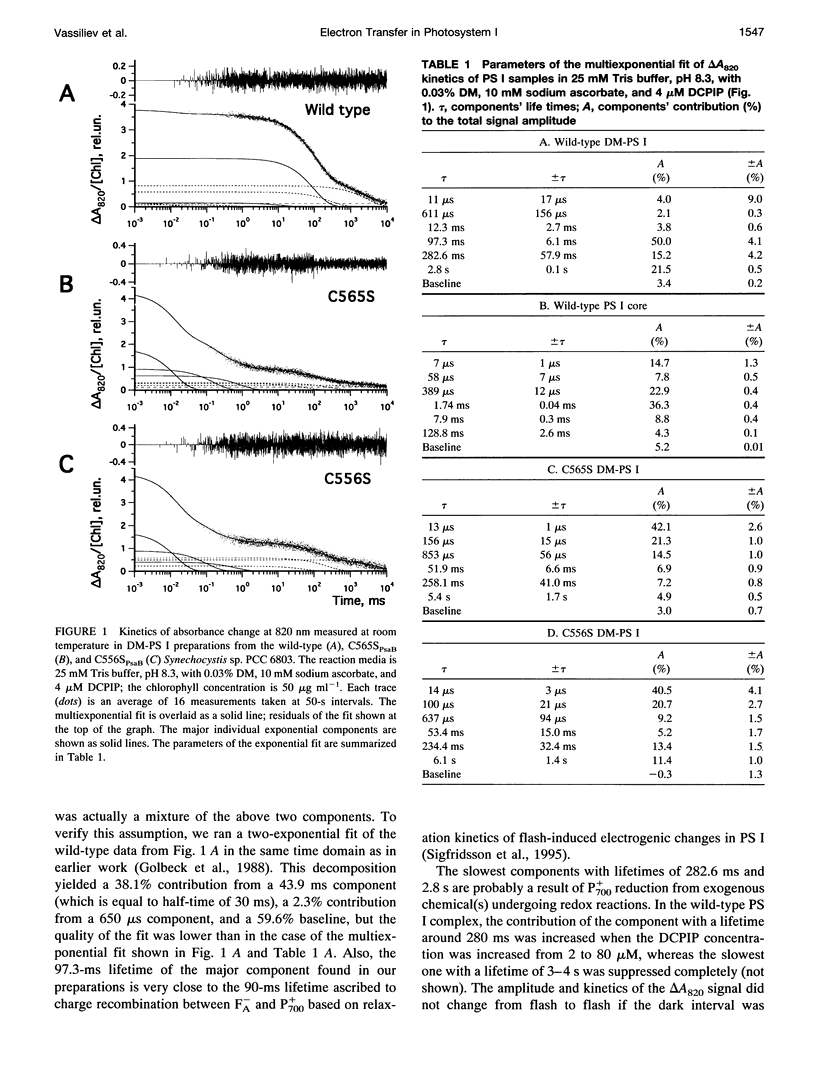
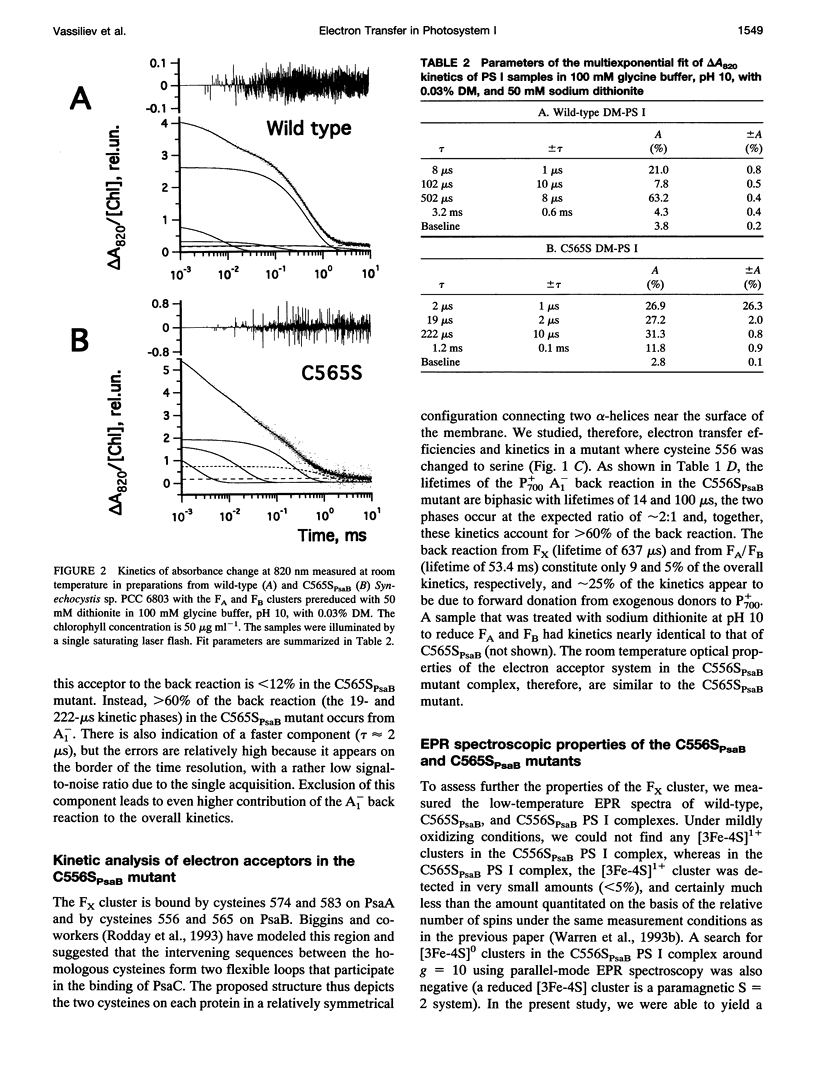
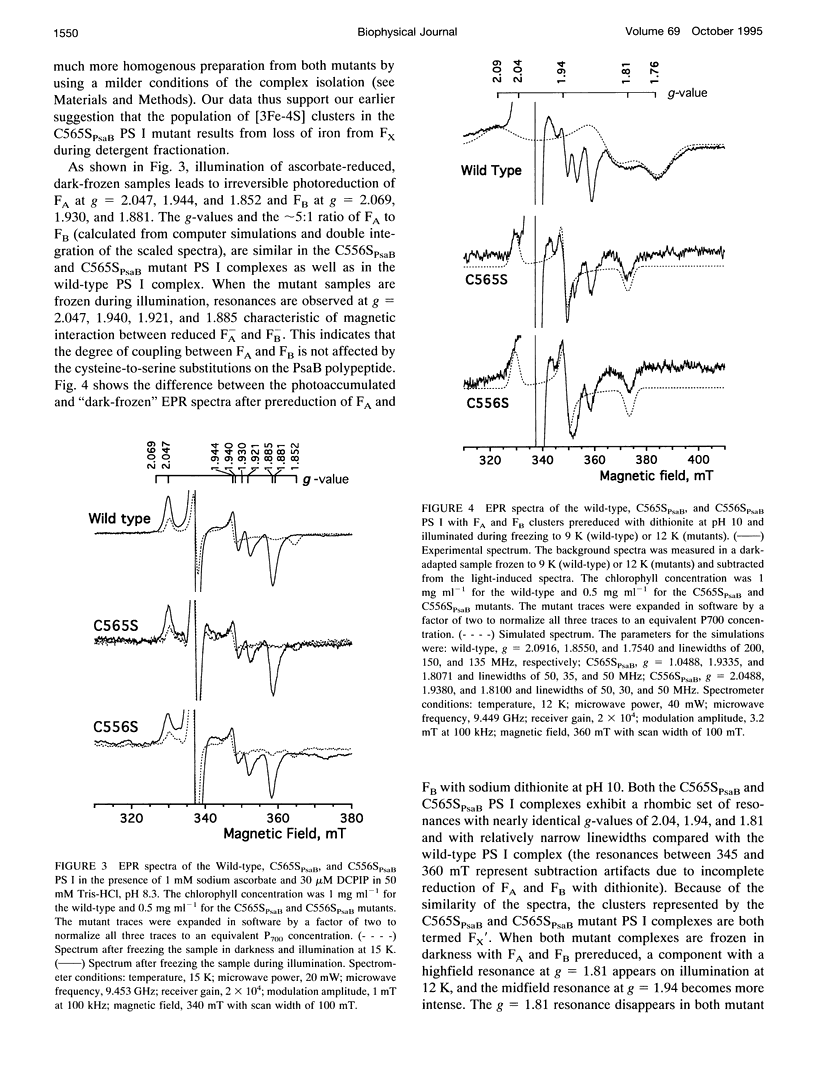
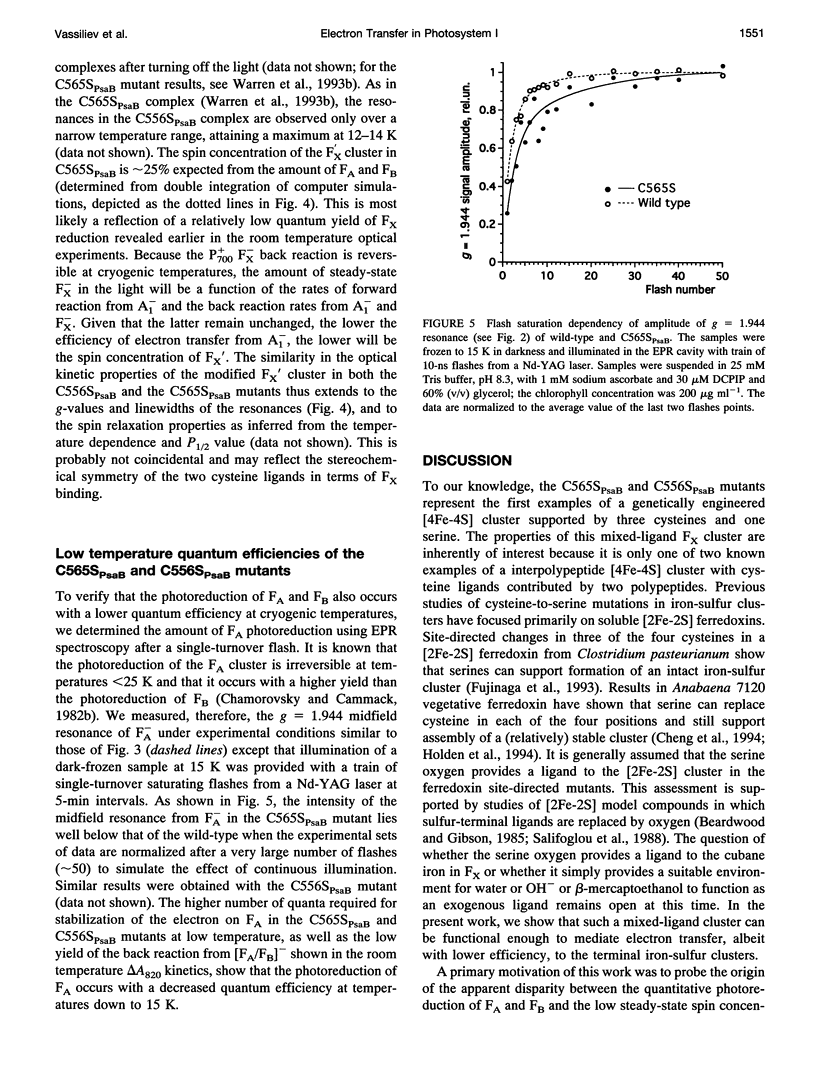
Abstract
The proposed structure of Photosystem I depicts two cysteines on the PsaA polypeptide and two cysteines on the PsaB polypeptide in a symmetrical environment, each providing ligands for the interpolypeptide Fx cluster. We studied the role of Fx in electron transfer by substituting serine for cysteine (C565SPsaB and C556SPsaB), thereby introducing the first example of a genetically engineered, mixed-ligand [4Fe-4S] cluster into a protein. Optical kinetic spectroscopy shows that after a single-turnover flash at 298 K, the contribution of A1- (lifetime of 10 microseconds, 40% of total and lifetime of 100 microseconds, 20% of total) and Fx- (lifetime of 500-800 microseconds, 10-15% of total) to the overall P700+ back reaction have increased in C565SPsaB and C556SPsaB at the expense of the back reaction from [FA/FB]-. The electron paramagnetic resonance spectrum of Fx shows g-values of 2.04, 1.94, and 1.81 in both mutants and a similarly decreased amount of FA and FB reduced at 15 K after a single-turnover flash. These results indicate that the mixed-ligand (3 cysteines, 1 serine) Fx cluster is an inefficient electron carrier, but that a small leak through Fx still permits FA and FB to be reduced quantitatively when the samples are frozen during continuous illumination. The data confirm that Fx is a necessary intermediate in the electron transfer pathway from A1 to FA and FB in Photosystem I.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. L., McIntosh L. Partial conservation of the 5' ndhE-psaC-ndhD 3' gene arrangement of chloroplasts in the cyanobacterium Synechocystis sp. PCC 6803: implications for NDH-D function in cyanobacteria and chloroplasts. Plant Mol Biol. 1991 Apr;16(4):487–499. doi: 10.1007/BF00023416. [DOI] [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Cheng H., Xia B., Reed G. H., Markley J. L. Optical, EPR, and 1H NMR spectroscopy of serine-ligated [2Fe-2S] ferredoxins produced by site-directed mutagenesis of cysteine residues in recombinant Anabaena 7120 vegetative ferredoxin. Biochemistry. 1994 Mar 22;33(11):3155–3164. doi: 10.1021/bi00177a003. [DOI] [PubMed] [Google Scholar]
- Chitnis V. P., Chitnis P. R. PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1993 Dec 27;336(2):330–334. doi: 10.1016/0014-5793(93)80831-e. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Heathcote P. Effects of glycerol on the redox properties of the electron acceptor complex in spinach photosystem I particles. Biochim Biophys Acta. 1980 Mar 7;590(1):89–96. doi: 10.1016/0005-2728(80)90148-6. [DOI] [PubMed] [Google Scholar]
- Fujinaga J., Gaillard J., Meyer J. Mutated forms of a [2Fe-2S] ferredoxin with serine ligands to the iron-sulfur cluster. Biochem Biophys Res Commun. 1993 Jul 15;194(1):104–111. doi: 10.1006/bbrc.1993.1791. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H. Structure, function and organization of the Photosystem I reaction center complex. Biochim Biophys Acta. 1987;895(3):167–204. doi: 10.1016/s0304-4173(87)80002-2. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Jacobson B. L., Hurley J. K., Tollin G., Oh B. H., Skjeldal L., Chae Y. K., Cheng H., Xia B., Markley J. L. Structure-function studies of [2Fe-2S] ferredoxins. J Bioenerg Biomembr. 1994 Feb;26(1):67–88. doi: 10.1007/BF00763220. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Møller B. L. The 110-kDa reaction center protein of photosystem I, P700-chlorophyll a-protein 1, is an iron-sulfur protein. J Biol Chem. 1986 Oct 25;261(30):14292–14300. [PubMed] [Google Scholar]
- Lüneberg J., Fromme P., Jekow P., Schlodder E. Spectroscopic characterization of PS I core complexes from thermophilic Synechococcus sp. Identical reoxidation kinetics of A1- before and after removal of the iron-sulfur-clusters FA and FB. FEBS Lett. 1994 Jan 31;338(2):197–202. doi: 10.1016/0014-5793(94)80364-1. [DOI] [PubMed] [Google Scholar]
- Moënne-Loccoz P., Heathcote P., Maclachlan D. J., Berry M. C., Davis I. H., Evans M. C. Path of electron transfer in photosystem 1: direct evidence of forward electron transfer from A1 to Fe-Sx. Biochemistry. 1994 Aug 23;33(33):10037–10042. doi: 10.1021/bi00199a030. [DOI] [PubMed] [Google Scholar]
- Newman T., de Bruijn F. J., Green P., Keegstra K., Kende H., McIntosh L., Ohlrogge J., Raikhel N., Somerville S., Thomashow M. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994 Dec;106(4):1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N., Hirschberg J. Mutations in the D1 subunit of photosystem II distinguish between quinone and herbicide binding sites. Plant Cell. 1992 Mar;4(3):273–282. doi: 10.1105/tpc.4.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrett K. G., Mehari T., Warren P. G., Golbeck J. H. Purification and properties of the intact P-700 and Fx-containing Photosystem I core protein. Biochim Biophys Acta. 1989 Feb 28;973(2):324–332. doi: 10.1016/s0005-2728(89)80439-6. [DOI] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Electron acceptors associated with P-700 in Triton solubilized photosystem I particles from spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):120–134. doi: 10.1016/0005-2728(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Sigfridsson K., Hansson O., Brzezinski P. Electrogenic light reactions in photosystem I: resolution of electron-transfer rates between the iron-sulfur centers. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3458–3462. doi: 10.1073/pnas.92.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart L. B., Warren P. V., Golbeck J. H., McIntosh L. Mutational analysis of the structure and biogenesis of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC 6803. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1132–1136. doi: 10.1073/pnas.90.3.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sétif P., Brettel K. Forward electron transfer from phylloquinone A1 to iron-sulfur centers in spinach photosystem I. Biochemistry. 1993 Aug 10;32(31):7846–7854. doi: 10.1021/bi00082a002. [DOI] [PubMed] [Google Scholar]
- Warren P. V., Golbeck J. H., Warden J. T. Charge recombination between P700+ and A1- occurs directly to the ground state of P700 in a photosystem I core devoid of FX, FB, and FA. Biochemistry. 1993 Jan 26;32(3):849–857. doi: 10.1021/bi00054a016. [DOI] [PubMed] [Google Scholar]
- Warren P. V., Smart L. B., McIntosh L., Golbeck J. H. Site-directed conversion of cysteine-565 to serine in PsaB of photosystem I results in the assembly of [3Fe-4S] and [4Fe-4S] clusters in Fx. A mixed-ligand [4Fe-4S] cluster is capable of electron transfer to FA and FB. Biochemistry. 1993 Apr 27;32(16):4411–4419. doi: 10.1021/bi00067a034. [DOI] [PubMed] [Google Scholar]
- Webber A. N., Gibbs P. B., Ward J. B., Bingham S. E. Site-directed mutagenesis of the photosystem I reaction center in chloroplasts. The proline-cysteine motif. J Biol Chem. 1993 Jun 15;268(17):12990–12995. [PubMed] [Google Scholar]
- van der Est A., Bock C., Golbeck J., Brettel K., Sétif P., Stehlik D. Electron transfer from the acceptor A1 to the iron-sulfur centers in photosystem I as studied by transient EPR spectroscopy. Biochemistry. 1994 Oct 4;33(39):11789–11797. doi: 10.1021/bi00205a015. [DOI] [PubMed] [Google Scholar]


