Abstract
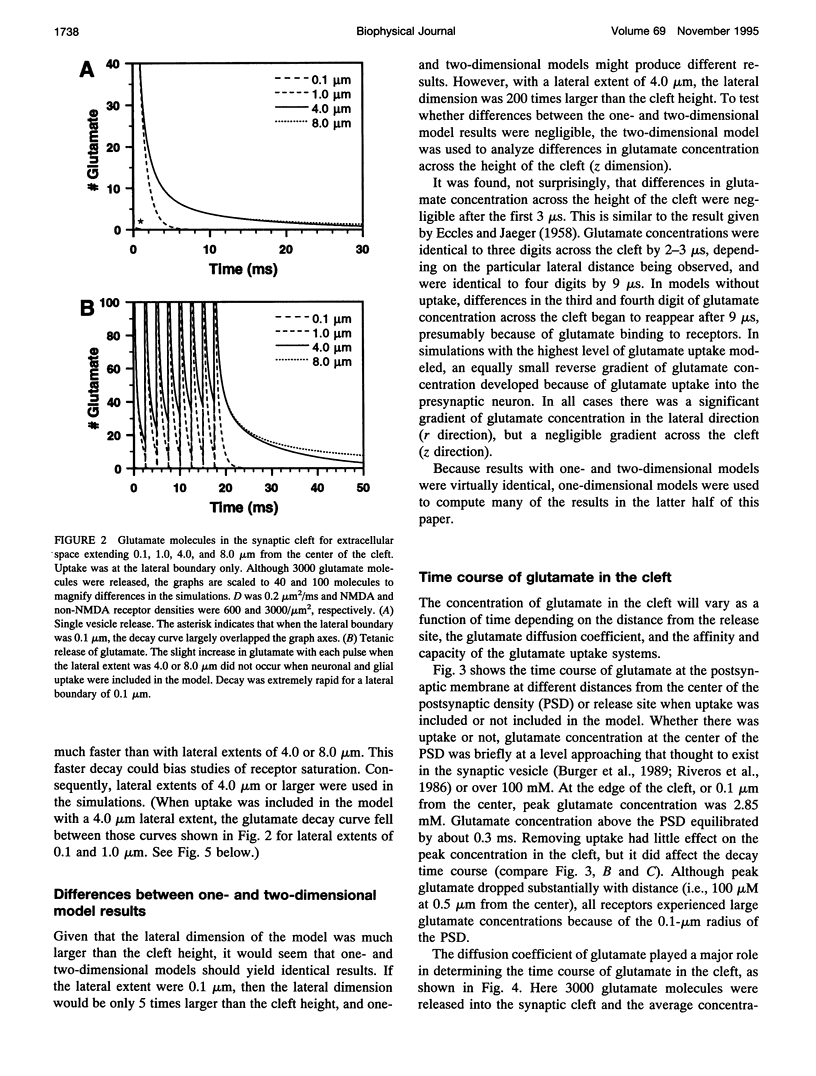
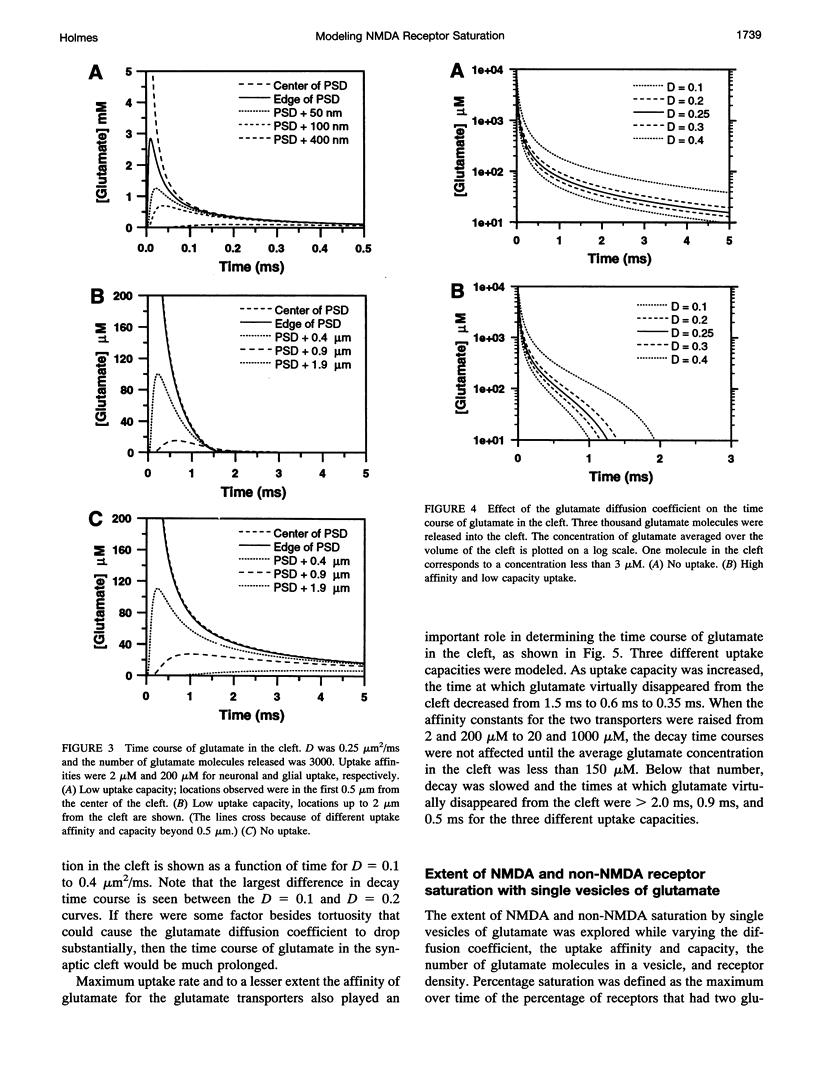
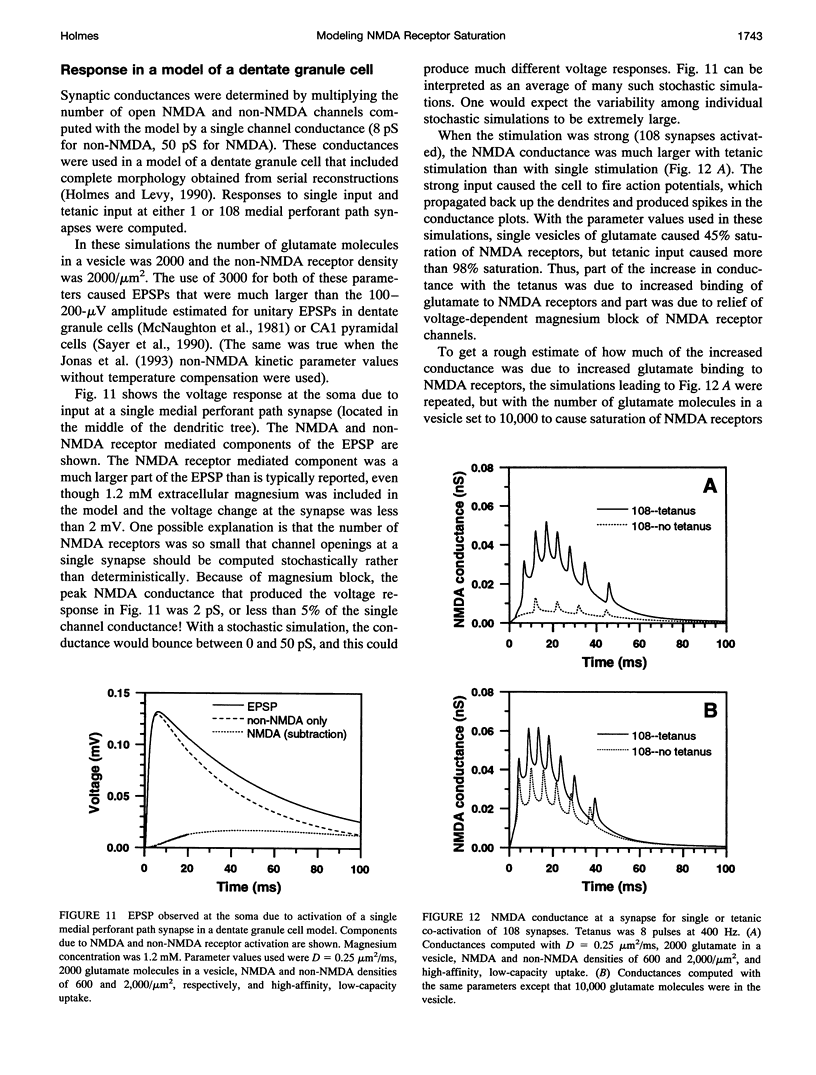
One- and two-dimensional models of glutamate diffusion, uptake, and binding in the synaptic cleft were developed to determine if the release of single vesicles of glutamate would saturate NMDA and non-NMDA receptors. Ranges of parameter values were used in the simulations to determine the conditions when saturation could occur. Single vesicles of glutamate did not saturate NMDA receptors unless diffusion was very slow and the number of glutamate molecules in a vesicle was large. However, the release of eight vesicles at 400 Hz caused NMDA receptor saturation for all parameter values tested. Glutamate uptake was found to reduce NMDA receptor saturation, but the effect was smaller than that of changes in the diffusion coefficient or in the number of glutamate molecules in a vesicle. Non-NMDA receptors were not saturated unless diffusion was very slow and the number of glutamate molecules in a vesicle was large. The release of eight vesicles at 400 Hz caused significant non-NMDA receptor desensitization. The results suggest that NMDA and non-NMDA receptors are not saturated by single vesicles of glutamate under usual conditions, and that tetanic input, of the type typically used to induce long-term potentiation, will increase calcium influx by increasing receptor binding as well as by reducing voltage-dependent block of NMDA receptors.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcar V. J., Johnston G. A. Glutamate uptake by brain slices and its relation to the depolarization of neurones by acidic amino acids. J Neurobiol. 1972;3(4):295–301. doi: 10.1002/neu.480030403. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Barbour B., Keller B. U., Llano I., Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994 Jun;12(6):1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Burger P. M., Mehl E., Cameron P. L., Maycox P. R., Baumert M., Lottspeich F., De Camilli P., Jahn R. Synaptic vesicles immunoisolated from rat cerebral cortex contain high levels of glutamate. Neuron. 1989 Dec;3(6):715–720. doi: 10.1016/0896-6273(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Westbrook G. L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991 Oct;7(4):605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. Changes in the postsynaptic density with long-term potentiation in the dentate gyrus. J Comp Neurol. 1986 Nov 22;253(4):476–482. doi: 10.1002/cne.902530405. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Colquhoun D. Rapid decay of averaged single-channel NMDA receptor activations recorded at low agonist concentration. Proc Biol Sci. 1992 Dec 22;250(1329):279–286. doi: 10.1098/rspb.1992.0160. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Young W. S., Legendre P., Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992 Nov 27;258(5087):1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. With an appendix: diffusion of transported amino acids into brain slices. Br J Pharmacol. 1985 May;85(1):297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Landis D. M. Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neuroscience. 1986 Nov;19(3):857–872. doi: 10.1016/0306-4522(86)90304-0. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13(3):277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Hertz L., Schousboe A., Boechler N., Mukerji S., Fedoroff S. Kinetic characteristics of the glutamate uptake into normal astrocytes in cultures. Neurochem Res. 1978 Feb;3(1):1–14. doi: 10.1007/BF00964356. [DOI] [PubMed] [Google Scholar]
- Holmes W. R., Levy W. B. Insights into associative long-term potentiation from computational models of NMDA receptor-mediated calcium influx and intracellular calcium concentration changes. J Neurophysiol. 1990 May;63(5):1148–1168. doi: 10.1152/jn.1990.63.5.1148. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Nicoll R. A. The uptake inhibitor L-trans-PDC enhances responses to glutamate but fails to alter the kinetics of excitatory synaptic currents in the hippocampus. J Neurophysiol. 1993 Nov;70(5):2187–2191. doi: 10.1152/jn.1993.70.5.2187. [DOI] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. D., Jones R. S. Activation of N-methyl-D-aspartate receptors contributes to the EPSP at perforant path synapses in the rat dentate gyrus in vitro. Neurosci Lett. 1989 Feb 27;97(3):323–328. doi: 10.1016/0304-3940(89)90618-6. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992 Feb;12(2):635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Tong G., Jahr C. E. Interactions between the glycine and glutamate binding sites of the NMDA receptor. J Neurosci. 1993 Mar;13(3):1088–1096. doi: 10.1523/JNEUROSCI.13-03-01088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A., Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol. 1981 Nov;46(5):952–966. doi: 10.1152/jn.1981.46.5.952. [DOI] [PubMed] [Google Scholar]
- Mennerick S., Zorumski C. F. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994 Mar 3;368(6466):59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L., Jane D. E., Watkins J. C. Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine. J Neurosci. 1992 Feb;12(2):595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman I. M., Trussell L. O. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992 Jul;9(1):173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Raman I. M., Trussell L. O. The mechanism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor desensitization after removal of glutamate. Biophys J. 1995 Jan;68(1):137–146. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. E., Gerhardt G. A., Hierl P. M., Nagy G., Adams R. N. Diffusion coefficients of neurotransmitters and their metabolites in brain extracellular fluid space. Neuroscience. 1985 Jul;15(3):891–902. doi: 10.1016/0306-4522(85)90087-9. [DOI] [PubMed] [Google Scholar]
- Riveros N., Fiedler J., Lagos N., Muñoz C., Orrego F. Glutamate in rat brain cortex synaptic vesicles: influence of the vesicle isolation procedure. Brain Res. 1986 Oct 29;386(1-2):405–408. doi: 10.1016/0006-8993(86)90181-2. [DOI] [PubMed] [Google Scholar]
- Sarantis M., Ballerini L., Miller B., Silver R. A., Edwards M., Attwell D. Glutamate uptake from the synaptic cleft does not shape the decay of the non-NMDA component of the synaptic current. Neuron. 1993 Sep;11(3):541–549. doi: 10.1016/0896-6273(93)90158-n. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Friedlander M. J., Redman S. J. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in the hippocampal slice. J Neurosci. 1990 Mar;10(3):826–836. doi: 10.1523/JNEUROSCI.10-03-00826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Glutamic acid and excitatory nerve endings: reduction of glutamic acid uptake after axotomy. Brain Res. 1977 Jan 21;120(2):379–386. doi: 10.1016/0006-8993(77)90918-0. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Margulis M., Shi Q. Y., Fielding A. Saturation of postsynaptic glutamate receptors after quantal release of transmitter. Neuron. 1994 Dec;13(6):1385–1393. doi: 10.1016/0896-6273(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994 Nov;13(5):1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994 Jan;12(1):51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Vyklicky L., Jr, Patneau D. K., Mayer M. L. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991 Dec;7(6):971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]