Abstract
Persistent antigen stimulation promotes differentiation of exhausted CD8+ T (TEX) cells. TEX cells are distinct from circulating memory T (TCIRCM) cells but share many features with tissue-resident memory (TRM) cells established following infection resolution. CD8+ T cells co-expressing residency- and exhaustion-associated molecules in chronic diseases often correlate with clinical outcomes. However, the relationship between these cells and conventional TRM or TEX cells remains unclear. Here, we show that chronic antigen stimulation drives development of tissue-resident TEX (TR-TEX) cells that are ontologically and functionally distinct from TRM cells generated after antigen clearance. TR-TEX phenotypically resembled TRM cells but were regulated by distinct transcriptional networks and were uniquely dependent on Tox for residency programming. Although TEX progenitor cells acquired residency features upon entering chronically infected tissues, they failed to generate conventional TRM cells after antigen withdrawal. Conversely, TRM cells were able to differentiate into TEX cells during chronic antigen stimulation. Deriving cell-state specific transcriptional signatures revealed a selective association of TR-TEX cells with patient responses to immune checkpoint blockade, and only TR-TEX but not TRM cells responded to PD-1 pathway inhibition in vivo. These data suggest that TR-TEX and TRM cells are developmentally distinct cell types that share a tissue-residency program but have distinct roles in disease control.
CD8+ T cell-mediated control of infection and cancer is central to the efficacy of immunotherapies. The CD8+ T cell compartment includes multiple cellular subsets with specialized roles in immune defense. Tissue-resident memory CD8+ (TRM) cells develop in peripheral non-lymphoid tissues following acutely resolved infections or vaccination, where they reside long-term without recirculating in blood. TRM cells express molecules that promote tissue retention including CD69 and CD103, depend on residency-promoting transcription factors (TFs) including Blimp1, Hobit and Runx3 and are transcriptionally and epigenetically distinct from circulating memory T (TCIRCM) cells1–6. Like other memory T cells (TMEM), canonical TRM cells formed after antigen clearance are long-lived, maintained in an antigen-independent manner, highly functional, and can provide rapid immune protection7–11. In contrast, during chronic infection or cancer, persistent antigen stimulation drives the development of CD8+ TEX cells. These cells are defined by sustained inhibitory receptor (IR) expression, reduced or altered effector function, inefficient antigen-independent maintenance, and compromised developmental plasticity12. Underlying these properties, TEX cells have a distinct epigenetic landscape compared to TCIRCM that is programmed by the TF Tox13–18. The TEX cell population is also heterogeneous and includes progenitor (TEX-PROG, also called stem-like) cells with increased plasticity, effector-like intermediate (TEX-INT), and terminally differentiated (TEX-TERM) cells19. Although TEX cells are suboptimal in controlling disease, these cells – particularly proliferative TEX-PROG – can be partially invigorated by immunotherapies such as PD-1 immune checkpoint blockade (ICB), often resulting in clinical benefit14,20.
Whereas TEX cells are distinct from TCIRCM cells12,14, the relationship between TEX and TRM cells has been less clear. Profiling antigen-specific CD8+ T cell responses in mouse models of vaccination, acute and chronic infection, or cancer, where the kinetics and duration of T cell receptor (TCR) stimulation can be precisely defined, has highlighted considerable phenotypic similarities between TRM formed after antigen clearance and TEX responding to persisting antigen. After resolution of acute infection, TRM cells generated in mice express IRs including PD-1, CD39, the TF Tox and other exhaustion-associated features in the absence of persisting antigen8,21–23. On the other hand, chronic infections and cancer drive accumulation of non-recirculating CD8+ T cells in non-lymphoid tissues24–27 and solid tumors28, as well as establishment of non-migratory TEX-PROG and TEX-TERM cell populations in secondary lymphoid organs25,29. In these settings, tissue-residence is not only closely associated with high IR expression but also with expression of many TRM-associated molecules including CD69, Blimp1 and Runx328,29. These shared features between TRM and TEX cells raise the possibility of potential overlap in their development and regulation.
CD8+ T cells co-expressing the tissue retention molecules CD69 and CD103 are widely present in healthy human tissues and in many chronic diseases, including human solid cancers1 where the presence of such cells often correlates with improved tumor control and immunotherapy responses30. Most CD69+CD103+ TIL co-express the TRM-associated TF Hobit (ZNF683) and multiple inhibitory receptors (IRs) including PD-1 and CD3931–33. These CD69+CD103+ TIL have a range of functional capacities in the tumor microenvironment (TME) 34,35, where they are often enriched for tumor-reactive T cell specificities32,36,37. Similar CD69+CD103+ CD8+ T cell populations are also prevalent in chronic autoimmune conditions including colitis38 and inflammatory skin disorders39. In these chronic diseases, CD8+ T cells expressing tissue-residency associated molecules are often classified as TRM cells40–45. However, whether these cells are ontologically or operationally equivalent to canonical TRM cells generated after resolution of acute infection or following vaccination is unclear. Key factors distinguishing antigen-independent TRM from chronically stimulated TEX cells remain poorly defined. Distinguishing between TRM and TEX cells is particularly challenging in human tissues or tumors where antigen and inflammation often persist, since the T cell receptor (TCR) specificity and the duration of antigen stimulation experienced by T cells in these environments is often unknown. As such, whether CD8+ T cells expressing tissue-residency features in these settings are developmentally related to TRM cells generated after antigen clearance and possess functional properties typical of memory T cells, or whether these cells are partially or fully exhausted is unclear. Addressing these questions has implications for chronic disease treatment, as TRM and TEX cells may respond differently to therapeutic intervention.
To explore these issues, we compared CD8+ T cells residing in peripheral tissues after acute-resolving versus chronic lymphocytic choriomeningitis virus (LCMV) infection in mice. Chronic infection drove the differentiation of a population of CD8+ tissue-resident TEX (TR-TEX) cells that expressed residency-associated molecules but was developmentally and functionally distinct from TRM cells formed after antigen clearance. Whereas both TRM and TR-TEX cells relied on overlapping residency-promoting TFs such as Blimp1 and Runx3, as well Hobit in certain tissues, only TR-TEX cells required Tox for residency programming and survival. TRM cells retained plasticity to generate TEX cells, whereas committed TEX cells could not give rise to TRM cells following antigen clearance and only acquired residency-associated features during chronic infection. TRM and TR-TEX cells engaged distinct gene regulatory networks, giving rise to cell-state specific transcriptional signatures that were stably maintained during inflammation. Finally, TRM and TR-TEX cells made distinct contributions to immunotherapy responses, with only TR-TEX cells and not TRM cells responding to PD-1 inhibition. These data reveal TRM and TR-TEX cells as developmentally distinct cellular lineages generated after clearance or persistence of antigen, respectively. Collectively, these findings highlight different roles for these cell types in disease settings and emphasize that immunotherapeutic interventions harnessing the unique properties of either cell subset could be developed to individually target TRM versus TEX cells.
Results
Phenotypically similar but functionally distinct CD8+ T cells develop in tissues during acute versus chronic infection
To explore the extent of phenotypic overlap between CD8+ T cells localizing to peripheral tissues after infection resolution or chronic infection, we compared expression of hallmark TRM and TEX associated molecules by virus-specific CD8 T cells responding to either acute-resolving (Armstrong, Arm) or chronic (clone 13, Cl13) strains of LCMV. Congenically marked (CD45.1+) P14 CD8+ T cells transgenic for a TCR recognizing the H-2Db-restricted gp33–41 epitope of LCMV were adoptively transferred to naïve mice prior to Arm or Cl13 infection. Parabiosis studies have shown that following resolution of Arm infection, the majority of tissue-localized P14 cells are non-recirculating TRM cells that can be distinguished from TCIRCM cells by a distinct surface phenotype that includes co-expression of the retention-coordinating molecules CD69, CXCR6, CD49a (VLA-1) and, in epithelial locations, CD1031,27,46–48. Here we used co-expression of CD69 and CD103 in epithelial sites (small intestine epithelium (SI) and salivary gland (SG)), or co-expression of CD69 and CXCR6 in non-epithelial tissues (liver and kidney) as proxies to identify ‘Arm TRM cells’, which we defined as non-migratory CD8+ T cells generated in tissues after antigen clearance. P14 cells recruited into non-lymphoid tissues during chronic Cl13 infection are also largely non-recirculating25,27. Using the LCMV system, we could therefore explore how the nature and duration of antigen stimulation impacts tissue-resident T cell programming by comparing Arm TRM cells generated after viral clearance to CD8+ T cells from matched non-lymphoid organs during chronic Cl13 infection.
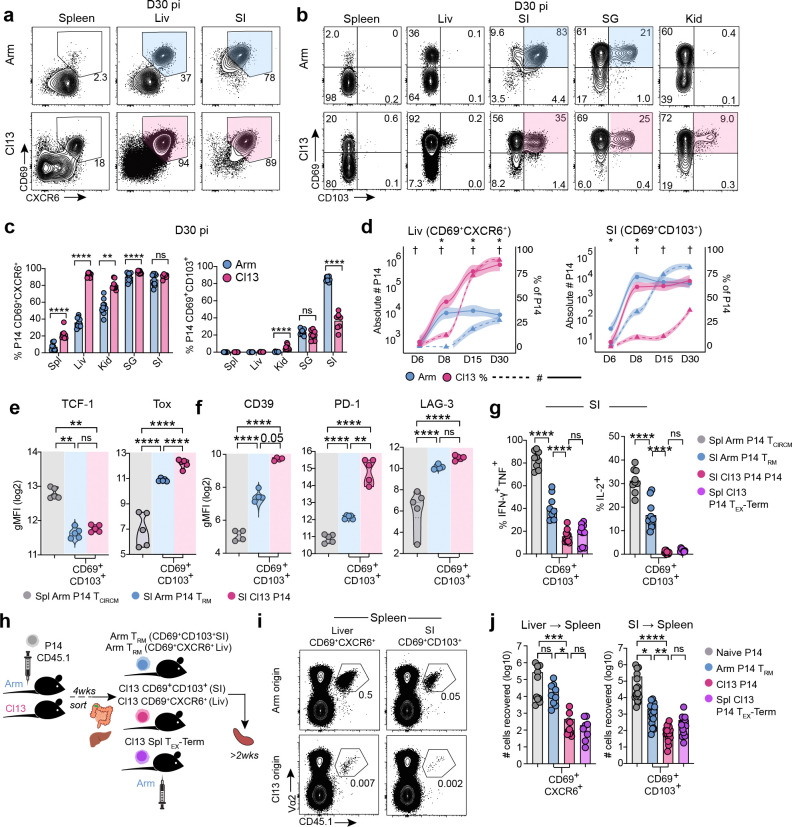
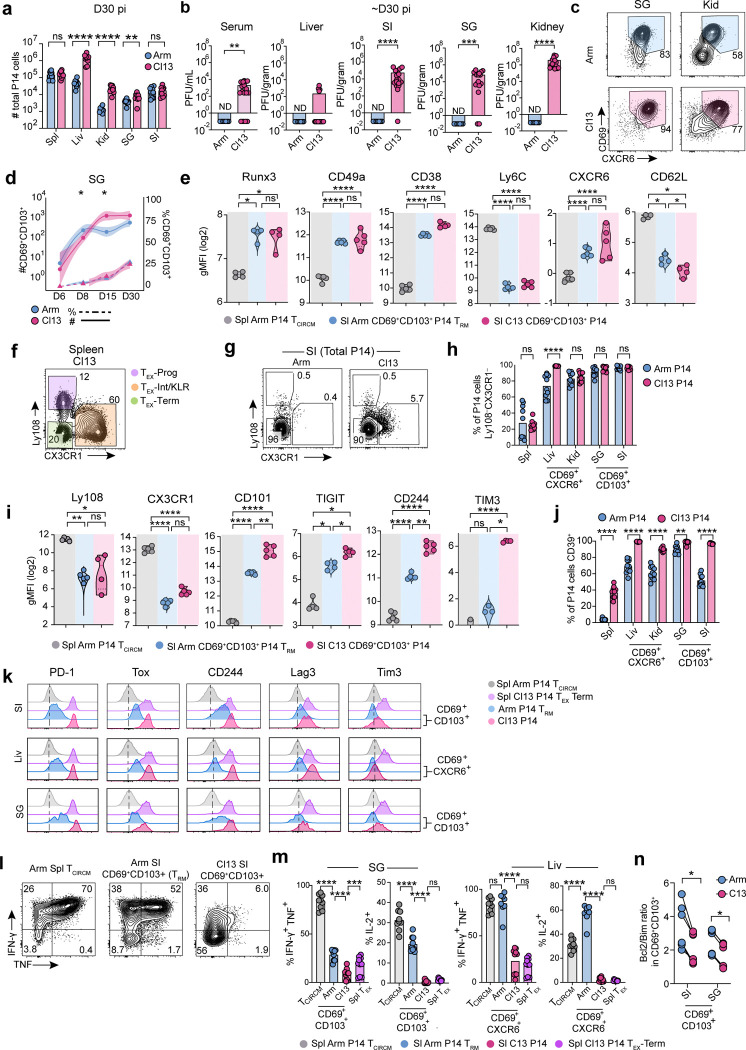
Four weeks post-infection, P14 cells had accumulated at similar or higher numbers in Cl13-infected tissues, where viral titers remained high, compared to tissues from mice that had cleared Arm infection (Extended Data Fig 1a–b). Across all peripheral tissues examined, both Arm and Cl13-derived P14 cells upregulated expression of the residency-associated molecules CD69 and CXCR6 compared to their splenic counterparts, although CD69 and CXCR6 were also co-expressed by a subset of terminally exhausted (TEX-TERM) P14 cells in the spleen of Cl13 infected mice29 (Fig 1a and Extended Data Fig 1c). Whereas the TRM-associated marker CD103 was not expressed by CD69+ P14 cells in the spleen and liver, CD69+CD103+ P14 cells were abundant in peripheral tissues including the small intestine epithelium (SI), salivary gland (SG), and kidney (Kid) at day 30 after both Arm and Cl13 infection (Fig 1b–c and Extended Data Fig 1c). Although CD69+CD103+ P14 cells were present at lower frequencies following chronic compared to acutely resolved infection in some tissues including the SI26,47,49,50, the frequencies of these cells in other organs were comparable (SG) or even higher (Kid) at day 30 of chronic infection (Fig 1b and c). Thus, similar populations of CD69+CXCR6+CD103+/− cells were found in non-lymphoid tissues after either acute or chronic infection.
Figure 1. Functionally distinct CD8+ T cells expressing residency and exhaustion molecules populate peripheral tissues in acute and chronic infection.
a, b Expression of CD69 and CXCR6 (a) or CD69 and CD103 (b) by P14 cells from the indicated tissues 30 days post-infection (dpi) with LCMV Arm or Cl13. Spl; spleen, Liv; liver, SG; salivary gland, SI; small intestine epithelium, Kid; kidney. Shading highlights tissue-localizing cells co-expressing both markers. c, Frequency of P14 cells expressing indicated molecules isolated from the indicated tissues 30 dpi with Arm or Cl13. d, Frequency (dashed lines, †) or absolute number (solid lines, *) of CD69+CXCR6+ (Liv) or CD69+CD103+ (SI) cells at indicated dpi with Arm (blue) or Cl13 (pink). Individual points represent mean; shading represents 95% confidence interval. e, f, Geometric Mean Fluorescence Intensity (gMFI) of indicated molecules in TCIRCM P14 cells isolated from the Spl of Arm mice (grey) or in CD69+CD103+ P14 cells isolated from the SI of Arm (TRM, blue) or Cl13 (pink) mice 30–40 dpi. g, Cytokine production by P14 TCIRCM cells from the Spl of Arm mice (grey), terminally exhausted P14 cells from the Spl of Cl13 mice (TEX-TERM), CD69+CD103+ P14 TRM cells from the SI of Arm infected mice (blue) or CD69+CD103+ P14 T cells from the SI of Cl13 infected mice (pink) 30–40 dpi following ex vivo gp33–44 peptide stimulation. h, Experimental schematic. CD45.2+ mice received naïve CD45.1+ P14 cells and were then infected with Arm or Cl13. The following populations were sort-purified 4–5 wks pi: CD69+CXCR6+ (Liv) or CD69+CD103+ (SI) P14 cells from Arm (TRM) or Cl13 infected mice, CD69+Ly108−TEX-TERM cells from the Spl of Cl13 mice, and CD44lo P14 naïve P14 cells from blood. Matched numbers of sorted cells (10–15,000) were adoptively transferred to separate naïve CD45.2+ recipients that were then rechallenged with Arm. P14 cells were re-isolated from the Spl of rechallenged mice >2 wks pi. i, j, Frequency (i) and absolute number (j) of total P14 cells from recovered from the Spl of Arm rechallenged recipients. Data are pooled from 2–3 independent experiments (c, d, g, j), or representative of 2–3 independent experiments (a, b, e, f, i) with n = 4–5 mice (a-g) or n = 5–7 (j-k) mice per group per experiment. * or † p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 Mann-Whitney test (c, d), Two-Way ANOVA (j) or Kruskal Wallis Test (e, f, g).
TRM cells develop rapidly after clearance of acute viral infection, with the TRM cell pool largely established by 8–14 days post-infection (dpi) and remaining numerically stable thereafter3,6,23. We therefore examined whether CD8+ T cells co-expressing residency-associated molecules emerged with similar kinetics in tissues during chronic versus acute infection. Although the relative frequencies of CD69+CXCR6+ P14 cells in the liver or CD69+CD103+ P14 cells in the SI differed in Arm compared to Cl13 infection, cells expressing residency-associated molecules initially (6–8 dpi) accumulated in similar numbers and were either numerically equivalent or present at higher numbers in tissues from chronically infected mice at later time points (30 dpi) (Fig 1d and Extended Data Fig 1d). P14 cells co-expressing CD69, CXCR6 and/or CD103 in Cl13-infected tissues also had similar induction of other residency-related molecules compared to Arm TRM cells from matched tissues, including high expression of CD38, CD49a (VLA-1) and the TF Runx3 as well as downregulation of Ly6C and CD62L (Extended Data Fig 1e). Thus, CD8+ T cells expressing residency-associated molecules used to identify TRM cells after infection resolution also populated peripheral tissues with comparable kinetics during chronic infection.
We next asked whether tissue-localizing P14 cells responding to acute or chronic infection differed in their expression of markers used to define TEX cells and TEX subsets (Extended Data Fig 1f). P14 cells expressing residency-associated molecules in Cl13-infected tissues phenotypically mirrored spleen-derived TEX-TERM cells, as they lacked Ly108 and CX3CR1 expression (Extended Data Fig 1g), consistent with previous studies29,51,52. However, Arm TRM cells also lacked expression of these markers (Extended Data Fig 1g–i), and both Arm TRM and Cl13-derived tissue P14 cells similarly downregulated the memory-associated TF TCF1 compared to splenic TCIRCM cells (Fig 1e). CD69+CD103+ SI and SG and CD69+CXCR6+ liver P14 cells generated after acute infection (Arm TRM cells) had higher expression of the exhaustion-pioneering TF Tox and IRs including PD-1, CD39, LAG-3, TIGIT, and CD101 compared to splenic TCIRCM cells. However, expression of these molecules by Arm TRM cells was lower than for CD69+CD103+ and CD69+CXCR6+ P14 cells from chronically infected tissues (Fig 1e–f and Extended Data Fig 1i–k). Thus, both Arm TRM cells and P14 cells expressing residency-associated molecules in Cl13 infected tissues expressed markers associated with TEX-TERM, with the highest expression of these molecules observed in the setting of chronic infection.
These results highlighted phenotypic similarities between TRM cells generated after acute infection and tissue-localized CD69+CXCR6+CD103+/− T cells in chronic infection, raising the possibility that these cell populations may be developmentally or functionally related. Unlike TEX cells, TRM cells are capable of robust cytokine production53,54, can locally expand8,55, and can transmigrate out of peripheral tissues following antigen-driven recall56,57. We therefore compared the functional capacities of T cells expressing residency-associated molecules in Cl13 infected tissues to TRM cells generated after acutely resolved infection and to TEX-TERM cells in the spleen of chronically infected mice. CD69+CD103+ Arm TRM cells from epithelial tissues were less polyfunctional than Arm TCIRCM cells (Fig 1g and Extended Data Fig 1l–m). However, chronically stimulated CD69+CD103+ P14 cells from the SI and SG and CD69+CXCR6+ P14 cells from the liver of Cl13 infected mice produced substantially lower levels of IFN-γ, TNF, and IL-2 than Arm TRM cells from matched tissues, mirroring limited cytokine production by splenic Cl13 TEX-TERM cells (Fig 1g and Extended Data Fig 1l–m).
We next directly compared the plasticity potential of Arm TRM cells to tissue-derived P14 cells expressing residency-associated molecules from chronically infected mice by sorting these populations and adoptively transferring each population to new congenically distinct recipient mice followed by rechallenge with Arm (Fig 1h and Supplementary Material 1a). Although CD69+CD103+ TRM cells from the SI were limited in their ability to differentiate into secondary effector cells compared to CD69+CXCR6+ TRM cells from the liver21,58,59, Arm TRM cells from both tissues had a greater potential for expansion compared to their phenotypically matched counterparts from chronically infected mice (Fig 1i–j). CD69+CD103+ P14 cells from the SI and SG of Cl13 infected mice also had a reduced Bcl2/Bim ratio compared to phenotypically matched Arm TRM cells from either tissue, suggesting impaired survival capacity (Extended Data Fig 1n). Thus, peripherally localized CD8+ T cells with tissue-residency features in chronically infected mice lacked quintessential functional properties characterizing memory T cells, including TRM cells generated after acutely resolved infection. Collectively, these data suggested that antigen-specific CD8+ T cells expressing canonical TRM-associated molecules including CD69, CXCR6 and CD103 develop in peripheral tissues following either acute or chronic infection and share some overlapping features of exhaustion but exhibit distinct functional potential. Notably, surface markers and TFs typically used to define TRM or TEX cells were insufficient to distinguish dysfunctional CD8+ T cells from functional memory T cells in tissues.
Distinct transcriptional and epigenetic regulation of TRM and tissue-resident exhausted T (TR-TEX) cells
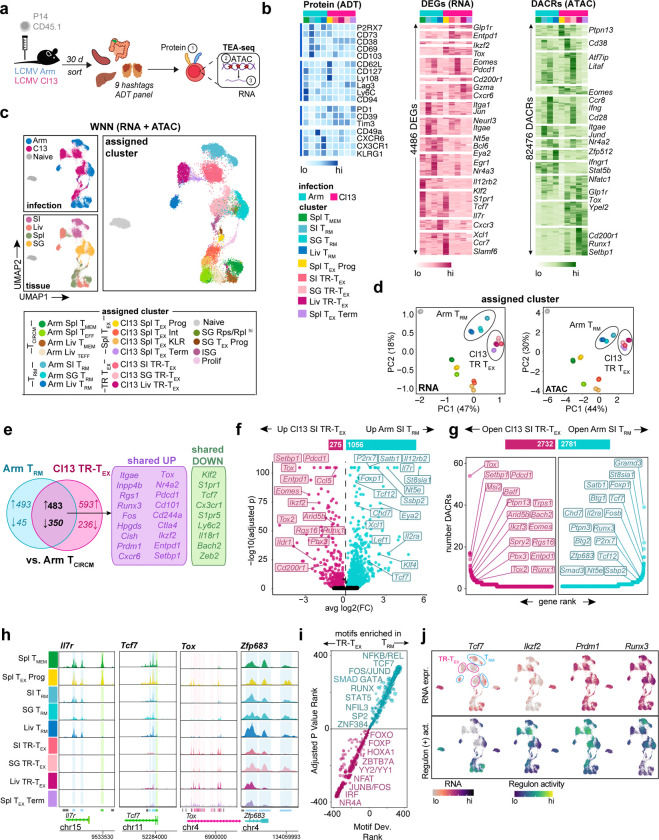
These results highlighted a population of CD8+ T cells localizing to tissues during chronic viral infection that phenotypically resembled TRM cells generated after antigen clearance but displayed reduced functional capacity. However, it remained unclear whether these chronically stimulated cells expressing residency-associated molecules were a subclass of dysfunctional TRM cell with reduced effector and proliferative potential or a developmentally distinct T cell state. To begin to address this question, we performed single-cell trimodal Transcriptome, Epitope and Accessibility (TEA) sequencing60 of total P14 cells from Arm or Cl13 infected mice across multiple tissues (spleen, liver, SI and SG) at 30 dpi (Fig 2a). Naïve P14 cells were also analyzed for comparison. This approach allowed us to simultaneously capture protein (via oligo-barcoded antibody derived tags or ADTs), RNA-seq and ATAC-seq profiles in individual P14 cells from matched tissues following Arm and Cl13 infection and deconvolute the impacts of tissue location and duration of antigen stimulation on T cell programming (Fig 2b).
Figure 2. Discrete transcriptional and epigenetic regulation of TRM and TEX cells across tissues.
a, Experimental schematic for single-cell TEA-seq of tissue P14 T cells. Naïve CD45.1+ P14 T cells were adoptively transferred to CD45.2+ mice that were then infected with Arm or Cl13. Total P14 cells were sort-purified from Spl, Liv, SI and SG 30 dpi or from naïve P14 blood, stained with Hashtag and Antibody-Derived-Tag (ADT) oligos then pooled and sequenced. b, Relative ADT intensity (blue) for all ADTs, RNA expression (pink) for the top 50 unique DEGs, or ATAC accessibility (green) for the top 5000 unique DACRs between indicated clusters. c, UMAP of Weighted Nearest Neighbor (WNN) analysis of RNA and ATAC modalities colored by infection (top left panel), tissue origin (bottom left panel) or assigned cluster (right panel, bottom key) based on gene expression and chromatin accessibility directed annotation of unsupervised clusters (see Supplementary Figure 2). d, Principal Component Analysis (PCA) of RNA and ATAC diversity between pseudobulk assigned clusters. e, Proportion of genes commonly up- or down-regulated by SI Arm TRM (blue) or SI Cl13 TR-TEX (pink) clusters compared to Arm Spl TCIRCM (Arm Spl TMEM and Arm Spl TEFF clusters). f, DEGs between SI Arm TRM (blue) and SI Cl13 TR-TEX (pink) clusters. Bar above shows the number of DEGs in pairwise comparison. g, Number of DACRs per gene loci between SI Arm TRM (blue) and SI Cl13 TR-TEX (pink) clusters. Bar above shows the number of DACRs in pairwise comparison. h, ATAC coverage plots of DACRs in indicated loci for assigned clusters. Peaks called are represented by bars below. Shading on plots or coloring of individual peaks indicates DACRs enriched in Arm Spl TMEM versus Arm TRM (green), Arm TRM versus Cl13 TR-TEX (blue) or Cl13 TR-TEX versus Arm TRM (pink) in at least one tissue. i, Motifs enriched in pairwise comparisons of tissue-matched TRM or TR-TEX clusters from each tissue (SI, Liv, SG) rank ordered by adjusted P value and motif deviation (Motif Dev.). k. RNA expression of indicated TFs (upper panel) and of genes in regulons predicted to be controlled by that TF via network analysis (positive regulon activity). Data are pooled from n = 20–25 mice per group, with significance in pairwise comparisons determined using a Wilcox Rank Sum Test (DEGs) or Logistic Regression Framework Test (DACRs, motifs). **** p < 0.01, two-sided Wilcox test.
Uniform manifold approximation and projection (UMAP) of combined RNA-seq and ATAC-seq data from all samples revealed P14 cells clustered separately based on infection history and tissue location (Fig 2c and Extended Data Fig 2a–b). Combining RNA and protein expression with chromatin accessibility features resolved known TEX cell heterogeneity in the spleen of Cl13 infected mice including progenitor (Cl13 Spl TEX-PROG, Tcf7hiSlamf6hi), effector-like (Cl13 Spl TEX-INT and Cl13 Spl TEX-KLR, Tcf7loCx3cr1hi) and terminally differentiated (Cl13 Spl TEX-TERM; Cxcr6hiEntpd1hi) subsets. In contrast, the spleen of Arm-immune mice contained effector- (Arm Spl TEFF, Klrg1hiCx3cr1hi) and memory-like (Arm Spl TMEM, Il7rhi) subsets, that we collectively annotated as Spl TCIRCM cells (Fig 2c and Extended Data Fig 2b–2c). Re-clustering P14 cells separately for each individual organ revealed tissue-derived P14 cells from Arm and Cl13 infected mice were minimally intermixed, with each population forming several heterogeneous clusters that segregated both from splenic cells and from one another (Extended Data Fig 2d–q). CD103 (Itgae) expression (detected via ADT or RNA expression) was not a major driver of clustering between either Arm or Cl13 P14 cells in the SI and SG (Extended Data Fig 2g and l). However, tissue P14 cell clusters from both infections had increased expression of the residency-associated molecules Cxcr6 and Cd69 and reduced expression of the tissue egress drivers Klf2 and S1pr1 compared to splenic Arm TCIRCM cells (Extended Data Fig 2f and k). Given that these RNA features are reliable predictors of long-term T cell tissue-residency in LCMV Arm and Cl13 parabiosis experiments27, we annotated Klf2loS1pr1loCd69hiCxcr6hiItgae+/− tissue-derived clusters containing Arm-stimulated P14 cells as ‘Arm TRM cells’ in our TEA-seq dataset (Fig 2c).
Principal Component Analysis (PCA) of global transcriptomic and epigenomic profiles revealed liver, SI and SG Arm TRM cells were more closely related to each other than to their tissue-matched counterparts from Cl13 infection or to Arm TCIRCM cells. In contrast, tissue-derived Cl13 P14 cells were positioned away from Arm TRM cells in both transcriptomic and epigenomic PCA space, acquiring a chromatin landscape most similar to that of Spl Cl13 TEX-TERM cells (Fig 2d). We therefore annotated clusters of chronically stimulated P14 cells in peripheral tissues as Cl13 tissue-resident TEX (Cl13 TR-TEX) cells because these cells expressed tissue residency-associated molecules such as CD69, CXCR6 and CD103 but were molecularly distinct from Arm TRM cells generated following acutely resolved infection.
Nevertheless, comparing either Arm TRM or Cl13 TR-TEX cells to Spl Arm TCIRCM cells revealed many transcriptional similarities between both tissue-derived populations, suggesting expression of a shared set of tissue residency-associated genes linked to tissue location (Extended Data Fig 3a). Of the 976 genes upregulated and 395 genes downregulated in SI Arm TRM cells compared to splenic TCIRCM cells, 50% and 90% respectively were also concordantly regulated in SI Cl13 TR-TEX compared to Spl Arm TCIRCM (Fig 2e). These shared genes included numerous canonical residency-related molecules known to be upregulated (Itgae, Fos, Jun, Runx3) or downregulated (Klf2, S1pr1, Tcf7, Ccr7) in TRM cells compared to TCIRCM cells, as well as increased expression of several hallmark T cell exhaustion genes (Tox, Nr4a2, Pdcd1, Setbp1 and others). Moreover, genes previously shown to distinguish TRM from spleen-derived TEX-TERM cells (e.g. Inpp4b, Cish, Hpgds) 61 were similarly expressed in Arm TRM and their Cl13 TR-TEX cell counterparts isolated from matched tissues (Fig 2e). Indeed, Arm TRM and Cl13 TR-TEX cells isolated from the same organs comparably upregulated many of the same tissue-specific genes (Extended Data Fig 3a–b), with tissue-derived TR-TEX cells expressing elevated levels of adhesion molecules and metabolic and stress pathway components compared to splenic TEX-TERM cells (Extended Data Fig 3c). Together, these findings highlight common patterns of gene expression adopted by tissue-localized TRM and TEX cells reflective of their local microenvironment. As such, many genes previously selectively associated with TRM cells were similarly expressed in TR-TEX cells isolated from the same tissues, suggesting they were more closely linked to tissue imprinting than to T cell differentiation state.
Therefore, to explore key differences between Arm TRM and Cl13 TR-TEX cell states in a manner that controls for tissue location, we performed pairwise comparisons between these populations from each individual organ. We identified >1000 differentially expressed genes (DEGs) and >2000 differentially accessible chromatin regions (DACRs) between tissue-matched Arm TRM and TR-TEX cells, with the majority of DEGs selectively upregulated in Arm TRM cells (Fig 2f–g, Extended Data Fig 3d–e and Extended Data Table 2 and 3). Similar results were obtained when the analysis was restricted to CD103+ Arm TRM and Cl13 TR-TEX cells identified by ADT expression (Extended Data Fig 3f). Although expression and accessibility of TCF1 (Tcf7) was lower in Arm TRM cells compared to Arm TCIRCM cells4,62 (Fig 1d and 2e), Arm TRM cells across tissues had higher gene expression and chromatin accessibility at the Tcf7 locus as well as at a variety of other canonical memory and stem-related gene loci (including Il7r, Foxp1, Il12rb2, Satb1, Btg1) compared to tissue-matched Cl13 TR-TEX (Fig 2g–h and Extended Data Fig 3d). In addition, Arm TRM cells had higher expression and accessibility at several gene loci previously associated with TRM cell biology (Xcl1, Smad3, Il2ra) and at numerous others with uncharacterized roles (Nt5e, Chd7, Tcf12, St8sia1, Klf4 and others) compared to Cl13 TR-TEX cells (Fig 2g–h). Whereas both Arm TRM and Cl13 TR-TEX cells were enriched for expression of exhaustion-related genes including Tox, Pdcd1 (PD-1) and Entpd1 (CD39) relative to Arm TCIRCM cells (Fig 2e), pairwise comparison of tissue-matched Arm TRM and Cl13 TR-TEX cells confirmed TR-TEX further enriched for expression and accessibility at these shared gene loci (Fig 2f–h, Extended Data Fig 3d and Extended Data Fig 4a). Arm TRM and Cl13 TR-TEX cells also differed in accessibility at loci encoding regulators of TRM cell commitment3,4, including Runx3, Zfp683 (Hobit) and Prdm1. For example, Cl13 TR-TEX cells from SI and liver lacked accessibility at sites in the Zfp683 locus that were open in TRM, TCIRCM, and TEX-PROG cells (Fig 2h and Extended Data Fig 4a).
These results suggested that despite shared expression of some residency- and exhaustion-associated molecules, tissue-matched Arm TRM and Cl13 TR-TEX cells were transcriptionally and epigenetically distinct, with many of the differences between these populations corresponding to memory and exhaustion lineage-associated genes. Indeed, TF motifs with increased accessibility in Arm TRM cells compared to tissue-matched Cl13 TR-TEX cells were highly correlated with those distinguishing Arm TCIRCM from Cl13 Spl TEX-TERM cells (Extended Data Fig 4b). Memory-associated motifs in the REL/NFKB and TCF/LEF families were the most significantly enriched in Arm TRM cells compared to Cl13 TR-TEX cells, whereas exhaustion-associated NR4A, NFAT and IRF family motifs were highly enriched in both Cl13 TR-TEX and Cl13 Spl TEX-TERM cells compared to Arm TRM cells (Fig 2i). Thus, Arm TRM and TR-TEX cells were characterized by induction of a common tissue-associated transcriptional framework but distinct memory (for TRM) or exhaustion (for TR-TEX) epigenetic programs.
To explore how gene expression might be controlled in each cell type, we used Pando63 TF-gene correlations to infer TF activity in TRM and TR-TEX cells. Differential regulome analysis predicted distinct TFs to be active in Arm TRM versus Cl13 TR-TEX cells from matched tissues, including several shared between Arm TRM and Arm TCIRCM cells (Lef1, TCF1), some selectively enriched in TRM cells compared to other cell states (Tcf12, Klf4) and others preferentially active in Cl13 TR-TEX cells (Ikzf2, Runx1, Nr3c1) (Fig 2j and Extended Data Fig 4c). The key residency-promoting TFs Blimp1 and Runx33,4 also showed differential predicted activity in Arm TRM compared to Cl13 TR-TEX cells, pointing to potential differences in the coordination of tissue residency-programming in either cell subset (Fig 2j). Together, these data suggested that TRM and TR-TEX cells are epigenetically distinct cellular states governed by divergent regulatory circuitry. Although both cell types acquire shared residency-driving transcriptional features linked to tissue location, TRM cells generated after infection resolution have a core memory gene program, whereas TR-TEX cells in chronically infected tissues adopt a canonical exhausted T cell epigenome.
Shared and distinct transcriptional drivers of TRM and TR-TEX cell programming
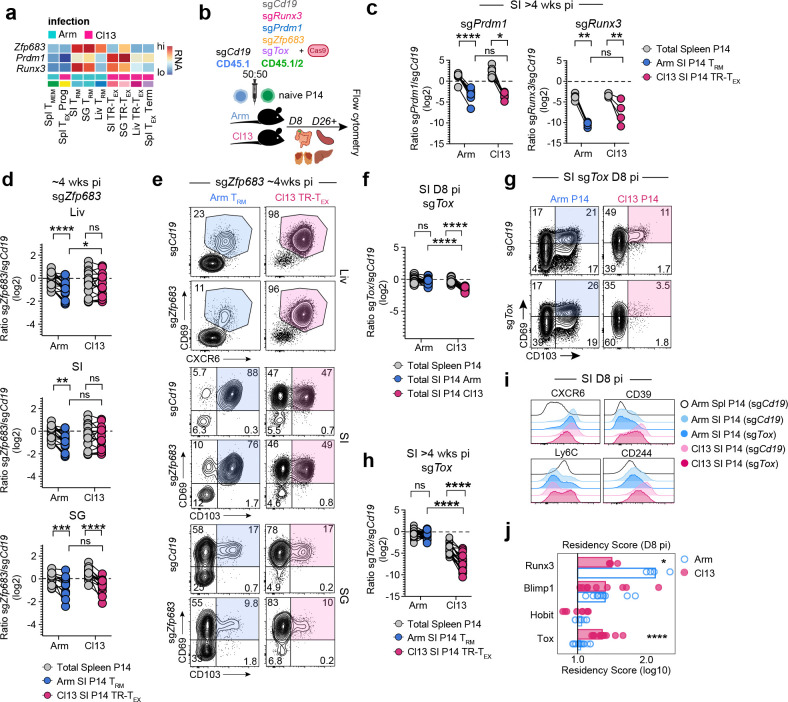
The results above suggested that Arm TRM and Cl13 TR-TEX cells were governed by distinct underlying molecular regulation, including potential differences in their use of master-regulator TFs of T cell state specification. Despite differences in chromatin accessibility at Runx3 and Prdm1 (Blimp1) loci, both tissue-matched Arm TRM and Cl13 TR-TEX cells from each organ had higher expression of these TFs compared to splenic Arm TCIRCM and Cl13 TEX-TERM cells. Of note, whereas Arm TRM cells in all tissues shared high Zfp683 (Hobit) expression, only SG Cl13 TR-TEX cells expressed this TF (Fig 3a). We therefore asked whether Arm TRM and Cl13 TR-TEX cells differed in their dependence on Blimp1, Hobit, or Runx3 for development or maintenance.
Figure 3. Divergent developmental requirements of TRM and TR-TEX cells during residency programming.
a, RNA expression of TFs in TEA-seq clusters at 30 dpi. b, Experimental schematic for CRISPR-Cas9 TF editing in naïve P14 cells. Naïve CD45.1+CD45.1+ or CD45.1+CD45.2+ P14 cells were electroporated with single-guide (sg) RNAs directed towards control (Cd19) or target genes with Cas9 protein and adoptively co-transferred at a 50:50 ratio to CD45.2+ recipient mice that were then infected with Arm or Cl13. c, Ratio of co-transferred SI-derived CD69+CD103+ Arm TRM (blue), SI-derived CD69+CD103+ Cl13 TR-TEX (pink) or total Spl-derived (grey) P14 cells edited with Prdm1 or Runx3 sgRNAs versus control Cd19 sgRNAs at 30–37 dpi. d, Ratio of co-transferred Arm TRM (blue) or TR-TEX (pink) P14 cells edited with Zfp683 sgRNAs or control Cd19 sgRNAs in indicated tissues (SI, SG P14 gated on CD69+CD103+, Liv P14 gated on CD69+CXCR6+) compared to total Spl P14 cells (grey) >26dpi. e, Co-expression of tissue-residency markers CD69 and CD103 (SI, SG) or CD69 and CXCR6 (Liv) by P14 T cells edited with sgCd19 or sgZfp683 >26 dpi with Arm (blue) or Cl13 (pink). f, Ratio of total co-transferred P14 cells edited with Tox sgRNAs or control Cd19 sgRNAs from the Arm (blue) or Cl13 (pink) infected SI (colored) or spleen (grey) at 8 dpi. g, Co-expression of CD69 and CD103 by sgTox or sgCd19 edited SI P14 T cells at 8 dpi. h, Ratio of co-transferred SI-derived CD69+CD103+ Arm TRM (blue), SI-derived CD69+CD103+ Cl13 TR-TEX (pink) or total Spl-derived (grey) P14 cells edited with Tox sgRNAs or control Cd19 sgRNAs >30 dpi. i, Expression of indicated molecules in total P14 cells edited with Tox or control Cd19 targeted sgRNAs in SI or Spl at 8–9 dpi. h, Normalized fold-change (FC) in residency score (directionally weighted normalized mean expression of CD103, CD69, CD39, CD38, CXCR6, CD49a and Ly6C compared to sgCd19 control) in total P14 cells edited with TF-targeting sgRNAs at 8–9 dpi with Arm (blue) or Cl13 (pink). Values indicate extent to which combined expression of markers is regulated by indicated TF. Data are pooled from or representative of 2–3 independent experiments with n = 4–5 (c) or n = 4–8 mice (d-j) per group per timepoint. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 paired T test (spleen versus tissue P14s) or two-tailed T test (Arm versus Cl13 tissue P14s) (c, d, f, h) or Mann-Whitney Test (i, j).
CRISPR-Cas9 electroporation targeting of Runx3, Prdm1 (Blimp1) and Zfp683 (Hobit) in naïve P14 cells prior to Arm or Cl13 infection (Fig 3b) revealed a clear role for Runx3 and Blimp1 in the generation (d8–9 pi) and maintenance (>4wks pi) of both Arm TRM and Cl13 TR-TEX cells across tissues (Fig 3c and Extended Data Fig 5a–e). Whereas Arm TRM cells from all tissues examined were moderately reduced in frequency and number following CRISPR-Cas9 targeting of Hobit, Cl13 TR-TEX cells in the liver and SI were unaffected (Fig 3d–e and Extended Data Fig 5e). However, both Cl13 TR-TEX cells and Arm TRM cells in the SG were similarly reduced following Hobit perturbation (Fig 3d–e), suggesting that Hobit supports TR-TEX cells in some settings and that dependency on (and expression of) this TF is not an exclusive property of TRM cells. A milder impact of targeting Hobit compared to Blimp1 in Arm TRM cells is consistent with some redundancy between these TFs4. Even so, partial reliance on Hobit by SG TR-TEX cells occurred despite elevated Prdm1 expression by TR-TEX in the SG relative to the SI and liver (Fig 3a), suggesting potentially non-redundant activities of Hobit and Blimp164. Selective use of Hobit by SG TR-TEX coincided with uniquely increased accessibility at the Zfp683 locus in these cells, supporting tissue-specific coordination of TR-TEX cell biology. Together, these data demonstrated that TRM and TR-TEX cells both enlist established regulators of residency programming, with particularly important roles for Blimp1 and Runx3. However, the observation that TR-TEX cells did not require Hobit in several tissues where this TF was required by TRM pointed to potential differences in the transcriptional circuits underlying residency programming in each cell type.
Given that similar residency-associated TFs drove differentiation of both TRM and TR-TEX cells, we next asked whether the exhaustion-associated TF Tox was required for the development and survival of each cell type. Tox is required for formation of circulating and lymphoid TEX but is dispensable for TCIRCM cell generation15–18. However, TRM cells have higher expression of Tox than TCIRCM cells (Fig 1e). As such, the role of Tox in TRM cells, and their TR-TEX counterparts, remained unclear. CRISPR-Cas9 targeting of Tox in naïve P14 cells (Fig 3b and Extended Data Fig 6a) resulted in a robust reduction in formation of TR-TEX cells in the SI as early as 8 dpi in Cl13 infected mice, even before the major impact of Tox deletion on developing splenic TEX cells was apparent (Fig 3f–g and Extended Data Fig 6b–c). In contrast, there was no detectable impact of Tox disruption on the development of early TRM precursors in tissues 8d after Arm infection (Fig 3f–g and Extended Data Fig 6b and c). At later time points (> 4wks pi), the frequency and numbers of mature Arm TRM cells across all tissues examined remained unaffected by Tox perturbation, whereas loss of Tox severely compromised generation and durability of Cl13 TR-TEX cells, to either a similar or greater extent than TEX in the spleen (Fig 3h and Extended Data Fig 6d–e). Similar results were obtained when gating on total CD69+ P14 cells in tissues without considering CD103 or CXCR6 co-expression (Extended Data Fig 6f). Thus, despite expressing Tox, TRM cells do not require this TF for development or survival. In contrast, TR-TEX cells in all tissues are highly Tox dependent.
These data suggested TRM and TR-TEX cells arise through independent differentiation pathways. Consistent with this idea, Tox perturbation selectively altered expression of many residency-associated molecules (CD103, CD69, CXCR6, CD39, CD38, Ly6C, CD244) in developing TR-TEX during Cl13 infection but had little to no impact on the expression of these molecules in developing Arm TRM cells (Fig 3i and Extended Data Fig 6g–h), despite early expression of Tox in both differentiating T cell populations (Extended Data Fig 6a). In contrast, most residency-related molecules were dysregulated in both developing Arm TRM and Cl13 TR-TEX cell precursors following disruption of Runx3 or Blimp1 (Extended Data Fig 6g–h). Examining induction of residency-associated molecules together with downregulation of circulatory-associated molecules in a combined ‘residency score’ revealed similar residency programming by Runx3 and Blimp1 in both Cl13 TR-TEX and Arm TRM cells, whereas Tox promoted acquisition of residency features exclusively in differentiating Cl13 TR-TEX cells (Fig 3j). Thus, in addition to selectively promoting the survival of TR-TEX cells, Tox played a central role in coordinating residency commitment within developing TR-TEX cells but not in TRM cells. Overall, these data supported the notion that TR-TEX cells are a separate cellular lineage diverging from TRM cells that develop after acutely resolved infection, with TR-TEX cells navigating a distinct Tox-dependent developmental pathway to ultimately converge on a common tissue-resident biology.
Developmental plasticity allows TRM cells to give rise to TEX cells during chronic antigen stimulation
The data above suggested that TRM and TR-TEX are distinct cell types that emerge through at least partially non-overlapping developmental pathways. To directly test the developmental relatedness of TRM and TR-TEX cells, we next asked whether cells committed to one differentiation state could give rise to the other. First, we asked whether established TRM cells generated after acutely resolved infection could become exhausted when chronically restimulated. We used a sort-rechallenge approach whereby equal numbers of liver or SI Arm TRM cells were sort-purified and adoptively transferred to naïve and congenically distinct recipient mice that were then infected with Cl13 (chronic rechallenge) or Arm (acute rechallenge) (Fig 4a and Supplementary Material 1b). For comparison, we also adoptively transferred naïve P14 cells and circulating CD127+CD62L+ central memory CD8+ T cells (TCM) to separate recipient mice that were also then infected with Arm or Cl13. Donor Arm TRM and TCM cells were analyzed by flow cytometry >3 weeks after Cl13 challenge and compared to: 1) in situ Arm TRM cells that had not been rechallenged, 2) donor Arm TRM and TCM cells rechallenged with acute Arm infection, and 3) naïve P14 cells responding to Cl13, to assess potential differentiation of donor populations into TEX cells during chronic infection. Following either Arm or Cl13 rechallenge, donor Arm TRM cells downregulated expression of residency-associated molecules compared to in situ Arm TRM cells (Fig 4b). In response to Arm rechallenge, both donor Arm TRM and TCM cells upregulated effector-associated molecules including KLRG1. In contrast, in response to Cl13 infection, donor Arm TRM and TCM cells robustly upregulated Tox and IRs to higher levels than in situ TRM cells (Fig 4b). In addition, donor Arm TRM and TCM cells rechallenged with Cl13 had impaired cytokine production and degranulation compared to both in situ TRM cells and donor cells responding to Arm rechallenge, with their defects in polyfunctionality resembling those seen in splenic TEX cells derived from naïve P14 cells (Figure 4c and Extended Data Fig 7a). Thus, established TRM cells generated after antigen clearance can become functionally exhausted when chronically restimulated with antigen.
Figure 4. TRM cells differentiate into heterogenous TEX cell subsets during chronic antigen exposure.
a, Experimental Schematic. Naïve CD45.1+ P14 cells were adoptively transferred to naïve CD45.2+ mice followed by Arm infection. Four to 6 wks pi, SI or Liv P14 TRM cells (CD69+CD103+ SI P14 cells or CD69+CXCR6+CD62L− Liv P14 cells), Spl TCM cells (CD127+CD62L+ Spl P14 cells) or CD44lo naïve P14 cells were sort-purified and 5000–9000 cells from each population were adoptively transferred to separate naïve recipient mice that were then rechallenged with Arm or Cl13. P14 cells were re-isolated from the spleen 21–28 dpi for flow cytometric analysis. Donor infection-matched SI and Liv TRM cells that were not transferred to new recipients were also isolated for comparison (no rechallenge, no rch). b, Relative gMFI of indicated molecules expressed by rechallenged P14 T cells originating from indicated donor origin populations (input cell) following rechallenge (stimulation). c, Cytokine production by rechallenged P14 cells originating from indicated donor populations following stimulation with gp33–41 peptide in vitro. Statistics indicate pairwise comparisons between Arm and Cl13 rechallenged cells from same input population. d-e, UMAPs of rechallenged P14 cells colored by rechallenge virus (infection, d upper panel), by phenograph clusters annotated based on surface marker expression (annotated clusters, d lower panel) or colored by degree of surface molecule expression (e). f, UMAPs highlighting cells derived from indicated input origin populations (colored) following rechallenge with Arm (upper panel) or Cl13 (lower panel). g-i, Frequency of cells derived from indicated input origin populations (x axis) giving rise to TEX subsets after Cl13 rechallenge, identified by phenograph cluster (g, with same legend key as in d lower panel) or by conventional flow cytometry gating (h, i). Data are representative of 3 independent experiments (b, d-f, h), 2 independent experiments (c) or pooled from 2–3 independent experiments (g) with n = 3–5 (b, d-f), n = 4–5 (c), n = 4–5 (g-h). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 Mann Whitney Test (c), unpaired parametric T test or Kruskal Wallis Test (g).
We next asked how TEX cells originating from established TRM cells compared to those derived from naïve T cells. Donor liver Arm TRM, SI Arm TRM, and TCM cells were all less capable of expansion than naïve P14 cells upon Cl13 infection (Extended Data Fig 7b). However, projection of these populations in flow-cytometric UMAP space revealed that regardless of prior identity, different donor cell populations clustered together based on Arm versus Cl13 rechallenge (Fig 4d–f). Unsupervised phenograph65 clustering revealed that whereas Arm-rechallenged donor Arm TRM and TCM cells predominantly gave rise to long-lived effector cells (LLEC) 56,57, Cl13-rechallenged donor Arm TRM and TCM cells primarily populated clusters corresponding to TEX-KLR and TEX-TERM subsets (Fig 4g and Extended Data Fig 7c–d). Cl13-rechallenged donor Arm TRM and TCM cells also gave rise to TEX-PROG and TEX-INT cells during Cl13 infection, though at a lower frequency than naïve P14 cells (Fig 4g–i). Although entirely new clusters did not emerge from donor Arm TRM and TCM compared to naïve P14 cells after Cl13 infection (Fig 4f–g), TEX cells originating from both donor Arm TRM or TCM were slightly enriched for expression of residency-associated molecules, including CXCR6 and CD49a, and expressed marginally lower Tox (Extended Data Fig 7e–g). These observations suggested that TEX cells derived from previously committed TRM and TCM cells may retain some hallmarks of their prior identity. Nevertheless, these data collectively indicated that persistent antigen exposure could drive differentiation of established TRM cells into TEX cells, highlighting the context-dependent developmental plasticity of TRM cells. Although TRM cells could give rise to multiple conventional TEX subsets including TEX-PROG and TEX-INT cells, TRM cells were skewed towards the generation of more terminally differentiated TEX-KLR and TEX-TERM subsets.
Committed TEX cells are unable to generate TRM cells following antigen withdrawal
Given that TRM cells could give rise to TEX cells following exposure to persisting antigen, we next asked whether established TEX could generate TRM cells after antigen clearance. We used a similar sort-rechallenge approach, this time isolating committed TEX-PROG, TEX-INT and TEX-TERM cells from the spleens of mice infected with Cl13 21d prior and adoptively transferring each subset to congenically distinct naïve recipient mice. These recipients were then infected with Arm, which is cleared by day 8–10 post-infection24, allowing us to track the fate of donor TEX cells following antigen withdrawal (Fig 5a). We further subdivided donor TEX-PROG cells into previously described lymphoid-resident CD69+Ly108+ TEX-Progenitor-1 (TEX-Pr1) and migratory CD69−Ly108+ TEX-Progenitor-2 (TEX-Pr2) populations29 to examine potential differences in their ability to undergo nonlymphoid residency programming (Supplementary Material 1c). Matched numbers of naïve P14 T cells were also adoptively transferred to separate mice as a control for conventional non-migratory TRM cell differentiation following Arm infection.
Figure 5. TEX progenitors are unable to generate TRM cells but can give rise to TR-TEX cells.
a, Experimental Schematic. Naïve CD45.1+ P14 cells were adoptively transferred to naïve CD45.2+ recipient mice followed by infection with Cl13. Ly108+ TEX-PROG (split into CD69+ TEX-Progenitor 1 [TEX-Pr1] or CD69− TEX-Progenitor 2 [TEX-Pr2] populations), CD69−Ly108− TEX-INT, CD69+Ly108− TEX-TERM from Spl and CD44lo naïve P14 cells were sort-purified 21–23 dpi and 10,000–15,000 cells from each population were adoptively transferred to separate congenic naïve mice that were then rechallenged with Arm. Sorted donor naïve or TEX P14 cells were re-isolated from the Spl and tissues 25–30 days post rechallenge. b, Absolute number of total P14 cells (left panel) or CD69+CD103+ P14 cells (right panel) derived from sorted naïve or TEX cell populations recovered from the SI of Arm rechallenged mice. c-e, Expression of indicated surface molecules by sorted naïve or TEX P14 cells rechallenged with Arm and recovered from the SI. f, Experimental Schematic. Naïve CD45.1+ P14 cells were adoptive transferred to CD45.2+ naïve recipient mice followed by infection with Cl13. TEX-PROG, TEX-INT, TEX-TERM from Spl and CD44lo naïve P14 cells gated as in a were sort-purified 21–23 dpi and 5000–10,000 cells from each population were adoptively transferred to separate congenic naïve recipient mice that were then rechallenged with Cl13. g, Absolute number of total P14 cells (left panel) or CD69+CD103+ P14 cells (right panel) recovered from the SI of Cl13 rechallenged mice. h-j, Expression of indicated surface molecules by sorted naïve or TEX cell populations rechallenged with Cl13 and recovered from the SI. k, Fold change in co-expression of CD69 and CD103 by TEX-derived SI P14 cells compared to naïve-derived SI P14 cells after Arm or Cl13 rechallenge. l, Naïve CD45.1+ P14 T cells were adoptively transferred to CD45.2+ recipient mice prior to infection with Arm or Cl13. Beginning 28–30 dpi, mice were treated with dasatinib or vehicle daily for 7d by oral gavage. Shown is the absolute number of CD69+CD103+ P14 T cells isolated from the SI or SG after treatment. Data are pooled from 2–3 independent experiments with n = 4–5 (b-e), n = 2–6 (g-k) or n = 5–8 (l) mice per group per experiment. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 unpaired parametric T test (l) or Kruskal Wallis Test (b, g, k).
Whereas donor TEX-INT and TEX-TERM cells survived poorly in Arm-rechallenged hosts, donor TEX-PROG populated tissues and were present at only slightly reduced numbers compared to naïve P14 cells responding to Arm infection (Fig 5b and Extended Data Fig 7h). There were no appreciable differences between the expansion capacity or phenotype of Arm-rechallenged TEX-Pr1 or TEX-Pr2 cells (Fig 5b and Extended Data Fig 7h–k). Intravascular labeling confirmed that Arm-rechallenged TEX-PROG cells localized to the parenchyma of epithelial tissues, mirroring the distribution of conventional TRM cells derived from naïve P14 cells (Extended Data Fig 7l–m). Nevertheless, although Arm-rechallenged TEX-PROG cells accumulated in peripheral tissues including the SI, these cells largely failed to upregulate hallmark surface markers defining non-migratory tissue-resident T cells27 – such as CD69, CD103, CD49a and CXCR6 – unlike Arm TRM cells derived from naïve P14 cells (Fig 5c–d and Extended Data Fig 7h–j). Arm-rechallenged TEX-PROG cells also poorly upregulated markers selectively expressed by Arm TRM cells compared to Cl13 TR-TEX cells (see Fig 2j), such as CD73 and P2RX7 (Extended Data Fig 7j). Instead, tissue-localizing cells derived from rechallenged TEX-PROG expressed markers characteristic of circulating T cells including Ly6C and CX3CR1, and maintained higher expression of Tox, Eomes and Ly108 compared to Arm TRM cells derived from naïve P14 cells (Fig 5d–e and Extended Data Fig 7j and k). In addition, compared to both Arm TCIRCM and naïve P14-derived Arm TRM cells, Arm-rechallenged TEX-PROG cells in the SI maintained elevated expression of the tissue-homing integrin α4β7 that supports continued CD8+ T cell migration49 (Extended Data Fig 7j), suggesting that tissue-localizing TEX-PROG cells retained trafficking potential. Altogether, these data suggested that despite infiltrating tissues, TEX-PROG cells were unable to acquire canonical tissue-residency features after antigen clearance, and instead upregulated or maintained expression of molecules associated with sustained T cell migration27,49. Thus, whereas TRM cells retain developmental flexibility and can convert into TEX cells when antigen persists, committed TEX cells are restrained in their differentiation potential and lose the capacity to acquire a tissue-residency program or generate conventional TRM cells following antigen withdrawal.
TR-TEX cells arise from committed TEX progenitors during chronic infection
These findings raised questions about the origins of TR-TEX cells that upregulate residency-associated molecules like CD69 and CD103 in the steady state during chronic infection. Specifically, it was unclear whether committed TEX cells could continuously generate TR-TEX cells in a process separate from TRM differentiation or whether TEX-PROG cells lost the ability to acquire certain residency features following commitment to the TEX lineage. We therefore performed a reciprocal experiment in which different TEX subsets were adoptively transferred to congenically distinct mice that were rechallenged with Cl13. We then examined the ability of donor TEX populations to give rise to TR-TEX during chronic antigen stimulation (Fig 5f). Donor TEX cells were compared to naïve P14 cells responding to Cl13 infection as a benchmark for typical TEX and TR-TEX cell differentiation. Consistent with findings after Arm rechallenge, donor TEX-PROG cells persisted in the spleen and peripheral tissues following Cl13 infection, whereas donor TEX-INT and TEX-TERM cells were nearly undetectable in recipient mice (Fig 5g and Extended Data Fig 7n). However, in contrast to findings after Arm rechallenge, progeny of TEX-PROG cells infiltrating Cl13-infected tissues efficiently upregulated residency-associated molecules like CD69, CD103 and CXCR6 and downregulated markers associated with circulating T cells including Ly6C and CX3CR1 in a manner comparable to previously naïve P14 cells responding to Cl13 (Fig 5h–i and Extended Data Fig 7o–q). Thus, established TEX-PROG readily upregulated residency-associated molecules and generated TR-TEX cells during chronic infection, despite inefficiently upregulating these same features after acute rechallenge (Fig 5k and Extended Data Fig 7r). Together, these data reveal a selective defect in the ability of TEX-PROG cells to undergo residency programming following antigen withdrawal (after acutely resolved infection) but not in the setting of chronic antigen exposure.
These results suggested that unlike Arm TRM cells11, Cl13 TR-TEX cells depend on chronic antigen stimulation. To test this idea, we treated Arm or Cl13 infected mice with the tyrosine kinase inhibitor dasatinib for 1 week starting approximately 30 dpi, to inhibit Lck signaling downstream of the TCR66,67 (Fig 5l). Whereas Arm TRM cells were minimally impacted by dasatinib treatment, Cl13 TR-TEX cells were substantially reduced in the SI and SG (Fig 5m and Extended Data Fig 7s–t). Thus, TR-TEX cells were more sensitive to inhibition of ongoing antigen stimulation and TCR signaling for their generation and/or maintenance compared to their Arm TRM counterparts. These data further reinforce the idea that TRM and TR-TEX cells are distinct cell types that originate through separate memory and exhaustion developmental pathways. Whereas established TRM cells retain the differentiation flexibility to become exhausted when exposed to chronic antigen, committed TEX cells require persistent antigen stimulation to undergo tissue residency programming.
Distinct TRM and TR-TEX cell state specific gene programs
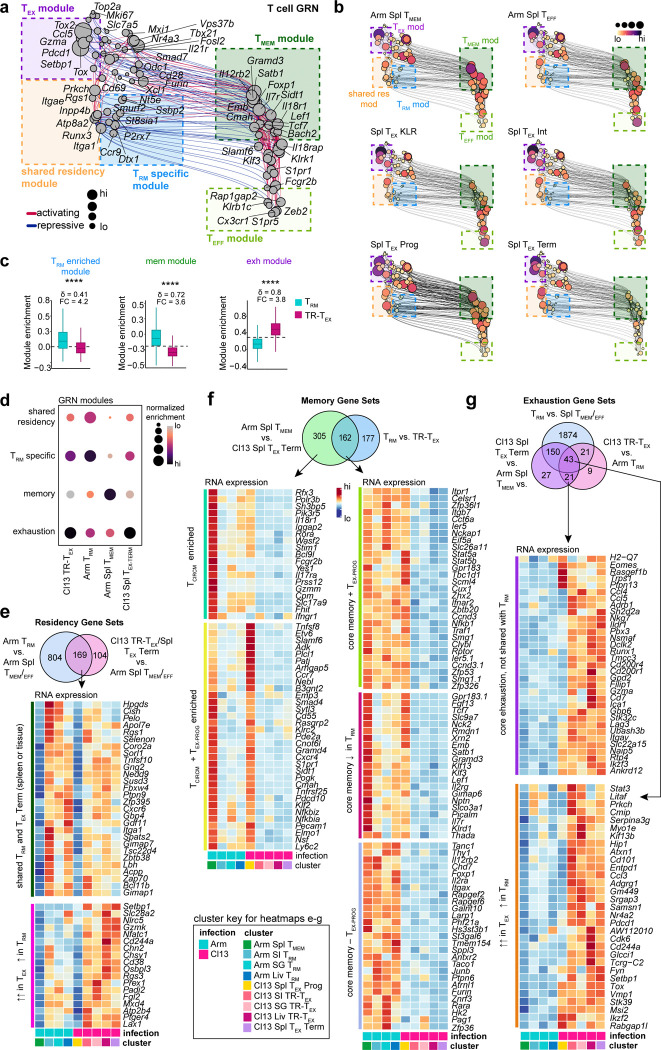
To better understand the molecular underpinnings of TRM and TR-TEX cell identity, we sought to define transcriptional programs that uniquely distinguish these distinct cell states from each other and alternative cell types. To this end, we leveraged our TEA-sequencing dataset to generate a global gene regulatory network (GRN) and compare underlying transcriptional circuits active in Arm TRM or Cl13 TR-TEX cells to those governing other CD8+ T cell fates. Global GRN projection in UMAP space (Fig 6a and Extended Data Fig 8a) and construction of subnetworks specific to each T cell subset (Fig 6b and Extended Data Fig 8b) identified expected core gene modules engaged in effector (enriched in Arm Spl TEFF), memory (enriched in Arm Spl TMEM) and spleen-derived exhausted (enriched in Cl13 Spl TEX-PROG, Cl13 Spl TEX-INT and Cl13 Spl TEX-TERM clusters) T cell subsets (Extended Data Fig 8b-d). We also identified a collection of core residency-related genes (shared residency module) that were engaged in both Arm TRM and Cl13 TR-TEX cells (including Rgs1, Itgae, Runx3, Itga1 and Inpp4b) (Fig 6a–b and Extended Data Fig 8e).
Figure 6. Identification of core transcriptional programs that distinguish TRM from TR-TEX in inflamed tissues.
a, UMAP of inferred single-cell Gene Regulatory Network (GRN) based on combined TF expression and motif accessibility in all non-naïve P14 cells. Size of nodes (genes) represents number of connections. b, Individual UMAP embeddings of genes in GRN in b that are actively engaged in either SI TRM (left panel) or SI TR-TEX (right panel) cells. Node size and color scale represent RNA expression. c, Relative enrichment for GRN-derived modules highlighted in a (highly connected gene clusters) in TRM or TR-TEX across tissues. d, Enrichment score for published core TRM4 and TEX-TERM68 signatures compared to TRM-specific or TR-TEX-specific signatures in TEA-seq clusters. Dashed line indicates the median score in all plotted cells. e, RNA expression of genes selectively upregulated in TRM compared to TCIRCM and TR-TEX cells from matched tissues (TRM-specific signature) or selectively upregulated in TR-TEX compared to TCIRCM and TRM from matched tissues (TR-TEX-specific signature). Venn diagrams indicate genes shared between TRM or TR-TEX cells across tissues with genes included in each signature colored. Blue text labels: TFs; red text labels: surface proteins. f, Expression of CD73 and CD200R by CD69+CD103+ SI Arm TRM or SI Cl13 TR-TEX cells or by non-naïve CD8+CD69+CD103+KLRG1− T cells from human tonsil or skin, or in head and neck cancer (HNSCC) or melanoma tumors. g, Frequency of CD69+CD103+ P14 T cells (LCMV) or non-naïve CD8+CD69+CD103+KLRG1− T cells (human samples) expressing CD73 and CD200R. h, Experimental schematic. Naïve CD45.1+CD45.2+ OT-I T cells were adoptively transferred to naïve CD45.2+ recipient mice that were then infected with the LM-OVA InlAm by oral feeding. Thirty days after LM infection, congenically distinct CD45.1+CD45.1+ naïve P14 cells were adoptively transferred to the same mice that were then infected with Arm or Cl13 or left uninfected (no inf). Tissues were analyzed 30d after LCMV infection. i, j Expression of molecules in SI CD69+CD103+ OT-I TRM cells without LCMV infection (grey, LM only), after Arm infection (blue) or after Cl13 infection (pink) compared to SI CD69+CD103+ Arm P14 TRM cells from Arm infected mice (green) or SI CD69+CD103+ Cl13 P14 TR-TEX cells from LCMV Cl13 infected mice (purple). k, Co-expression of CD69 and CD103 by total OT-I T cells or P14 T cells isolated from LM and LCMV infected mice (top panel) and expression of CD73 and CD200R by each indicated CD69+CD103+ transgenic T cell population. Data are pooled from n = 20–25 mice per group (a-e), representative of at least 2 independent experiments with n = 4–5 mice per group or n = 5–7 patient samples per tissue (f, g) or representative of 2 independent experiments with n = 4–7 mice per group (i-k). * p < 0.05, ** p < 0.01, **** p < 0.001 Kruskal Wallis Test.
Arm TRM cells were distinguished from other T cell fates including Cl13 TR-TEX cells by combinatorial and selective engagement of: i) a core memory gene module shared with TCIRCM cells (including Il7r, Satb1, Lef1 and Foxp1) and ii) a TRM-specific gene module comprising several genes (including Nt5e, St8sia1, Ssbp2 and P2rx7) that were selectively active in Arm TRM cells compared to alternate cell types, including Arm TCIRCM cells and each Cl13 TEX cell subset (Fig 6b–c and Extended Data Fig 8c–d). Comparing gene expression between Arm TRM, Arm Spl TCIRCM and Cl13 TEX cells resolved a similar but expanded foundational set of memory genes expressed by all memory T cells regardless of location (Arm TRM and Arm Spl TCIRCM) and lacking in all TEX cells (Extended Data Fig 8f). In contrast, TR-TEX cells more strongly engaged the core exhaustion gene module relative to TRM cells without engaging core memory or TRM-specific gene modules (Fig 6b–c and Extended Data Fig 8c–d). In addition, comparative analyses identified a set of exhaustion-related genes increased in both Arm TRM and Cl13 TEX cells compared to Arm TCIRCM cells, but more highly expressed in TEX cells (Tox, Stat3, Cd101, Nr4a2 and others). These genes were distinct from a separate set that was selectively enriched in all TEX cells including TR-TEX cells, but not expressed by TRM cells (Eomes, Pbx3, Ubash3b, Gbp6 and others) (Extended Data Fig 8g). Thus, although TRM and TR-TEX cells shared some core tissue-residency features, TRM were uniquely distinguished from TR-TEX cells and other cell states by selective expression of a TRM-specific gene program. On the other hand, although TRM and TR-TEX cells both engaged some exhaustion-associated genes, other genes were restricted to the TEX lineage and were not upregulated by TRM cells. TRM and TR-TEX cell identities were therefore shaped by distinct gene regulatory networks, with the integrated engagement of cell type-specific as well as shared core gene modules defining each cell type.
Despite these key differences in transcriptional specification, gene signatures typically used to identify TRM or TEX cells conflated these T cell populations: Arm TRM and Cl13 TR-TEX cells from our TEA-Seq dataset were similarly enriched for the core TRM cell gene signature previously reported to distinguish TRM cells from TCIRCM cells4, and Arm TRM cells substantially enriched for gene sets previously shown to distinguish TEX-TERM cells from TCIRCM cells68 (Fig 6d). Thus, existing transcriptional signatures lacked the resolution required to distinguish TRM cells from TR-TEX cells. To address this issue, we derived new transcriptional signatures based on the cell-state specific features of each subset. We identified 92 and 53 genes that were selectively upregulated in either TRM or TR-TEX cells from at least two tissues, respectively, compared to TCIRCM cells (Figure 6e and Extended Data Fig 9a and Extended Data Table 4). Of note, neither cell-state specific signature included Itgae or Zfp683 typically used to annotate TRM in human TIL datasets32,69. Instead, the TRM cell signature included several genes in the GRN TRM-selective gene module (Ssbp2, Nt5e, St8sia1, P2rx7, Klf4, Tcf12) as well as multiple genomic regulators (Tet3, Zfp592, Nfatc2), enzymes (Pdk1, Ptpn3) and signaling molecules (Sh2b1, Gpr55) not previously linked to TRM cell regulation. Genes comprising the TR-TEX cell-specific signature included TFs previously associated with T cell dysfunction (Tox2, Eomes and Batf) and TEX-lineage specific regulators of cell signaling and stimulation (Ubash3b, Naip5, Sh2d2a), but notably lacked Tox, Entpd1 and other shared exhaustion features (Figure 6e and Extended Data Fig 9a). Unlike published signatures, the TRM cell-specific gene signature derived here was selectively enriched in Arm TRM cell clusters from our LCMV TEA-seq dataset and in healthy human intestinal tissue70, whereas the TR-TEX cell signature preferentially enriched for Cl13 TR-TEX and splenic Cl13 TEX-TERM populations and for CD39+PD-1+ TEX-like cells in human blood71 (Figure 6d and Extended Data Fig 9b–d).
Given that these selective TRM and TR-TEX cell signatures included several surface molecules (Extended Data Figure 9e), we next asked if these cells could be distinguished using surface proteins. The ectoenzyme CD73 (Nt5e) was highly expressed by SI and liver Arm TRM cells but not by Cl13 TR-TEX cells. In contrast, the IR CD200R was selectively upregulated by Cl13 TR-TEX cells but not by Arm TRM cells (Fig 6f–g and Extended Data Fig 9f–g). Likewise, CD69+CD103+ CD8+ T cells isolated from healthy human tonsil or skin epidermis were enriched for CD73 expression, whereas CD69+CD103+ CD8+ TIL from either head and neck squamous cell carcinoma (HNSCC) or melanoma expressed little CD73 and had high expression of CD200R (Fig 6g, Extended Data Fig 9g and Supplementary Material 1d). Indeed, CD8+CD69+CD103+CD200R+CD73− TIL had higher expression of multiple IRs compared to CD8+CD69+CD103+CD73+ cells from healthy tissues, paralleling expression patterns observed for Arm TRM and Cl13 TR-TEX cells after LCMV infection (Extended Data Fig 9h–i). Thus, we identified TRM and TR-TEX specific gene signatures and putative surface molecules that could potentially better deconvolute these cell states in human tissues.
Inflammatory cues can influence the biology of memory T cells72,73, potentially complicating efforts to distinguish TRM and TR-TEX cells in chronic disease. We therefore next tested whether chronic bystander inflammation impacted expression of molecules distinguishing TRM from TR-TEX cells in a setting where resting TRM cells were exposed to an unrelated chronic infection. In these experiments, we adoptively transferred naïve CD45.1+CD45.2+ transgenic OT-I T cells specific for Ovalbumin (OVA) to mice that were then infected with recombinant Listeria monocytogenes (LM-OVA IlnAmut), generating OT-I TRM cells in the SI (CD69+CD103+) and liver (CXCR6+CD69+)74. Thirty days post-LM-OVA infection, the same mice received naïve CD45.1+CD45.1+ P14 cells and were either left uninfected (no LCMV), infected with Arm to generate P14 TRM cells or infected with Cl13 to generate P14 TR-TEX cells (Fig 6h). This approach allowed us to directly compare the phenotype of resting OT-I TRM cells exposed to acute (Arm) or chronic (Cl13) bystander infection to P14 TR-TEX cells actively responding to chronic infection in the same mice.
OT-I TRM cells maintained similar expression of tissue-residency associated molecules including CD69 and CD103 in Cl13 infected mice with persisting infection compared to Arm infected mice or mice with no LCMV infection (Extended Data Fig 10a–b). In contrast, some molecules associated with TEX or TMEM biology were altered by chronic bystander inflammation. For example, OT-I TRM cells exposed to chronic infection downregulated expression of CD127 and upregulated expression of Tox compared to OT-I TRM cells from Arm infected mice or mice with no LCMV infection (Fig 6i). However, the expression of key surface molecules and TFs demarcating TRM from TR-TEX cells in the cell-state specific gene signatures derived above (CD73, P2RX7, CD200R, Eomes, Batf and others) remained unchanged in bystander OT-I TRM cells from Cl13 infected mice compared to Arm infected mice or mice with no LCMV infection (Fig 6j–k and Extended Data Fig 10c). Together, these data indicated that although some conventional memory and exhaustion-associated features were modestly impacted by bystander inflammatory cues, the TRM and TR-TEX cell-specific gene programs defined here remained relatively stable despite bystander chronic inflammation. These data further reinforced the idea that TR-TEX and TRM cells are distinct cell types, with duration of antigen exposure – rather than persistent inflammation – being the key determinant of their differentiation.
Divergent roles for TRM and TR-TEX cells in chronic disease and immunotherapy
The results above indicated TRM and TR-TEX cells have distinct ontogeny and divergent functional capacities. These observations raised the possibility that each cell type might contribute differently to disease control or immunotherapy responses. TIL expressing CD103 and/or Hobit have been implicated in cancer control and ICB responsiveness42,43,75, but the extent to which this correlation reflects the presence of TRM versus TR-TEX cells is unclear. We therefore compared the ability of the cell-state specific TRM or TR-TEX signatures derived above to stratify cancer patient survival in cohorts for which protective associations with TIL displaying TRM-associated features had previously been reported33,43,76,77. Both TRM and TR-TEX signatures highly correlated with CD8 expression in immunotherapy-naïve patients from The Cancer Genome Atlas (TCGA) skin cutaneous melanoma (SKCM) and METABRIC triple-negative breast cancer (TNBC) cohorts78. As a result, either signature predicted survival when considering all patients, possibly reflecting a surrogate for overall T cell infiltration (Extended Data Fig 10d–f). However, pre-stratifying patients into CD8hi and CD8lo groups revealed differential association of distinct TRM and TR-TEX cell-state specific signatures with survival in each cohort. Whereas there was a trend towards improved survival in CD8hi melanoma patients with higher TR-TEX cell but not TRM cell signature scores, only the TRM cell signature was highly correlated with survival in CD8hi TNBC patients (Fig 7a–b and Extended Data Fig 10d–e).
Figure 7. Differential contribution of TRM and TR-TEX cells to disease control.
a, Kaplan-Meier survival curves for overall survival of metastatic melanoma patients from the TCGA database stratifying by TRM and TR-TEX signatures (hi = top 25%, lo = bottom 25%) within CD8hi patients compared to CD8lo patients (hi = top 50%, lo = bottom 50% for CD8A expression). b, Kaplan-Meier survival curves for overall survival of triple-negative breast cancer (TNBC) patients from METABRIC database stratifying by TRM and TR-TEX signatures (hi = top 25%, lo = bottom 25%) within CD8hi patients compared to CD8lo patients (hi = top 50%, lo = bottom 50% for CD8A expression). c, Kaplan-Meier survival curves for overall survival of urothelial carcinoma patients treated with α-PDL1 from IMvigor210 database stratifying by ITGAE expression (left panel; hi = top 25%, lo = bottom 25%) or TRM and TR-TEX signatures (hi = top 10%, lo = bottom 10%). d, Kaplan-Meier survival curves for overall survival of melanoma patients treated with α-PD1 and/or α-CTLA479 stratifying by TRM and TR-TEX signatures (hi = top 25%, lo = bottom 25%). e, Experimental Schematic. Naïve CD45.1+ P14 cells were adoptively transferred to naïve CD45.2+ recipient mice prior to Arm or Cl13 infection. Daily i.p. treatments with FTY720 commenced 4 weeks p.i.. Three days after the first FTY720 treatment mice received one dose of α-PD-L1 or vehicle (PBS) i.p. with or without co-injection of 25μg gp33–44 peptide i.v. Tissues were collected 48h later and stimulated ex vivo with gp33–41 peptide. f, Frequency of CD69+CD103+ SI P14 TRM (Arm) or TR-TEX (Cl13) cells producing IFN-γ and TNF after α-PD-L1 treatment. g, h, Frequency of Arm TRM or Cl13 TR-TEX cells (CD69+CD103+ in SI or CD69+CXCR6+ in Kid) producing IFN-γ after α-PD-L1 treatment without (g) or with (h) i.v. gp33–41 peptide treatment. i, Viral titers in Cl13 infected FTY720-treated mouse tissues 48h after one dose of α-PD-L1. j, Model schematic. TRM and TR-TEX cells derive from divergent differentiation trajectories but acquire a common residency program. TRM cells maintain heightened plasticity potential whereas TR-TEX cells are responsive to PD-1 pathway blockade. Data are pooled from 2–3 independent experiments (f-i) with n = 4–8 (f-h) or n = 9–10 (i) mice per group. Shading on survival curves represents 50% confidence interval. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 Mann Whitney Test (g-i) or Kaplan-Meier estimate (a-d).
We then examined a cohort of urothelial carcinoma patients treated with α-PD-L1 therapy (IMvigor210), where an association between ITGAE (CD103) expression and improved survival was previously reported33 (and Fig 7c left panel). Whereas the TR-TEX cell-specific signature strongly enriched for improved patient survival following α-PD-L1 treatment, the TRM cell-specific signature was not associated with disease outcome (Fig 7c). Similarly, only the TR-TEX cell-specific signature was associated with improved outcome in metastatic melanoma patients treated with α-PD-1 alone or in combination with α-CTLA479, whereas the TRM cell-specific signature showed no predictive association (Fig 7d). These associations therefore suggested that although abundance of TRM cells can correlate with improved cancer outcomes, it was TR-TEX cells rather than TRM cells that were preferentially associated with improved disease control following PD-1-directed ICB.
These data raised questions about whether TR-TEX and TRM cells might be differentially modulated by ICB. We therefore asked whether TRM and TR-TEX cells would differ in their response to PD-1 pathway blockade (α-PD-L1) in vivo in the LCMV setting. To control for the presence of antigen, we devised a system where Arm TRM cells could be restimulated at the time of PD-1 pathway inhibition via intravenous (i.v.) infusion of cognate gp33–41 peptide. We generated populations of TRM and TR-TEX cells by infecting mice with Arm or Cl13, treated these mice with the sphingosine-1-phosphate (S1P) inhibitor FTY720 to restrict T cell migration between blood and tissues80, and then administered α-PD-L1 blocking antibodies to Arm or Cl13-infected mice with or without simultaneous co-infusion of gp33–41 peptide i.v. (Fig 7e).
Delivery of gp33–41 peptide to Arm-infected mice triggered PD-1 upregulation in reactivated TRM cells, as well as upregulation of PD-L1 on local antigen-presenting cells in tissues by 48h post-infusion (Extended Data Fig 10g), without prompting CD8+ T cell infiltration (Extended Data Fig 10h). After 48h of α-PD-L1 treatment, there was no change in cytokine production by Arm TCIRCM or Cl13 TEX cells in the spleen (Extended Data Fig 10i–j). However, CD69+CD103+ SI and CD69+CXCR6+ Kid Cl13 TR-TEX cells from α-PD-L1 treated mice had enhanced IFN-γ production and degranulation compared to control-treated mice, both with and without gp33–41 peptide co-infusion (Fig 7f–h and Extended Data Fig 10k–n). In contrast, cytokine production and degranulation by both resting and peptide-reactivated Arm TRM cells was unaffected by α-PD-L1 treatment (Fig 7f–h and Extended Data Fig 10k–n). Enhanced cytokine production by Cl13 TR-TEX cells coincided with locally reduced Cl13 viral titers in the kidney but not serum 48h after a single dose of α-PD-L1 compared to control treated mice (Fig 7i), suggesting that TR-TEX cells can enhance local viral control following ICB. Thus, the restrained effector functions of TR-TEX cells can be partially enhanced by α-PD-L1 blockade, whereas TRM cell activity is not enhanced by ICB in vivo. These findings underscore a fundamental functional divergence between TRM and TR-TEX cells, pointing to distinct roles for each subset in disease control and immunotherapy responses.
Discussion
CD69+CD103+CD39hi CD8+ T cells that acquire transcriptional features of TRM cells are abundant in autoimmunity, chronic infections or cancer where they often associate with disease outcomes. Despite many reports identifying such cells, their ontogeny has remained unclear. A key question was whether TRM-like cells expressing IRs found in tissues in chronic disease settings are a subset of pre-committed TRM cells experiencing dysfunction or whether these cells are a separate T cell state. The data presented here support a model where persistent antigen stimulation induces a population of CD69+CD103+/− TEX cells within peripheral tissues that are developmentally and functionally distinct from TRM cells formed after antigen clearance. Despite engaging a common tissue-residency program, TRM and TR-TEX cells have different underlying transcriptional circuitry and arise from divergent developmental pathways. TR-TEX cells acquire an epigenome most similar to splenic TEX-TERM cells and are derived from committed TEX-PROG cells that are unable to form conventional TRM cells following antigen clearance. Moreover, whereas TR-TEX cells depend on the exhaustion-driving TF Tox for both tissue-residency programming and survival, TRM cells generated after infection resolution do not require Tox. TRM and TR-TEX cells were stable differentiation states shaped by antigen clearance or persistence, respectively, with each cell type maintaining its intrinsic identity even after exposure to bystander inflammation. Thus, these findings support a model where TRM and TR-TEX cells are orthogonal cellular states belonging to separate memory and exhaustion CD8+ T cell differentiation trajectories, respectively (Fig 7j). Such a model has implications for understanding T cell programming, the dynamics of CD8+ T cell responses in chronic disease and the therapeutic responsiveness of CD8+ T cell subsets.
CD8+ T cells in nonlymphoid tissues acquire a set of characteristic transcriptional features including elevated Hobit (Zfp683) and CD103 expression, initially described for cells generated after acute-resolving infections3,4,6. This transcriptional module is often used to identify and annotate TRM cells in chronic diseases including tumors. However, our data demonstrates that this residency-associated transcriptional framework can also be co-opted by TEX cells in non-lymphoid tissues. These TR-TEX cells activate a foundational exhaustion transcriptional program shared with other TEX cells together with this residency module, whereas TRM cells are characterized by the combinatorial engagement of core memory T cell, common tissue-residency, and TRM-cell specific gene modules. Despite both subsets expressing a shared set of residency-associated features, TRM and TR-TEX cells have distinct functional capacities that reflect their underlying memory or exhaustion lineage identity. Memory T cells formed after antigen clearance – including TRM cells – retain the developmental plasticity required to contribute to secondary immune responses. Indeed, not only do TRM cells have the capacity to generate new effector and memory T cells upon acute rechallenge8,55–57,81, they can also differentiate into TEX cells following chronic antigen stimulation. In contrast, TEX cells – including those with residency features – are programmed to develop an epigenetic landscape that restricts their fate flexibility and differentiation plasticity. Thus, although both TRM and TR-TEX cells can populate tissues during chronic infection, cancer and other diseases, these are biologically distinct cell types with unique properties that may differentially impact disease trajectories and therapeutic responsiveness.
Although these new data and related studies82 suggest that distinguishing between TRM and TR-TEX may be of interest in chronic disease, identifying memory versus exhausted CD8 T cells in tissues has been challenging. Use of individual genes (or previous gene signatures) comprising a common residency module may be useful to identify cells with residency properties, but such signatures were insufficient to distinguish TRM from TR-TEX cells. Our analyses derived new cell-state specific transcriptional signatures and surface markers that can distinguish these cell types with improved resolution. These transcriptional programs were stably maintained during chronic inflammation, indicating that it is now possible to use these signatures to separate TRM from TR-TEX cells in chronic disease settings. Such an approach could be informative in human tissues, where factors such as T cell specificity and the timing or duration of antigen stimulation are often unknown. Our data suggest that TRM cells with memory-like attributes arise in situations where antigen has been completely cleared or compartmentalized in a manner that limits continuous T cell stimulation. Such scenarios could include after resolution of acutely resolved infection, in some settings of latent infections83,84, during tumor-immune equilibrium where antigen is available intermittently and T cell stimulation is low10, following tumor antigen mutation or loss, or after vaccination. In contrast, sustained antigen stimulation such as occurs in chronically infected tissues or in established tumors drives the formation of TEX cells that combine residency features with a backbone of exhaustion. This combination leads to the development of a different resident CD8+ T cell type (TR-TEX) with distinct regulatory circuitry that shapes cellular behavior, durability and response to immunotherapy. When the biological properties and origins of T cells in tissues cannot be explicitly defined or tested, broader less prescriptive terms – such as ‘tissue-resident T cells’ – may better capture the idea of tissue-residency biology without inferring memory versus exhaustion properties.
The distinct functional properties of TRM and TR-TEX cells described here may influence their respective contributions to disease control. For example, the transcriptional signatures of TRM or TR-TEX cells differentially correlated with patient survival in distinct cancer settings. Whether TRM or TR-TEX cells can directly inhibit human cancer growth, or whether protective associations between either cell type and disease outcomes reflects more broadly the presence of a productive anti-tumor CD8+ T cell response, remains an open question. Studies in preclinical models suggest antigen-specific TRM cells generated prophylactically via infection or vaccination can directly inhibit cancer growth10,85. TRM cells may also drive cancer-immune equilibrium and prevent tumor progression10. Thus, antigen-specific TRM cells generated after tumor clearance or after a reduction in tumor burden may persist and reactivate to protect against cancer recurrence or metastasis. Likewise, TR-TEX TIL may also be capable of contributing to cancer control, but in different settings. Although TEX-PROG cells are necessary to sustain durable anti-tumor CD8+ T cell responses their role is to give rise to downstream TEX subsets such as TEX-INT and/or TEX-TERM that can mediate some tumor cell killing by producing effector cytokines and cytotoxic molecules locally86–88. Indeed, TEX-TERM and/or TEX-INT cells found in the TME are capable of slowing tumor growth independently of TEX-PROG cells in mouse models86,88–90. Thus, both TRM and TR-TEX cells could act through distinct pathways to reduce tumor burden and curtail cancer progression.
Nevertheless, the differences in TRM and TR-TEX functionality observed here have implications for immunotherapy, suggesting distinct approaches could be tailored to effectively harness or modulate each population. Prior work has shown TEX-PROG cells are key targets of PD-1 pathway blockade, as these are the cells that are reinvigorated and expand to give rise to more effector-like TEX and more terminally differentiated TEX cells following ICB91–93. One implication of this idea is that associations between TR-TEX cells and beneficial responses to immunotherapy in cancer patients might reflect increased abundance of upstream TEX-PROG cells that respond to ICB, in turn amplifying TR-TEX cell abundance. Our data now add to this understanding of ICB effects on TEX by demonstrating that the effector functions of terminally exhausted TR-TEX cells themselves are also reinvigorated by PD-1 pathway blockade in vivo, highlighting a secondary mechanism through which ICB may promote cancer control. Although these TR-TEX cannot expand numerically, the increase in local effector function of these cells elicited following PD-1 pathway blockade was sufficient promote some disease control during chronic infection. These data are consistent with results in tumor explant cultures from human melanoma where ex vivo tumor-resident TIL function can be reinvigorated by PD-1 blockade even in tumor fragments where TEX-PROG were unlikely to be present94. Thus, TR-TEX cells have the potential to provide local benefit following PD-1 pathway blockade.
In contrast, although TRM cells generated after antigen clearance express IRs including PD-1, the functions of these cells are not directly enhanced by PD-1 targeted ICB. Instead, TRM cells retain higher baseline functionality and plasticity compared to TEX cells, which may allow these cells to contribute to disease control in part via the generation of multiple TEX subsets following chronic antigen stimulation (Fig 7j). Thus, TRM cells might participate more effectively in cancer prevention, cancer vaccination, or adoptive cellular therapy settings that depend on flexible and durable T cell responses, whereas TR-TEX cells may be more amenable to transient intervention using ICB. This latter notion is consistent with our analyses that indicated TRM-like TIL associated with responses to ICB across diverse tumor contexts42,45,76,95,96 are more closely aligned with TR-TEX cells than to TRM cells generated after antigen clearance. Overall, these new findings suggest a re-evaluation of how to define tissue CD8+ T cell states in pathological settings, with implications for understanding how to best leverage CD8+ T cell responses to improve treatment of chronic disease.
Methods
Mice.
Female C57BL/6 recipient mice were purchased from Charles River or Jackson Laboratories and used at 6–8 weeks of age. CD45.1+ × P14 (B6.SJL.PtprcaPep3b/BoyJ) and CD45.1+CD45.2+ × P14 transgenic mice expressing a TCR specific for H2-Db-restricted LCMV gp33–41, and CD45.1+ × OT-I and CD45.1+CD45.2+ × OT-I transgenic mice expressing a TCR specific for H2-Kb-restricted OVA257–264 were bred in-house at the University of Pennsylvania and backcrossed to either NCI C57BL/6 or Jackson Laboratory C57BL/6J mice and used at 6–18 weeks of age. Mice were maintained in a specific-pathogen-free animal facility at the University of Pennsylvania at ~20°C with 55% humidity and a light-dark cycle of 12h/12h. All experiments and breeding were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines for the University of Pennsylvania and complied with ethical US and international animal ethical guidelines.
Human samples.
All human specimens were collected with informed consent and approval from the University of Pennsylvania Institutional Review Board (IRB#844642, IRB#417200 and IRB#808244). Healthy human peripheral blood mononuclear cell (PBMCs, n = 3), skin (n = 5), tonsil (n = 6), melanoma (n = 4) and HNSCC (n = 7) samples were obtained through the University of Pennsylvania. Age, sex and tissue site information can be found in Extended Data Table 5.
Adoptive T cell transfer.
500–1000 CD45.1+ naïve P14 T cells (in LCMV Arm and LCMV Cl13 experiments) and 10,000 CD45.1+ naïve OT-I T cells (in LM-OVA experiments) were isolated from the peripheral blood of donor mice via density gradient centrifugation with Histopaque-1083 (Sigma-Aldrich) and adoptively transferred to recipients in PBS intraveneously (i.v.) through the tail vein or retro-orbitally (r.o.) 1–2 days prior to infection. In sort-rechallenge experiments using only Arm donors, 5000 naïve P14 T cells were adoptively transferred.
Infections and plaque assays.
LCMV Arm and Cl13 were grown in BHK cells (American Type Culture Collection (ATCC), CL-10) and titrated by performing plaque assays on Vero cells (ATCC, CCL-81) as described97. Recipient mice were infected intraperitonealy (i.p.) with LCMV Arm (2 × 105 PFU) or i.v. with LCMV Cl13 (4 × 106 PFU) diluted in 1% FBS/RPMI. Listeria monocytogenes infection was performed using the recombinant LM-OVA IlnAmut strain expressing OVA and a mutated internalin A protein. Mice were infected with 109 CFU LM via the oral feeding route in bread, as described74.
Mouse tissue processing.
For flow cytometric analysis, lymphocytes were isolated from the spleen and lymph nodes by pushing organs through a 70μm nylon filter and treating single-cell suspensions with ACK Lysing Buffer (Thermofisher) for 3 min at room temperature (RT) before washing cells with 5% FBS/RPMI. In experiments requiring cell-sorting (TEA-seq and sort-rechallenge experiments), lymphocytes were isolated from the spleen by pushing organs through a 70μm nylon filter then performing EasySep magnetic negative selection for CD8+ T cells using a Stem Cell Technologies kit (cat # 19858) according to the manufacturers’ instructions. Lymphocytes were isolated from the liver by pushing organs through a 70μm nylon filter to create single cell suspensions, resuspending cells in 44% Percoll/RPMI solution and performing density centrifugation for 20 mins at 500G at RT and then incubating cells in ACK Lysing Buffer for 5 mins at RT before washing with 5% FBS/RPMI. Lymphocytes were isolated from the salivary gland, kidney and small intestine epithelium as described21,74. Briefly, the salivary gland and kidney were finely chopped in 2% FBS/RPMI containing Collagenase Type III (Worthington, 180,000 U/mL) and incubated for 1 hour in a 37°C water bath. After quenching with 5% FBS/RPMI, digested samples were pushed through a 70μm nylon filter and resuspended in a 44%/70% Percoll density gradient before centrifuging for 20 min at 500G at RT and washing cells with 5% FBS/RPMI. Lymphocytes were isolated from the small intestine epithelium by submerging the small intestine in ice cold CMF solution (10%FBS/Hank’s Balanced Salt Solution (HBSS)/Hepes Bicarbonate Buffer), removing Peyer’s Patches, cutting the intestine open lengthwise, and removing mucus and intestinal content before cutting into ~0.5inch pieces and placing in ice cold CMF. Intestinal pieces were incubated in CMF containing dithioerythritol (DTE, 0.155mg/mL) for 30 mins at 37°C with shaking at 220rpm. Single cell suspensions were vortexed, filtered through a 70μm nylon filter and washed with 5% FBS/RPMI before being overlaid on a 44%/70% Percoll/RPMI density gradient and centrifuged for 20 min at 500G at 18°C prior to washing cells with 5% FBS/RPMI. To perform in vivo intravenous fluorescent antibody labeling, mice were injected with 3μg CD8α-BV650 antibody in PBS i.v. 5 minutes prior to sacrifice as described98.
Human tissue processing.
Lymphocyte isolation from blood was performed as described99. For lymphocyte isolation from tonsil and HNSCC tumor samples, tissue was finely chopped then incubated at 37°C for 60 minutes in DMEM supplemented with DNase I (Sigma Aldrich, 50mg/mL) and Liberase (Roche, 25mg/mL) then passed through a 70mm cell strainer. For lymphocyte isolation from healthy human skin, subcutaneous fat was removed using scissors and the epidermal layer of skin gently scored using a razor blade before incubating samples overnight in Dispase II/PBS solution (Roche, 2.5mg/mL) at 4°C. Dermal and epidermal fractions were separated, finely chopped and incubated separately in Collagenase Type III solution (Worthington, 180,000U/mL) for 90 min in a 37°C water bath. Following incubation, samples were agitated by mixing with a transfer pipette in 10%FBS/RPMI solution and single cell suspensions filtered through a 70μm nylon filter prior to cryopreservation in 10%DMSO/FBS. Melanoma samples were processed as described100. For analysis, cryopreserved samples were thawed at 37°C and washed with 10% FBS/RPMI prior to staining.
Flow cytometry.
Single cell suspensions were stained with amine-reactive Zombie dyes (Biolegend) diluted in PBS for 10 mins at RT before staining with surface antibodies (see Extended Data Table 6) diluted in a 1:1 mixture of Brilliant Stain Buffer (BD) and 2%FBS/PBS for 60 mins on ice. Samples were then fixed and permeabilized with an eBioscience Foxp3 Staining Kit (Thermofisher Scientific) according to the manufacturers’ instructions before staining with intracellular antibodies for 60–90 mins at RT. To determine absolute cell counts, SPHERO Blank Calibration Particles (6–6.4μm, BD) were added to each sample before acquisition on BD Symphony A5 or Cytek Aurora flow cytometers. Samples were analyzed in Flowjo v10 or in OMIQ. For mean fluorescence intensity calculations in Fig 1 and Extended Data Fig 1, Arm and Cl13 P14 cells from tissue or spleen were stained separately with anti-CD45.1-Biotin antibodies, barcoded with unique streptavidin tags then mixed prior to cell surface staining to control for staining intensity variability between tissue single-cell suspensions. P14 cell ratios in CRISPR-Cas9 experiments were normalized back to the exact input ratio of naïve cells injected at the time of adoptive cotransfer. Combined residency scores were determined by summing the fold-change in MFI of markers typically upregulated in TRM cells (CD69, CD103, CD49a, CXCR6, CD38, CD39) in control sgCd19 T cells compared to test guide perturbed T cells, subtracting the fold-change in MFI Ly6C and normalizing back to the average score in the sgCd19 versus sgCd19 control group.
In vitro stimulation of CD8+ T cells.
For in vitro restimulation experiments, mice were injected with 50μg of anti-mouse ARTC2 nanobody (Treg protector, Biolegend, clone s+16a) r.o. 10–30 mins prior to euthanasia to limit P2RX7-mediated cell death. Following tissue processing, cells were resuspended in cRPMI (10%FBS/RPMI containing 1% Penn/Step, 10mM HEPES, 1% MEM Nonessential Amino Acids, 1% L-glutamine, 1mM Sodium Pyruvate, 0.05mM β-mercaptoethanol), CD107a AF647 (Biolegend), GolgiStop (1:250, BD, 554724), GolgiPlug (1:500, BD, 555029) and 2 × 10−4 μg/mL gp33–41 peptide (KAVYNFATM) and incubated at 37°C for 5 hours. Cells were washed, stained with surface antibodies and fixed and permeabilized with a BD Biosciences Cytofix/Cytoperm kit according to the manufacturers’ instructions prior to intracellular antibody staining.
Flow cytometric sorting and viral rechallenge.
For sort-retransfer and TEA-seq experiments, mice were injected with 50μg of anti-mouse ARTC2 nanobody (Treg protector, Biolegend, clone s+16a) r.o. 10–30 mins prior to euthanasia to limit P2RX7-mediated cell death. Following tissue processing, for sort-retransfer experiments cells were resuspended in PBS containing amine-reactive Zombie dyes (Biolegend) and surface antibodies and incubated on ice for 30 mins before being resuspended in 2%FBS/PBS containing 2mM EDTA. For TEA-sequencing experiments, cells were first stained with surface antibodies on ice then resuspended in 2% FBS/PBS containing 7-aminoactinomycin D (7AAD) for discrimination of live cells. Cells were sorted into 50% FBS/RPMI on a BD FACS Aria III using a 100μm nozzle with the sorting chamber maintained at 4°C. For sort-retransfer experiments, cells were washed twice with PBS prior to adoptive transfer to recipient mice via i.v. injection. Mice were either rechallenged with LCMV Arm i.p. 1 hour later or with LCMV Cl13 i.v. 12 hours later.
Transcriptomic, Epitope and Accessibility (TEA) sequencing.
TEA-sequencing was performed using a modified version of the standard 10x Genomics Chromium Single Cell Multiome ATAC + Gene Expression Kit protocol, as described60. Briefly, live CD8β+CD44hiCD45.1+Vα2+ P14 cells from each sample (pooled organs from 20 Arm-infected mice or 20 Cl13-infected mice) were sorted into separate FBS-coated FACS tubes containing 50%RPMI/FBS and washed once with Cell Staining Buffer (Biolegend) before staining samples with individual TotalSeq-A Hashtag Oligo (HTO) antibodies and a custom pool of TotalSeq-A Antibody Derived Tag (ADT) antibodies (Biolegend; see Extended Data Table 6). Samples were washed with Cell Staining Buffer (850G, 7 mins, 4°C) before resuspending cells in 2%BSA/DPBS and pooling cells. Cells were resuspended in custom TEA-seq Wash Buffer (20mM Tris-HCl, 150mM NaCl, 2mM MgCl2 and 1% BSA in H2O) containing RNase Inhibitor (1U/mL, Roche) and 0.01% Digitonin (Thermofisher) and incubated for 5 minutes on ice to permeabilize cell and nuclear membranes. Cells were topped with TEA-seq Wash Buffer and spun for 7 mins at 850G at 4°C before washing with TEA-seq Tagmentation Buffer (20mM Tris-HCl, 150mM NaCl, 2mM MgCl2 in H2O). 30,000 cells per reaction were removed and subjected to transposition and GEM generation as described in the 10x Chromium Single Cell Multiome ATAC + Gene Expression Kit. Library preparation was performed as described60, with the following customizations. Indexing of ADT and HTO libraries was performed using Biolegend-recommended Illumina D70x (i7 long; HTO) or RNA PCR Index (RPIx short; ADT) adapter sequences (IDT), with three additional rounds of amplification performed on purified HTO/ADT libraries using P5/P7 primers specific for generic Illumina adapter sequences (IDT) prior to sequencing (see Extended Data Table 7). Libraries were assessed using D1000 or D5000 High Sensitivity DNA ScreenTape (Agilent) on an Agilent Tapestation and quantified using a KAPA Illumina Library Quantification Kit (KK4824, Roche) prior to sequencing on S1 and S4 Flow Cells (Illumina) using a NovaSeq 6000 Instrument (Illumina).
Single-cell RNA and ATAC sequencing processing and quality control.
BCL files were demultiplexed using cellranger-atac-2.0.0 for ATAC-seq data, bcl2fastq for HTO and ADT data and cellranger-arc for RNA-seq data. Alignment and quantification of reads was performed using cellranger_arc (v2.0.2) for RNA-seq and ATAC-seq data and in Barcounter (v1.0) for HTO and ADT data. Analysis was then performed in R (v4.2) using ArchR (v1.0.2), Seurat (v5.1.0) and Signac (v1.13) using custom R scripts. Quantified HTO and ADT reads were combined with gene expression reads derived from cellranger to create Seurat objects. Cells were filtered out based on the following metrics: less than 500 RNA features, less than 2000 ATAC counts, less than 1500 ATAC features, a nucleosome signal greater than 5, a Transcription Site Enrichment (TSS) enrichment score greater than 4, more than 30% mitochondrial reads, and outliers with high numbers of HTO reads. HTOs were normalized using the Seurat CLR method and manually demultiplexed in using the multimode package (v1.5) by inspecting the distribution of each HTO signal against all others profiled. Negative droplets and doublets identified during HTO calling were removed. ATAC peaks were then called in ArchR using all cells that had passed quality control in Seurat and Signac. Briefly, additional QC was first performed by executing AddDoubletScores() then removing doublets with a filter ratio of 0.5 and filtering additional cells with a TSS Enrichment Score less than 10. Clustering based on ATAC fragments was performed in ArchR using the function addIterativeLSI() with the default parameters except for use of 25,000 variable features and a resolution of 2 with 2 iterations and then executing the addClusters() function with a resolution of 3.4. Pseudobulk replicates were created using the function addGroupCoverages() with a minimum cell cutoff of 30, maximum cell cutoff of 774 and the default parameters. Peaks were then called on clusters using macs2 and the ArchR function addReproduciblePeakSet() using the default parameters. This ArchR-derived peak set was then added to a Seurat object containing filtered cells merged from both wells using FeatureMatrix() and CreateChromatinAssay() and peaks were annotated against the mm10 genome using EnsDb.Mmusculus.v79 (v2.99) and the ClosestFeature() function. Barcounter derived ADT counts from filtered cells were processed and normalized using dsb (v1.0.4) using the default parameters and isotype control antibodies (see Extended Data Table 6) then added to the Seurat object.
Single-cell RNA and ATAC sequencing analysis.
Following quality control and peak calling, combined RNA and ATAC analysis was performed in Seurat (v5.1.0) and Signac (v1.13) on 1) the entire object containing all samples (23,338 cells) and 2) individual subsetted objects containing cells from each tissue and spleen in both infections. For RNA normalization and integration, SCTransform (SCT) v2 was performed on each well individually using 3500 variable features with regression of mitochondrial read proportions. Supervised reciprocal PCA (RPCA) integration of wells was performed on SCTransformed RNA using a k.anchor of 20, an anchor matrix disallowing matches between inconsistent HTOs and by excluding a subset of genes shown to be modulated by heat treatment during sample digestion101, heatshock genes (beginning with ‘Hsp’ and ‘Dnaj’) and ribosomal-related and mitochondrial genes (beginning with ‘Rps’, ‘Rpl’ or ‘mt’) from the AnchorFeatures. For ATAC normalization and integration, standard processing was performed using FindTopFeatures(), RunTFIDF() and RunSVD() and integration performed using PCA dimensions 2:50 and FindIntegrationAnchors() by subsetting the anchor matrix to disallow matches between inconsistent HTOs then performing IntegrateEmbeddings() using the default settings. Weighted Nearest Neighbor (WNN) analysis was performed using FindMultimodalNeighbors() using the integrated RNA (SCT dimensions 1:30) and ATAC (dimensions 2:50) reductions followed by RunUMAP(). Clustering on WNN within each object was then performed with the following resolutions: 2.3 (whole object), 0.7 (SI and spleen), 0.6 (SG and spleen) and 1.2 (liver and spleen). Clusters were either annotated within the whole object (splenic and naïve P14 cells) or within individual organ subsetted objects, with annotations from the latter then projected back onto the whole object for tissue-derived P14 cells. Principal Component Analysis was performed in edgeR (v4.2.1) and DEseq2 (v1.44) by pseudobulking each cluster according 10x lane origin. CD103-ADT+ cells were annotated as cells with normalized expression of CD103-ADT >1 from each tissue. Most RNA-seq and ATAC-seq based clusters comprised cells from a single tissue and infection origin, but clusters defined by proliferation (Prolif) interferon-stimulated genes (ISG) or by high expression of Rps/Rpl stress-related genes (SG Rps/Rplhi) contained a mixture of cells from acutely and chronically infected mice and so were excluded from downstream analysis.
Differentially expressed genes (DEGs) were calculated in Seurat using the SCT assay and functions FindAllMakers() and FindMarkers() for pairwise comparisons using a Wilcoxon Test and log2 fold-change cutoff of 0.125 and adjusted P value of less than 0.05 with a min.pct of 0.05. Gene signature enrichment was determined using the function AddModuleScore(), except for analysis of bulk RNA-seq human PBMC samples in Extended Data Figure 11b, where single-sample gene set enrichment analysis (ssGSEA) was used (GSVA_1.50.5) to calculate enrichment scores. Cliff’s delta values to assess effect size of enrichment were calculated using the effsize package. The established core TRM cell signature4 was defined as all genes upregulated in TRM cells across the SI, skin and liver. The TEX-TERM signature68 was defined as the top 300 genes enriched in TEX-TERM cells (by average log2 fold-change) isolated 30 dpi with Cl13 compared to splenic T cells isolated from Arm infected mice. DACRs were calculated in Signac using the functions FindAllMarkers() and FindMarkers() for pairwise comparisons using the LR test and a log2 fold-change cutoff of 0.125 and adjusted P value of less than 0.05 with a min.pct of 0.01, including the number of counts as a latent variable. Differentially motif enrichment (motif deviation) was calculated in Seurat using the JASPAR2024 motif set (for all vertebrates, extracted from the JASPAR2024 package v0.99.6 and added as a custom pfm) and the function RunChromvar() with BSgenome.Mmusculus.UCSC.mm10, using FindMarkers() for pairwise comparisons using the LR test and a log2 fold-change cutoff of 0.125 and adjusted P value of less than 0.05 with a min.pct of 0.05, including the number of counts as a latent variable. All heatmaps were generated using pheatmap (v1.0.12) by calculating average feature expression for each cluster using the AverageExpression() function in Seurat. Venn Diagrams and upset plots were generated using VennDetail (v1.20). Feature Plots and Joint Density Plots were generated in Seurat or with scCustomize (v2.1). Genome coverage tracks were generated using the Signac functions CoveragePlot(), PeakPlot(), TilePlot() and AnnotationPlot(). Peak-gene linkages for coverage tracks were calculated in Seurat using the default parameters. Adjusted P values < 5−150 were mutated to 5−150 for display in volcano plots to permit visualization.
Gene regulatory network (GRN) analysis was performed on all non-naïve cells in Pando63 (v1.1.1) using the top 3500 variable SCTransform normalized RNA features excluding heat-modulated, heatshock and Rsp/Rpl genes (as above) and using a union of selected candidate regulatory chromatin regions including conserved regulatory elements from ENCODE102, phastCons elements conserved across vertebrates103 (Vert 35 El) and additional domains of regulatory chromatin (DORCs) identified through peak-gene correlation for all P14 cells performed in FigR104 (v0.1.0). Motif identification was performed using the JASPAR2024105 motif set (for all vertebrates) and TFs identified using TFDB106 and the genome BSgenome.Mmusculus.UCSC.mm10. The GRN was inferred using the function infer_grn() using the xgb method with the peak to gene method Signac, a TF correlation threshold of 0.05 and extending 105 bases up and downstream of the transcription start site. GRN UMAP visualization was performed in Pando and included all genes inferred in the GRN. Cell-specific gene regulatory networks were generated using tidygraph (v1.3.1) using the Pando coefficient matrix, UMAP coordinates from the global GRN and by aggregating RNA expression determined by SCTransform in Seurat for each cluster. Regulatory modules were identified using the function find_modules() with a P threshold of 0.05, R2 threshold of 0.05 and an nvar_threshold of 6. Regulons were extracted from the Pando SeuratPlus object using the function NetworkModules(), filtered to modules containing more than 7 genes and regulon activity was determined using the AddModuleScore() function in Seurat and assigned to cells using the CreateAssayObject() function. Differential regulon activity was calculated using FindMarkers() in Seurat using a Wilcoxon Test and log2 fold-change cutoff of 0.125 and adjusted P value of less than 0.05.
CRISPR-Cas9 electroporation of naïve CD8+ T cells.
Electroporation of naïve CD8+ transgenic T cells was performed as described107. Briefly, CD45.1+ and CD45.1+CD45.2+ P14 T cells were isolated from the spleen or LN and subjected to EasySep magnetic CD8+ T cell enrichment (StemCell Technologies, kit 19858) according to the manufacturers’ instructions. RNP complexes were generated by incubating 0.3nmol sgRNA (2 guides per target obtained from IDT Technologies, see Extended Data Table 7) with 0.6μl Cas9 protein (IDT, 1081059) for 10 mins at RT. 2–10 × 106 CD8+ T cells were resuspended in supplemented P3 Buffer (Lonza P3 Primary Cell 4D-Nucleofector X electroporation kit, Lonza, V4XP-3032), mixed with RNP complexes and electroporated in a Lonza 4D-Nucleofector TM 4 Core Unit (Lonza, AAF-1002B) using the program DN100. Cells were quenched with cRPMI and rested for 10 mins at 37°C. 1000 congenically distinct control-targeted and test-targeted cells were then mixed at a 1:1 ratio and adoptively co-transferred i.v. to CD45.2+ recipient mice that were infected 48h post-cell infusion. Exact co-transfer ratios were recorded and used to normalize ratios of test to sgCd19 control cells observed in each organ back to the starting input ratio for analysis.
In vivo immune modulator, antibody and gp33 peptide administration.
For Dasatinib treatment, mice were delivered 50mg/kg Dasatinib (MedChem Express) in 0.9%saline/10%DMSO/20%Sulfobutylether-β-Cyclodextrin (MedChem Express) solution daily for 7 days by oral gavage. For FTY720 treatment, mice were injected i.p. with 1mg/kg Fingolimod (Cayman Chemical) in 2%(2-hydroxypropyl)-beta-Cyclodextrin/PBS (Sigma Aldrich). For α-PD-L1 treatment, mice were injected once i.p. with 200ug of α-PD-L1 antibody (10F.9G2, BioXCell, BE0101) diluted in PBS and analyzed 48 hours later. For i.v. gp33–41 peptide administration, mice were injected i.v. with 25μg gp33–41 peptide diluted in 10% DMSO/PBS. In all cases, control mice received the vehicle solution only.
Construction of cell state-specific gene signatures.
The TRM cell-specific signature was derived by taking the union of genes significantly upregulated in TRM cells from at least two tissues compared to TR-TEX cells from matched tissue sites and compared to TCIRCM cells (Arm Spl TMEM and Arm Spl TEFF clusters) from the spleen with a log2 fold-change threshold of 0.5 and adjusted P value cutoff of 0.05, excluding genes that were also upregulated in TR-TEX cells compared to TCIRCM cells. The TR-TEX cell-specific signature was derived by taking the union of genes significantly upregulated in TR-TEX cells from at least two tissues compared to TRM cells from matched tissue sites and compared to TCIRCM cells from the spleen with a log2 fold-change threshold of 0.5 and adjusted P value cutoff of 0.05, excluding genes that were also upregulated in TRM cells compared to TCIRCM cells.
Survival probability analysis in tumor patient cohorts.
Clinical metadata and gene expression count files were obtained from cBioPortal (TCGA SKCM and METABRIC datasets), the R package IMvigor210CoreBiologies (v1.0.1, IMvigor210 dataset) or from Liu et al. 201979 (α-PD-L1/α-CTLA4 metastatic melanoma dataset). The METABRIC dataset was pre-filtered to select for triple negative breast cancer (TNBC) patients. Kaplan Meier survival analysis was performed in R using the packages survminer (v0.4.9), survival (v3.7.0) and GSVA (v1.48.3). Where indicated, samples were divided into the top and bottom 50% (CD8hi and CD8lo) based on ranked expression of the gene CD8A, before the CD8hi fraction was further divided into signaturehi (top 10 or 25% as indicated), signaturemed (middle 50 or 80% as indicated) or signaturelo (bottom 10 or 25% as indicated) fractions based on ranked scores for each signature. In other analyses, all samples were split into signaturehi, signaturemed or signaturelo fractions without CD8A pre-stratification. Where relevant, the IMvigor210 cohort was stratified based on clinical response using RECIST criteria. Signature score correlation analyses with CD8A expression were performed using the Pearson correlation method in R.
Statistical analysis.
Statistical analysis was performed in Graphpad Prism (v10.2) or in R (v4.2) using a Mann Whitney Test, Wilcoxon Paired Sum Rank Test, Two Way ANOVA or Kaplan Meier Test where indicated in the figure legends and Methods. All statistical tests performed were two-sided tests. Where parametric tests were used, a Kolmogorov-Smirnov test was used to confirm normal distribution of samples. In all other cases, non-parametric tests were used. Except where indicated, individual data points are indicated with symbols and bars on plots indicate the mean. For box plots, the center line indicates the median, the box limits represent the upper 75th and lower 25th percentile and interquartile range and whiskers extend to 1.5x the interquartile range. Samples with fewer than 10 P14 cells were excluded from flow cytometric frequency calculations. Samples for which no or low titers of LCMV Cl13 could be detected in the serum or kidney >15 dpi were excluded from chronically infected groups in rechallenge experiments and as sort donors for rechallenge experiments and TEA-sequencing experiments. Mice were allocated to groups randomly or by quantifying LCMV Cl13 titers in serum by plaque assay and evenly distributing mice between groups based on viral titers.
Data availability.
Multimodal ADT, HTO, RNA and ATAC raw sequencing data generated in this study will be deposited in the National Center for Biotechnology Information Gene Expression Omnibus prior to publication. Raw differential gene expression, differential accessibility data and unique gene signatures generated in this study are available as Extended Data Tables. All other raw and processed data are available from the authors upon reasonable request. Publicly available datasets used for analysis in this manuscript are available at the following accession numbers: GSE70813, GSE179613, GSE199565, or via gutcellatlas.org.
Extended Data
Extended Data Figure 1. Phenotype and function of tissue-infiltrating CD8+ T cells during acute and chronic infection.
a, Absolute number of Vα2+CD45.1+ P14 T cells isolated from spleen (Spl), liver (Liv), kidney (Kid), salivary gland (SG) or small intestine epithelium (SI) 30 dpi with acute LCMV Arm or chronic LCMV Cl13 infection. b, Viral titers detected by plaque assay in serum or indicated tissues 28–32 dpi. c, Expression of CD69 and CXCR6 by P14 cells in indicated tissues 30 dpi with Arm or Cl13. d, Frequency (dashed lines, †) or absolute number (solid lines, *) of CD69+CD103+ cells in SG at indicated dpi with Arm (blue) or Cl13 (pink). Individual data points represent mean; shading represents 95% confidence interval. e, Geometric mean-fluorescence intensity (gMFI) of residency-associated molecules by CD69+CD103+ P14 cells isolated 30 dpi from the SI of Arm (TRM) or Cl13 infected mice or TCIRCM cells from Arm Spl. f,g, Expression of Ly108 and CX3CR1 by P14 T cells isolated from Spl (f) or SI (g) 30 dpi. TEX-Prog; TEX Progenitor, TEX-Int; TEX-Intermediate, TEX-KLR; TEX-Killer cell lectin-like receptor, TEX-Term; TEX Terminal. h, Frequency of Ly108−CX3CR1− cells within total P14 cells (Spl), CD69+CXCR6+ P14 cells (Liv or Kid) or CD69+CD103+ P14 cells (SG, SI) 30d post-infection with Arm or Cl13. i, Geometric mean-fluorescence intensity (gMFI) of exhaustion-associated molecules by CD69+CD103+ P14 cells isolated 30 dpi from the SI of Arm (TRM) or Cl13 infected mice or TCIRCM cells from Arm Spl. j, Expression of CD39 by P14 cells with indicated surface phenotype 30 dpi with Arm or Cl13 across tissues. k, Expression of indicated surface markers by CD69+CD103+ (SI, SG) or CD69+CXCR6+ (Liv) P14 TRM cells (Arm, blue) or P14 T cells (Cl13, pink) compared to Arm Spl TCIRCM cells (grey) and Cl13 Spl TEX-Term cells (purple) 30–40 dpi. Dashed line indicates average expression in Arm TCIRCM P14 cells. l, Cytokine production by TCIRCM P14 cells from Arm Spl or by CD69+CD103+ TRM cells from Arm SI or CD69+CD103+ P14 cells from Cl13 SI 30–40 dpi following in vitro gp33–44 peptide stimulation. m, Cytokine production by P14 TCIRCM cells isolated from the Spl of Arm infected mice (grey), CD69+CXCR6+ terminally exhausted P14 cells from the Spl of Cl13 infected mice (TEX-TERM), or by CD69+CD103+ (SG) or CD69+CXCR6+ (Liv) P14 cells from Arm (TRM, blue) or Cl13 (pink) infected mice 30–40 dpi following in vitro gp33–44 peptide stimulation. n, Ratio of Bim and Bcl2 gMFI in CD69+CD103+ P14 T cells isolated from the SI or SG of Arm (TRM, blue) or Cl13 (pink) infected mice 15–25 dpi. Data are pooled from 2–3 independent experiments (a, b, d, h, j, m, n), or representative of 2–3 independent experiments (c, e, f, g, i, k, l) with n = 4–5 mice (a-m) or n = 3 mice per group per experiment (n). * or † p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 Mann-Whitney test (a, c, d, e, h, i, j, m) or Wilcoxon signed-rank test (n).
Extended Data Figure 2. Resolution of T cell states across tissues in acute and chronic infection.
a, Projection of all P14 cells analyzed by TEA-seq in UMAP space based on RNA expression only (upper panel) or chromatin only (lower panel) colored by sample (Hashtag; HT). b, Distribution of P14 cells assigned to each annotated cluster derived from each infection (upper panel), tissue (middle panel) or sorted sample (lower panel). c, Weighted-nearest neighbor (WNN) UMAP based on combined RNA and ATAC expression in P14 cells colored by ADT (protein, top row), RNA expression (middle row) or by gene activity (bottom row, chromatin accessibility) for indicated markers. d, Re-clustered WNN UMAP based on combined gene expression and chromatin in P14 cells from SI and Spl samples only, colored by annotated Seurat subcluster (cluster key d-h). e RNA expression of top 10 marker genes and selected key genes by each indicated SI-derived subcluster and Spl-derived cluster. f,g Expression of CD103 protein (ADT) and RNA (Itgae), joint RNA expression of Klf2 and S1pr1 or joint CD69 and CXCR6 protein (ADT) expression in SI and Spl derived P14 cells. Purple shaded area in g indicates cells annotated as CD103-ADT+. h, Distribution of cells from SI and Spl assigned to each subcluster. i, Re-clustered WNN UMAP based on combined gene expression and chromatin in P14 cells from SG and Spl samples only, colored by annotated Seurat subcluster (cluster key i-m). j, RNA expression of top 10 marker genes and selected key genes by each indicated SG-derived subcluster and Spl-derived cluster. k, l, Expression of CD103 protein (ADT) and RNA (Itgae), joint RNA expression of Klf2 and S1pr1 or joint CD69 and CXCR6 protein (ADT) expression in SI and Spl derived P14 cells. Purple shaded area in l indicates cells annotated as CD103-ADT+. m, Distribution of cells from SG and Spl assigned to each subcluster. n, Re-clustered WNN UMAP based on combined gene expression and chromatin in P14 cells from Liv and Spl samples only, colored by annotated Seurat subcluster (cluster key n-q). o, RNA expression of top 10 marker genes and selected key genes by each indicated Liv subcluster and Spl-derived cluster. p, Expression of Gzma RNA or joint RNA expression of Klf2 and S1pr1 in Liv and Spl derived P14 cells. q, Distribution of cells from Liv and Spl assigned to subcluster. Data are pooled from 20–25 mice per infection per tissue.
Extended Data Figure 3. Transcriptional and epigenetic differences between TRM and TR-TEX cells.
a, Heatmap of RNA expression of genes that are upregulated in Arm TRM cells versus Arm Spl TCIRCM cells and are also upregulated in Cl13 TR-TEX cells versus Cl13 Spl TEX-TERM cells. b, Violin plots displaying gene expression in indicated Seurat clusters. c, Volcano plot of differentially expressed genes (DEGs) between Arm SI TR-TEX and Cl13 Spl TEX-TERM cells. d, Volcano plots displaying pairwise DEGs (left column) and rank-ordered plots displaying pairwise differentially accessible chromatin regions (DACRs) (right column) between Arm TRM cells (blue) and Cl13 TR-TEX cells (pink) from SG (top panel) or Liv (bottom panel). e, Number of DEGs (left panel) and DACRs (right panel) between Arm Spl TMEM and Cl13 Spl TEX-Term or tissue-matched Arm TRM (blue) and Cl13 TR-TEX (pink) P14 cells. e, Proportion of genes upregulated or downregulated in CD103-ADT+ Arm TRM or CD103-ADT+ Cl13 TR-TEX cells from the SI or SG that are also up- or down-regulated by total Arm TRM or Cl13 TR-TEX cells from the same tissue. Data are pooled from 20–25 mice per infection per tissue.
Extended Data Figure 4. Differential regulation of exhaustion, memory and tissue-residency associated factors in TRM and TR-TEX cells.
a, ATAC coverage plots of DACRs in indicated gene loci for each cluster. SI and SG Arm TRM and Cl13 TR-TEX are subsetted to CD103-ADT+ cells. Green bars indicate DACRs enriched in Arm Spl TMEM compared to Arm TRM cells, blue bars indicate DACRs enriched in Arm TRM cells compared to location-matched Cl13 TR-TEX cells from at least one tissue, pink bars indicate DACRs enriched in Cl13 TR-TEX compared to location-matched Arm TRM cells from at least one tissue. b, Pairwise transcription factor (TF) motif enrichment in SI (left panel), SG (middle panel) or Liv (right panel) Arm TRM cells compared to tissue-matched Cl13 TR-TEX cells (x axis) plotted against pairwise TF motif enrichment in Arm Spl TMEM cells compared to Cl13 Spl TEX-TERM cells (y axis). MeanDiff = mean difference determined by chromvar motif deviation analysis. c, RNA expression of indicated TFs (upper panel) and of genes in Pando-defined regulons predicted to be controlled by each TF (positive regulon activity). Data are pooled from 20–25 mice per infection per tissue.
Extended Data Figure 5. Requirements for Runx3, Blimp1 and Hobit for residency programming across tissues.
a, Ratio of total P14 cells electroporated with indicated sgRNAs versus control Cd19 sgRNAs from Arm (blue) or Cl13 (pink) infected SI (colored) or spleen (grey) at 8–9 dpi..b, Co-expression of CD69 and CD103 by SI P14 T cells electroporated with control sgCd19 or indicated TF-targeting sgRNAs at 8–9 dpi with Arm (blue) or Cl13 (pink). c, Ratio of congenically distinct and co-transferred P14 Arm TRM (blue), TR-TEX (pink) cells electroporated with identical control Cd19 sgRNAs (same guide in each congenic population) in indicated tissues (SI, SG P14 gated on CD69+CD103+, Liv P14 gated on CD69+CXCR6+) compared to co-transferred total Spl-derived P14 cells (grey) at 26–37 dpi. d, Ratio of co-transferred Arm TRM (blue), Cl13 TR-TEX (pink) or total Spl-derived (grey) P14 cells electroporated with Prdm1 or Runx3 sgRNAs versus control Cd19 sgRNAs at 30–37 dpi. Arm TRM and Cl13 TR-TEX were gated as CD69+CD103+ in the SG and as CD69+CXCR6+ in the Liv. e, Ratio of total CD69+ P14 cells electroporated with indicated sgRNAs versus control Cd19 sgRNAs from Arm (blue) or Cl13 (pink) infected tissues (SI, Liv, SG; colored) compared to ratio of total Spl-derived P14 cells (grey) at 26–37 dpi. Data are pooled from 2 independent experiments with 4–8 mice per group per experiment. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 paired T test (spleen versus tissue P14s) or two-tailed T test (Arm versus Cl13 tissue P14s) (a, c-e).
Extended Data Figure 6. Tox coordinates TR-TEX cell residency programming but is not required for TRM cell development.
a, Expression of Tox in total sgCd19 and sgTox electroporated P14 cells isolated from the SI at 8–9 dpi with Arm or Cl13. b, Ratio of total P14 cells electroporated with Cd19 control or Tox targeting sgRNAs isolated from the Spl or SI at 8d (left panel) or >4wks (right panel) pi with Arm or Cl13. c, Frequency of CD69+CD103+ sgCd19 (grey) or sgTox (purple) electroporated P14 cells isolated from the SI at 8 dpi. d, Ratio of co-transferred Arm TRM (blue), Cl13 TR-TEX (pink) or total Spl-derived (grey) P14 cells electroporated with Tox sgRNAs versus control Cd19 sgRNAs >4wks pi. Arm TRM and Cl13 TR-TEX were both gated as CD69+CD103+ in the SG or as CD69+CXCR6+ in the Liv. e, Frequency of P14 cells that were CD69+CD103+ (SI, SG) or CD69+CXCR6+ (Liv) following electroporation with Tox (purple) or Cd19 (grey) targeting sgRNAs isolated from indicated tissues >4wks pi. f, Ratio of total CD69+ P14 cells electroporated with Tox sgRNAs versus control Cd19 sgRNAs from Arm (blue) or Cl13 (pink) infected tissues (SI, Liv, SG; colored) compared to ratio of total P14 cells in the spleen (grey) >4wks pi. g, Expression of indicated surface molecules by total P14 cells electroporated with indicated sgRNAs and isolated from the SI 8–9 dpi with Arm or Cl13. h, Fold change (FC) in expression of indicated surface molecules in total P14 cells electroporated with sgRNAs directed towards genes encoding indicated TFs compared to control cells electroporated with sgRNAs directed towards Cd19 at 8–9 dpi with Arm or Cl13. Data are pooled from 2–3 experiments with 4–8 mice per group per experiment. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 paired T test (spleen versus tissue P14s) or two-tailed T test (Arm versus Cl13 tissue P14s) (b-f) or Mann Whitney test (h).
Extended Data Figure 7. Developmental plasticity and lineage relationships between TRM and TEX cells.
a, Expression of CD107a by sort-transferred donor P14 cells rechallenged with Arm or Cl13 and re-isolated from the Spl 21–28 dpi following ex vivo gp33–44 stimulation, compared to TRM cells that were not transferred or rechallenged (no rch). Statistics indicate comparison between Arm and Cl13 rechallenged cells of same origin. b, Absolute number of total P14 cells recovered from the Spl of mice receiving donor cells from indicated origin following Cl13 rechallenge. c, UMAP of unsupervised phenograph (pg) clustering of donor P14 cells from Arm or Cl13 rechallenged mice; numbers in key indicate pg cluster determined by kmeans clustering. d, Heatmap of marker expression in pg clusters. Rechallenge infection giving rise to each cluster is indicated by pink (Cl13) or blue (Arm). TEX-TERM clusters are highlighted in purple (pg 8 and pg 4). e, Proportion of Spl TEX-TERM cells derived from donor P14 cells of indicated origin in pg clusters after Cl13 rechallenge. f, g, Expression of markers by Spl TEX-TERM cells from indicated donor origin after Cl13 rechallenge compared to Arm rechallenge. h, Absolute number of total P14 cells (Spl) or CD69+CD103+ P14 cells (SG) derived from donor P14 cells following Arm rechallenge. i, Proportion of Arm-rechallenged P14 cells co-expressing CD69 and CD103 in SG. j, Expression of indicated surface molecules by Arm-rechallenged donor P14 cells of indicated origin isolated from the Spl or SI. k, Proportion of Arm-rechallenged P14 cells in the Spl or SI expressing CX3CR1 and Ly6C. l, m, Proportion of Arm-rechallenged donor P14 cells staining positive for CD8a-BV650 during intravascular labelling. Bl; blood. n, Absolute number of total P14 cells (Spl) or CD69+CD103+ P14 cells (SG) derived from donor P14 cells following Cl13 rechallenge. o, Proportion of Cl13-rechallenged donor P14 cells co-expressing CD69 and CD103 in SG. p, Expression of Ly6C and CX3CR1 by Cl13-rechallenged donor P14 cells recovered from the SI. q, Expression of indicated surface molecules by Cl13 rechallenged donor P14 cells isolated from the Spl or SI. r, Proportion of donor P14 cells expressing CD69 and CD103 in the SI after Arm or Cl13 rechallenge. s, Absolute number of total P14 cells isolated from the Spl of Arm or Cl13 infected mice following 1wk dasatinib treatment. t, Frequency of P14 cells co-expressing CD69 and CD103 in SI and SG of Arm or Cl13 infected mice following 1wk dasatinib treatment. Dashed lines on absolute number plots (h, n) indicate threshold limit of detection for plotting in frequency plots. Dashed lines in histograms (l, q) indicate average expression in total naïve P14-derived cells in Spl. Data are pooled from or representative of 2 independent experiments (a, h-r) or 3 independent experiments (b-g, s-t), with n = 3–5 mice per group per experiment. TN; naïve P14 cells. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 Mann Whitney Test.
Extended Data Figure 8. Cell-state specification of TRM and TR-TEX cells.
a, Detailed UMAP embedding of inferred single-cell Gene Regulatory Network (GRN) based on combined transcription factor (TF) expression and motif accessibility in all non-naïve P14 cells analyzed by TEA-sequencing. Size of nodes (genes) represents the number of connections in network. b, Individual UMAP embeddings of genes and gene modules from inferred single-cell Gene Regulatory Networks (GRNs) engaged in each T cell subset. Node size and color scale represent degree of RNA expression for each gene in network. c, d Enrichment for GRN modules in merged TRM and TR-TEX cell Seurat clusters from all tissues, or in Arm Spl TMEM and Cl13 Spl TEX-TERM clusters. FC = fold change, δ = Cliff’s delta effect size. Circle size and heat scale indicate relative enrichment per cluster. e, Heatmap of RNA expression of genes commonly upregulated in Arm TRM and Cl13 TR-TEX cells versus Arm Spl TCIRCM cells (pooled Arm Spl TMEM and Arm Spl TEFF clusters). f, Heatmap of RNA expression of genes uniquely upregulated in Arm Spl TCIRCM cells versus Cl13 Spl TEX-TERM cells but not in Arm TRM cells versus Cl13 TR-TEX cells (left panel), or commonly upregulated in Arm Spl TCIRCM cells versus Cl13 Spl TEX-TERM cells and Arm TRM cells versus Cl13 TR-TEX cells (right panel). g, Comparative analysis of RNA expression of genes upregulated in 1) Cl13 Spl TEX-TERM cells versus Arm Spl TCIRCM cells, 2) Cl13 Spl TR-TEX cells versus Arm TRM cells from each tissue and 3) TRM from each tissue versus Arm Spl TCIRCM cells. Heatmaps display genes that are uniquely enriched in TEX (top panel) or are shared with Arm TRM cells (bottom heatmap) compared to Spl TCIRCM cells. Data are pooled from 20–25 mice per infection per tissue.
Extended Data Figure 9. Defining cell-state specific gene and surface protein signatures of TRM and TR-TEX cells.
a, Heatmaps displaying relative RNA expression of indicated genes comprising TRM cell-specific (upper panel) or TR-TEX cell-specific (lower panel) signatures identified through TEA-seq. b, UMAP projections showing Seurat enrichment for published Mackay 2016 TRM core and Giles 2022 TEX-TERM signatures in TRM (blue circled clusters) and TR-TEX (pink circled clusters) compared to newly derived TRM and TR-TEX cell-state specific signatures in TEA-seq dataset. c, Cliff’s delta effect size and fold-change of Seurat enrichment for published Mackay 2016 TRM core and Giles 2022 TEX-TERM signatures and newly derived TRM and TR-TEX cell-state specific signatures in indicated P14 cell populations from TEA-seq dataset. d, Seurat enrichment for cell-state specific TRM or TR-TEX signature scores within CD8+ T cells isolated from human SI epithelium or non-naïve CD8+ T cells from healthy donor peripheral blood60 or GSEA enrichment for cell-state specific signatures within CD39+PD-1+ TEX cells compared to all non-naïve CD8+ T cells isolated from healthy donor peripheral blood61. δ = Cliff’s delta, FC = fold-change. e, RNA expression of genes encoding indicated surface proteins in LCMV-generated P14 cell clusters identified via TEA-seq. Circle size indicates proportion of cells expressing each gene; scale bar indicates relative expression. f, Expression of indicated molecules in Arm Spl TCIRCM, Cl13 Spl TEX-PROG or Cl13 Spl TEX-TERM cells and in TRM or TR-TEX cells isolated from SI (CD69+CD103+) or Liv (CD69+CXCR6+) 30–40 dpi with Arm or Cl13, respectively. g, Proportion of TRM (blue) or TR-TEX cells (pink) expressing CD73 and CD200R in Liv (CD69+CXCR6+) or SG (CD69+CD103+) after Arm or Cl13 infection, respectively (left and middle panel) or proportion of TRM-like cells in human epidermal or melanoma samples (KLRG1−CD69+CD103+) expressing CD73 and CD200R (right panel). h, IR expression by total P14 TCIRCM from the Spl of Arm-infected mice or by CD69+CD103+CD73+ TRM cells (blue) or CD69+CD103+CD73−CD200R+ TR-TEX cells (pink) 30–40 dpi with Arm or Cl13, respectively. i, IR expression by KLRG1−CD69+CD103+CD73+ TRM-like cells from human tonsil or epidermal skin or KLRG1−CD69+CD103+CD73−CD200R+ TRM-like TIL from HNSCC or melanoma. Data are pooled from 3–6 human donors (g, i) or representative of at least 2 experiments with 4–5 mice per group per experiment (e, f, g). TEA-seq data are pooled from 20–25 mice per infection per tissue. * p < 0.05, Mann Whitney test.
Extended Data Figure 10. TRM and TR-TEX cells differentially contribute to immune responses.
a, Frequency of LM-OVA generated OT-I cells in the SI co-expressing CD69 and CD103 following LCMV Arm or Cl13 reinfection, or with no LCMV infection (no inf). b, Viral titers in the serum of LM-OVA immune mice 30d after infection with Arm or Cl13. LOD = limit of detection. c, Proportion of CD69+CD103+ OT-I TRM (LM-derived), P14 TRM (Arm-derived) or P14 TR-TEX (Cl13-derived) cells expressing CD73 or CD200R following LCMV infection or without LCMV infection (LM only). d, e Kaplan-Meier survival curves for overall survival of metastatic melanoma patients from the TCGA database (d) or TNBC patients from the METABRIC database (e) displaying stratification by TRM and TR-TEX specific signatures (hi = top 25%, lo = bottom 25%) within all patients (upper panel) or within the top 50% of CD8hi patients compared to CD8lo patients (lower panel). f, Correlation analysis comparing TRM (blue) and TR-TEX (pink) signature scores and CD8A expression in TCGA melanoma patients. g, Geometric mean fluorescence intensity (gMFI) of PD-1 in CD69+CD103+ Arm SI P14 TRM or Cl13 TR-TEX cells (left panel) and of PD-L1 in MHCII+CD11c+ SI-derived dendritic cells (DCs) at baseline (no peptide treatment) and 48h after treatment with gp33–44 peptide i.v.. h, Number of total P14 cells isolated from the SI (right panel) of Arm or Cl13 infected mice treated with FTY720 and α-PD-L1 with or without i.v. gp3333–41 peptide administration 48h earlier. i, j, Cytokine production by total Spl P14 cells (i) or by CD69+CXCR6+ Cl13 Spl TEX-TERM cells (j) treated with α-PD-L1 with or without i.v. gp3333–41 peptide treatment. k, Frequency of CD69+CD103+ SI P14 TRM (Arm) or TR-TEX (Cl13) cells producing IFN-γ and TNF following α-PD-L1 treatment with gp33–44 peptide i.v. co-infusion in all mice. l-n, Degranulation (CD107a expression, l) or cytokine production by SI CD69+CD103+ P14 Arm TRM cells or Cl13 TR-TEX cells from mice treated with α-PD-L1 with or without gp3333–41 peptide i.v.. Data are pooled from and representative of 3 independent experiments with n = 4–7 mice per group per experiment (a-c) and 2–3 independent experiments with 3–8 mice per group per experiment (g-n). * p < 0.05, ** p < 0.01, Kruskal Wallist Test (a-c, g), Mann Whitney test (h-n), Kaplan-Meier estimate (d, e) or Pearson correlation test (f).
Supplementary Material
Acknowledgments.
We thank all members of the Wherry Laboratory for helpful discussions and critical analysis of this manuscript. We thank the Penn Cytomics and Cell Sorting Shared Resource Laboratory and Children’s Hospital of Philadelphia Flow Cytometry Core for providing technical support and instrumentation. We thank the Penn Dermatology Skin Biology and Diseases Resource-based Center (SBDRC) for providing human skin samples. We thank Brian Sheridan for providing the IlnAmut strain of LM-OVA.
Funding.
S.L.P. was supported by a Cancer Research Institute Irvington Postdoctoral Fellowship. M.M.P. was supported by an NIH F32 grant (AI181343). M.A.S. was supported by the NIH NIAID grant 5F30AI174776 and the University of Pennsylvania Medical Scientist Training Program. Y.J.H. was supported by a National Science Foundation Graduate Research Fellowship. D.B.R was supported by an MD fellowship of the Boehringer Ingelheim Fonds. V.F. was supported by NIH 5T32AR007465–40, the University of Pennsylvania Colton Center for Autoimmunity, and the Dermatology Foundation’s Dermatologist Investigator Research Fellowship. J.E.W. was supported by an NIH T32 grant (AR007442) and Parker Institute for Cancer Immunotherapy Scholar Award. D.B. received funding from an NIH/NIDCR grant (R01DE034056). A.C.H. was supported by NIH grants P50CA261608 and R01CA273018. C.T.E. was supported by NIH/NIAMS K08-AR0802666 and the Colton Center for Autoimmunity. A.D. was supported in part by Grant IRG-22–150- 41- IRG from the American Cancer Society, and by the Breakthrough Challenge Foundation. This work was supported by grants from the NIH, AI155577; AI115712; AI117950; AI108545; AI082630 and CA210944 (to E.J.W.), the Mark Foundation, the Colton Center at Penn, and the Parker Institute for Cancer Immunotherapy which fund work in the Wherry laboratory. Work involving human skin samples was supported by funding from NIH/NIAMS grant P30-AR069589.
Footnotes
Competing Interests. A.C.H received research funding from Bristol Myers Squibb and Merck. C.T.E. holds equity in Cabaletta Bio and has licensed patents with Cabaletta Bio and Novartis. J.R.G. is a consultant for Arsenal Biosciences, Cellanome, Seismic Therapeutics, and GVM1. E.J.W. is a member of the Parker Institute for Cancer Immunotherapy which supported this study. E.J.W. is an advisor for Arsenal Biosciences, Coherus, Danger Bio, IpiNovyx, New Limit, Marengo, Pluto Immunotherapeutics, Prox Biosciences, Related Sciences, Santa Ana Bio, and Synthekine. E.J.W. is a founder of Prox Biosciences, Danger Bio, and Arsenal Biosciences. E.J.W. holds stock in Coherus.
References
- 1.Christo SN, Park SL, Mueller SN, Mackay LK. The Multifaceted Role of Tissue-Resident Memory T Cells. Annual Review of Immunology. 2024;42(1):317–345. [DOI] [PubMed] [Google Scholar]
- 2.Szabo PA, Miron M, Farber DL. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. Apr 5 2019;4(34)doi: 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milner JJ, Toma C, Yu B, et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552(7684):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackay LK, Minnich M, Kragten NAM, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459–463. doi: 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- 5.Crowl JT, Heeg M, Ferry A, et al. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat Immunol. Jul 2022;23(7):1121–1131. doi: 10.1038/s41590-022-01229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Article. Nature Immunology. 12//print 2013;14(12):1294–1301. doi: 10.1038/ni.2744 http://www.nature.com/ni/journal/v14/n12/abs/ni.2744.html#supplementary-information [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. 10.1038/ni.1718. Nat Immunol. 05//print 2009;10(5):524–530. doi:http://www.nature.com/ni/journal/v10/n5/suppinfo/ni.1718_S1.html [DOI] [PubMed] [Google Scholar]
- 8.Park SL, Zaid A, Hor JL, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nature Immunology. 2018/February/01 2018;19(2):183–191. doi: 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- 9.Schenkel J, Fraser K, Beura L, Pauken K, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014/October// 2014;346(6205):98–101. doi: 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SL, Buzzai A, Rautela J, et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature. 2018/December/31 2019;565(7739):366–371. doi: 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- 11.Wijeyesinghe S, Beura LK, Pierson MJ, et al. Expansible residence decentralizes immune homeostasis. Nature. 2021/April/01 2021;592(7854):457–462. doi: 10.1038/s41586-021-03351-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLane L, Abdel-Hakeem M, Wherry E. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annual review of immunology. 2019:ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Sen DR, Kaminski J, Barnitz RA, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354(6316):1165. doi: 10.1126/science.aae0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160. doi: 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019/July/01 2019;571(7764):211–218. doi: 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfei F, Kanev K, Hofmann M, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019/July/01 2019;571(7764):265–269. doi: 10.1038/s41586-019-1326-9 [DOI] [PubMed] [Google Scholar]
- 17.Scott AC, Dündar F, Zumbo P, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019/July/01 2019;571(7764):270–274. doi: 10.1038/s41586-019-1324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao C, Sun HW, Lacey NE, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8(+) T cell persistence in chronic infection. Nat Immunol. Jul 2019;20(7):890–901. doi: 10.1038/s41590-019-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan X, Zebley CC, Youngblood B. Cellular and molecular waypoints along the path of T cell exhaustion. Sci Immunol. Sep 2023;8(87):eadg3868. doi: 10.1126/sciimmunol.adg3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016/September/01 2016;537(7620):417–421. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christo SN, Evrard M, Park SL, et al. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nature Immunology. 2021/September/01 2021;22(9):1140–1151. doi: 10.1038/s41590-021-01004-1 [DOI] [PubMed] [Google Scholar]
- 22.Isaacs JF, Degefu HN, Chen T, et al. CD39 Is Expressed on Functional Effector and Tissue-resident Memory CD8+ T Cells. The Journal of Immunology. 2024;213(5):588–599. doi: 10.4049/jimmunol.2400151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurd NS, He Z, Louis TL, et al. Early precursors and molecular determinants of tissue-resident memory CD8(+) T lymphocytes revealed by single-cell RNA sequencing. Sci Immunol. May 15 2020;5(47)doi: 10.1126/sciimmunol.aaz6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proceedings of the National Academy of Sciences. 2020;117(8):4292. doi: 10.1073/pnas.1917298117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beura LK, Anderson KG, Schenkel JM, et al. Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution. J Leukoc Biol. February 1, 2015. 2015;97(2):217–225. doi: 10.1189/jlb.1HI0314-154R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott MC, Steier Z, Pierson MJ, et al. Deep profiling deconstructs features associated with memory CD8(+) T cell tissue residence. Immunity. Jan 14 2025;58(1):162–181.e10. doi: 10.1016/j.immuni.2024.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavil NV, Scott MC, Weyu E, et al. Chronic antigen in solid tumors drives a distinct program of T cell residence. Sci Immunol. Jun 8 2023;8(84):eadd5976. doi: 10.1126/sciimmunol.add5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltra JC, Manne S, Abdel-Hakeem MS, et al. Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity. May 19 2020;52(5):825–841.e8. doi: 10.1016/j.immuni.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavil NV, Cheng K, Masopust D. Resident memory T cells and cancer. Immunity. 2024;57(8):1734–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boddupalli CS, Bar N, Kadaveru K, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1(21):e88955. doi: 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caushi JX, Zhang J, Ji Z, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. Aug 2021;596(7870):126–132. doi: 10.1038/s41586-021-03752-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banchereau R, Chitre AS, Scherl A, et al. Intratumoral CD103+ CD8+ T cells predict response to PD-L1 blockade. J Immunother Cancer. Apr 2021;9(4)doi: 10.1136/jitc-2020-002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengsch B, Ohtani T, Khan O, et al. Epigenomic-Guided Mass Cytometry Profiling Reveals Disease-Specific Features of Exhausted CD8+ T Cells. Immunity. 2018;48(5):1029–1045. doi: 10.1016/j.immuni.2018.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Gunasegaran B, Singh HD, et al. Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity. 2021;54(8):1825–1840.e7. doi: 10.1016/j.immuni.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 36.Duhen T, Duhen R, Montler R, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nature Communications. 2018;9(1):2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. [DOI] [PubMed] [Google Scholar]
- 38.Boland BS, He Z, Tsai MS, et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Science Immunology. 2020/August/21 2020;5(50):eabb4432. doi: 10.1126/sciimmunol.abb4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan GE, Harris JE, Richmond JM. Resident Memory T Cells in Autoimmune Skin Diseases. Front Immunol. 2021;12:652191. doi: 10.3389/fimmu.2021.652191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amsen D, van Gisbergen KPJM, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nature Immunology. 2018/June/01 2018;19(6):538–546. doi: 10.1038/s41590-018-0114-2 [DOI] [PubMed] [Google Scholar]
- 41.Okła K, Farber DL, Zou W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J Exp Med. Apr 5 2021;218(4)doi: 10.1084/jem.20201605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luoma AM, Suo S, Wang Y, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. Aug 4 2022;185(16):2918–2935.e29. doi: 10.1016/j.cell.2022.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nature Medicine. 2018/July/01 2018;24(7):986–993. doi: 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 44.Clarke J, Panwar B, Madrigal A, et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. The Journal of Experimental Medicine. 2019:jem.20190249. doi: 10.1084/jem.20190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corgnac S, Malenica I, Mezquita L, et al. CD103+CD8+ TRM Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Reports Medicine. 2020;1(7)doi: 10.1016/j.xcrm.2020.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. The Journal of Experimental Medicine. March 15, 2010. 2010;207(3):553–564. doi: 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey KA, Fraser KA, Schenkel JM, et al. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. The Journal of Immunology. May 15, 2012. 2012;188(10):4866–4875. doi: 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinert E, Schenkel J, Fraser K, et al. Quantifying Memory CD8+ T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161(4):737–749. doi: 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. Oct 17 2013;39(4):687–96. doi: 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macleod BL, Elsaesser HJ, Snell LM, et al. A network of immune and microbial modifications underlies viral persistence in the gastrointestinal tract. J Exp Med. Dec 7 2020;217(12)doi: 10.1084/jem.20191473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandu I, Cerletti D, Oetiker N, et al. Landscape of Exhausted Virus-Specific CD8 T Cells in Chronic LCMV Infection. Cell Reports. 2020;32(8)doi: 10.1016/j.celrep.2020.108078 [DOI] [PubMed] [Google Scholar]
- 52.Daniel B, Yost KE, Hsiung S, et al. Divergent clonal differentiation trajectories of T cell exhaustion. Nature Immunology. 2022/November/01 2022;23(11):1614–1627. doi: 10.1038/s41590-022-01337-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariotti S, Beltman JB, Chodaczek G, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proceedings of the National Academy of Sciences. November 27, 2012. 2012;109(48):19739–19744. doi: 10.1073/pnas.1208927109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Article. Nat Immunol. 05//print 2013;14(5):509–513. doi: 10.1038/ni.2568 http://www.nature.com/ni/journal/v14/n5/abs/ni.2568.html#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beura LK, Mitchell JS, Thompson EA, et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nature Immunology. 2018;19(2):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behr FM, Parga-Vidal L, Kragten NAM, et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nature Immunology. 2020/September/01 2020;21(9):1070–1081. doi: 10.1038/s41590-020-0723-4 [DOI] [PubMed] [Google Scholar]
- 57.Fonseca R, Beura LK, Quarnstrom CF, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nature Immunology. 2020/April/01 2020;21(4):412–421. doi: 10.1038/s41590-020-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Hoesslin M, Kuhlmann M, de Almeida GP, et al. Secondary infections rejuvenate the intestinal CD103(+) tissue-resident memory T cell pool. Sci Immunol. Nov 11 2022;7(77):eabp9553. doi: 10.1126/sciimmunol.abp9553 [DOI] [PubMed] [Google Scholar]
- 59.Fung HY, Teryek M, Lemenze AD, Bergsbaken T. CD103 fate mapping reveals that intestinal CD103(−) tissue-resident memory T cells are the primary responders to secondary infection. Sci Immunol. Nov 11 2022;7(77):eabl9925. doi: 10.1126/sciimmunol.abl9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson E, Lord C, Reading J, et al. Simultaneous trimodal single-cell measurement of transcripts, epitopes, and chromatin accessibility using TEA-seq. eLife. 2021/April/09 2021;10:e63632. doi: 10.7554/eLife.63632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buquicchio FA, Fonseca R, Yan PK, et al. Distinct epigenomic landscapes underlie tissue-specific memory T cell differentiation. Immunity. Sep 10 2024;57(9):2202–2215.e6. doi: 10.1016/j.immuni.2024.06.014 [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Madi A, Mieg A, et al. T Cell Factor 1 Suppresses CD103+ Lung Tissue-Resident Memory T Cell Development. Cell Reports. 2020/April/07/ 2020;31(1):107484. doi: 10.1016/j.celrep.2020.03.048 [DOI] [PubMed] [Google Scholar]
- 63.Fleck JS, Jansen SMJ, Wollny D, et al. Inferring and perturbing cell fate regulomes in human brain organoids. Nature. 2023/September/01 2023;621(7978):365–372. doi: 10.1038/s41586-022-05279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kragten NAM, Behr FM, Vieira Braga FA, et al. Blimp-1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur J Immunol. Oct 2018;48(10):1644–1662. doi: 10.1002/eji.201847771 [DOI] [PubMed] [Google Scholar]
- 65.Levine Jacob H, Simonds Erin F, Bendall Sean C, et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015/July/02/ 2015;162(1):184–197. doi: 10.1016/j.cell.2015.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber EW, Parker KR, Sotillo E, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. Apr 2 2021;372(6537)doi: 10.1126/science.aba1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schade AE, Schieven GL, Townsend R, et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. Feb 1 2008;111(3):1366–77. doi: 10.1182/blood-2007-04-084814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giles JR, Ngiow SF, Manne S, et al. Shared and distinct biological circuits in effector, memory and exhausted CD8+ T cells revealed by temporal single-cell transcriptomics and epigenetics. Nature Immunology. 2022/November/01 2022;23(11):1600–1613. doi: 10.1038/s41590-022-01338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng L, Qin S, Si W, et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. Dec 17 2021;374(6574):abe6474. doi: 10.1126/science.abe6474 [DOI] [PubMed] [Google Scholar]
- 70.Elmentaite R, Kumasaka N, Roberts K, et al. Cells of the human intestinal tract mapped across space and time. Nature. 2021/September/01 2021;597(7875):250–255. doi: 10.1038/s41586-021-03852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giles JR, Manne S, Freilich E, et al. Human epigenetic and transcriptional T cell differentiation atlas for identifying functional T cell-specific enhancers. Immunity. Mar 8 2022;55(3):557–574.e7. doi: 10.1016/j.immuni.2022.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stelekati E, Shin H, Doering TA, et al. Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity. May 15 2014;40(5):801–13. doi: 10.1016/j.immuni.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge C, Monk IR, Pizzolla A, et al. Bystander Activation of Pulmonary Trm Cells Attenuates the Severity of Bacterial Pneumonia by Enhancing Neutrophil Recruitment. Cell Rep. Dec 24 2019;29(13):4236–4244.e3. doi: 10.1016/j.celrep.2019.11.103 [DOI] [PubMed] [Google Scholar]
- 74.Sheridan Brian S, Pham Q-M, Lee Y-T, Cauley Linda S, Puddington L, Lefrançois L. Oral Infection Drives a Distinct Population of Intestinal Resident Memory CD8+ T Cells with Enhanced Protective Function. Immunity. May/15/ 2014;40(5):747–757. doi: 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park SL, Gebhardt T, Mackay LK. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends in Immunology. 2019/August/01/ 2019;40(8):735–747. doi: 10.1016/j.it.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 76.Edwards J, Wilmott JS, Madore J, et al. CD103+ Tumor-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clinical Cancer Research. 2018;24(13):3036–3045. doi: 10.1158/1078-0432.Ccr-17-2257 [DOI] [PubMed] [Google Scholar]
- 77.Jaiswal A, Verma A, Dannenfelser R, et al. An activation to memory differentiation trajectory of tumor-infiltrating lymphocytes informs metastatic melanoma outcomes. Cancer Cell. May 9 2022;40(5):524–544.e5. doi: 10.1016/j.ccell.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curtis C, Shah SP, Chin S-F, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012/June/01 2012;486(7403):346–352. doi: 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu D, Schilling B, Liu D, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nature Medicine. 2019/December/01 2019;25(12):1916–1927. doi: 10.1038/s41591-019-0654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. Jan 22 2004;427(6972):355–60. doi: 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- 81.Beura LK, Wijeyesinghe S, Thompson EA, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. 2018;48(2):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burn TN, Schröder J, Gandolfo LC, et al. Antigen reactivity defines tissue-resident memory and exhausted T cells in tumours. bioRxiv. 2025:2025.07.23.666465. doi: 10.1101/2025.07.23.666465 [DOI] [Google Scholar]
- 83.Mackay LK, Wakim L, van Vliet CJ, et al. Maintenance of T Cell Function in the Face of Chronic Antigen Stimulation and Repeated Reactivation for a Latent Virus Infection. The Journal of Immunology. January 23, 2012. 2012:2173–2178. doi: 10.4049/jimmunol.1102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu J, Peng T, Johnston C, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. May 23 2013;497(7450):494–7. doi: 10.1038/nature12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malik BT, Byrne KT, Vella JL, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Science Immunology. 2017/April// 2017;2(10):eaam6346. doi: 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nature Immunology. 2019/March/01 2019;20(3):326–336. doi: 10.1038/s41590-019-0312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minnie SA, Waltner OG, Zhang P, et al. TIM-3+ CD8 T cells with a terminally exhausted phenotype retain functional capacity in hematological malignancies. Science Immunology. 9(94):eadg1094. doi: 10.1126/sciimmunol.adg1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LaFleur MW, Nguyen TH, Coxe MA, et al. PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat Immunol. Oct 2019;20(10):1335–1347. doi: 10.1038/s41590-019-0480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishna S, Lowery FJ, Copeland AR, et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. Dec 11 2020;370(6522):1328–1334. doi: 10.1126/science.abb9847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo Y, Xie YQ, Gao M, et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. Jun 2021;22(6):746–756. doi: 10.1038/s41590-021-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proceedings of the National Academy of Sciences. 2008/September/30 2008;105(39):15016–15021. doi: 10.1073/pnas.0801497105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral Tcf1+ PD-1+ CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity. 2019;50(1):195–211. [DOI] [PubMed] [Google Scholar]
- 94.Voabil P, de Bruijn M, Roelofsen LM, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nature Medicine. 2021/July/01 2021;27(7):1250–1261. doi: 10.1038/s41591-021-01398-3 [DOI] [PubMed] [Google Scholar]
- 95.Lee YJ, Kim JY, Jeon SH, et al. CD39(+) tissue-resident memory CD8(+) T cells with a clonal overlap across compartments mediate antitumor immunity in breast cancer. Sci Immunol. Aug 26 2022;7(74):eabn8390. doi: 10.1126/sciimmunol.abn8390 [DOI] [PubMed] [Google Scholar]
- 96.Virassamy B, Caramia F, Savas P, et al. Intratumoral CD8+ T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell. 2023;41(3):585–601.e8. doi: 10.1016/j.ccell.2023.01.004 [DOI] [PubMed] [Google Scholar]
- 97.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. Jun 29 2015;212(7):1125–37. doi: 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson KG, Mayer-Barber K, Sung H, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–222. doi: 10.1038/nprot.2014.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Painter MM, Johnston TS, Lundgreen KA, et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nature Immunology. 2023/October/01 2023;24(10):1711–1724. doi: 10.1038/s41590-023-01613-y [DOI] [PubMed] [Google Scholar]
- 100.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nature Medicine. 2019/March/01 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Flanagan CH, Campbell KR, Zhang AW, et al. Dissociation of solid tumor tissues with cold active protease for single-cell RNA-seq minimizes conserved collagenase-associated stress responses. Genome Biology. 2019/October/17 2019;20(1):210. doi: 10.1186/s13059-019-1830-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abascal F, Acosta R, Addleman NJ, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020/July/01 2020;583(7818):699–710. doi: 10.1038/s41586-020-2493-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siepel A, Bejerano G, Pedersen JS, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. Aug 2005;15(8):1034–50. doi: 10.1101/gr.3715005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kartha VK, Duarte FM, Hu Y, et al. Functional inference of gene regulation using single-cell multi-omics. Cell Genomics. 2022/September/14/ 2022;2(9):100166. doi: 10.1016/j.xgen.2022.100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rauluseviciute I, Riudavets-Puig R, Blanc-Mathieu R, et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Research. 2024;52(D1):D174–D182. doi: 10.1093/nar/gkad1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen W-K, Chen S-Y, Gan Z-Q, et al. AnimalTFDB 4.0: a comprehensive animal transcription factor database updated with variation and expression annotations. Nucleic Acids Research. 2023;51(D1):D39–D45. doi: 10.1093/nar/gkac907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nüssing S, House IG, Kearney CJ, et al. Efficient CRISPR/Cas9 Gene Editing in Uncultured Naive Mouse T Cells for In Vivo Studies. The Journal of Immunology. 2020:ji1901396. doi: 10.4049/jimmunol.1901396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Multimodal ADT, HTO, RNA and ATAC raw sequencing data generated in this study will be deposited in the National Center for Biotechnology Information Gene Expression Omnibus prior to publication. Raw differential gene expression, differential accessibility data and unique gene signatures generated in this study are available as Extended Data Tables. All other raw and processed data are available from the authors upon reasonable request. Publicly available datasets used for analysis in this manuscript are available at the following accession numbers: GSE70813, GSE179613, GSE199565, or via gutcellatlas.org.