. doi: 10.1016/0092-8674(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Lepault J., Dubochet J., Baschong W., Kellenberger E. Organization of double-stranded DNA in bacteriophages: a study by cryo-electron microscopy of vitrified samples. EMBO J. 1987 May;6(5):1507–1512. doi: 10.1002/j.1460-2075.1987.tb02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre M. F. Transient electric birefringence studies of T2 bacteriophage and T2 ghost. Biopolymers. 1968;6(3):415–430. doi: 10.1002/bip.1968.360060313. [DOI] [PubMed] [Google Scholar]
- NORTH A. C., RICH A. X-ray diffraction studies of bacterial viruses. Nature. 1961 Sep 23;191:1242–1245. doi: 10.1038/1911242a0. [DOI] [PubMed] [Google Scholar]
- Reilly K. E., Thomas G. J., Jr Hydrogen exchange dynamics of the P22 virion determined by time-resolved Raman spectroscopy. Effects of chromosome packaging on the kinetics of nucleotide exchanges. J Mol Biol. 1994 Aug 5;241(1):68–82. doi: 10.1006/jmbi.1994.1474. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Rill R. L. Liquid crystalline phases in concentrated aqueous solutions of Na+ DNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):342–346. doi: 10.1073/pnas.83.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Arrangement of double-stranded DNA packaged in bacteriophage capsids. An alternative model. J Mol Biol. 1986 Aug 5;190(3):509–512. doi: 10.1016/0022-2836(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Serwer P., Hayes S. J., Watson R. H. Conformation of DNA packaged in bacteriophage T7. Analysis by use of ultraviolet light-induced DNA-capsid cross-linking. J Mol Biol. 1992 Feb 20;223(4):999–1011. doi: 10.1016/0022-2836(92)90258-l. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Serwer P., Ross M. J. Assembly of bacteriophage T7. Dimensions of the bacteriophage and its capsids. Biophys J. 1981 Dec;36(3):743–757. doi: 10.1016/S0006-3495(81)84763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Cantor C. R. Studies on the arrangement of DNA inside viruses using a breakable bis-psoralen crosslinker. J Mol Biol. 1987 Nov 5;198(1):63–71. doi: 10.1016/0022-2836(87)90458-x. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Tests of spool models for DNA packaging in phage lambda. J Mol Biol. 1983 Dec 25;171(4):419–437. doi: 10.1016/0022-2836(83)90038-4. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]