Abstract
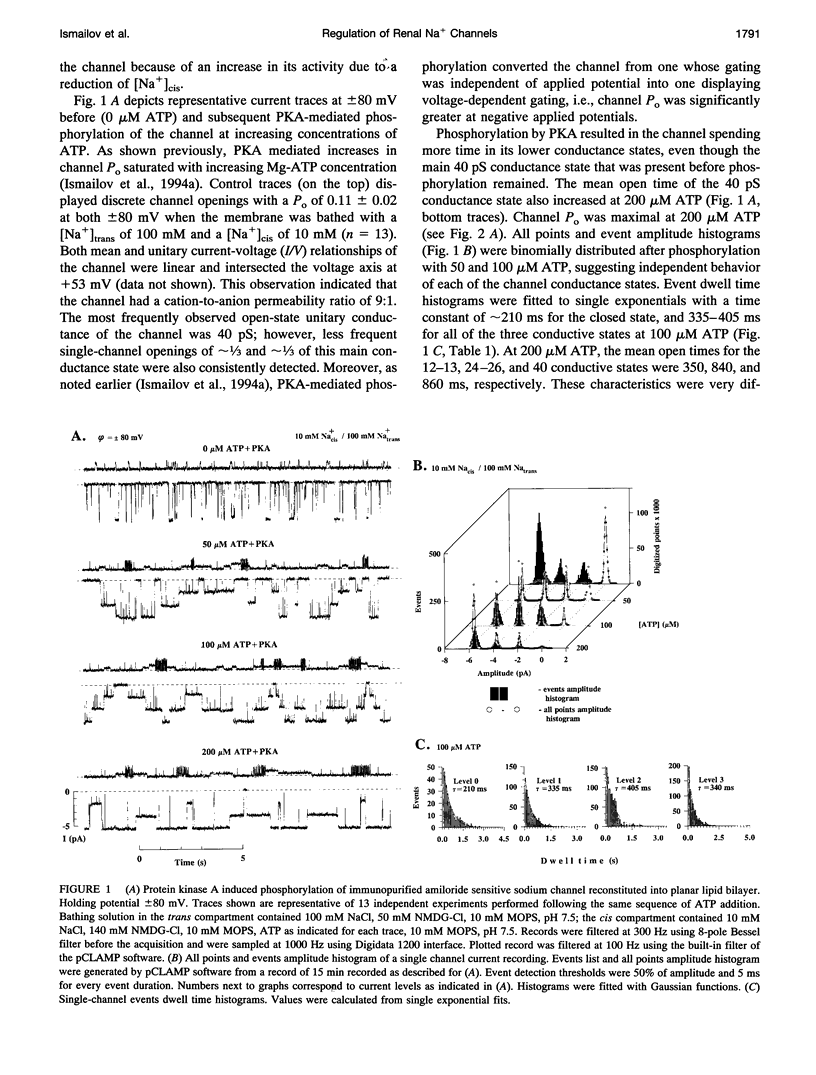
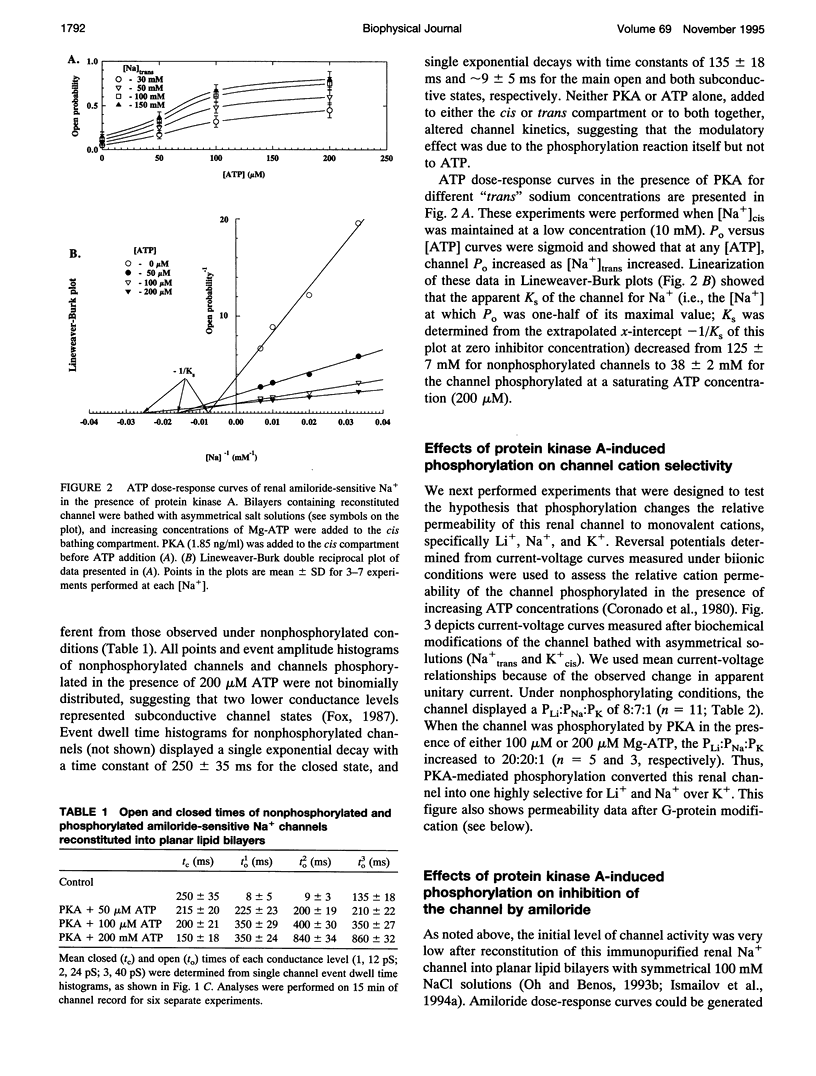
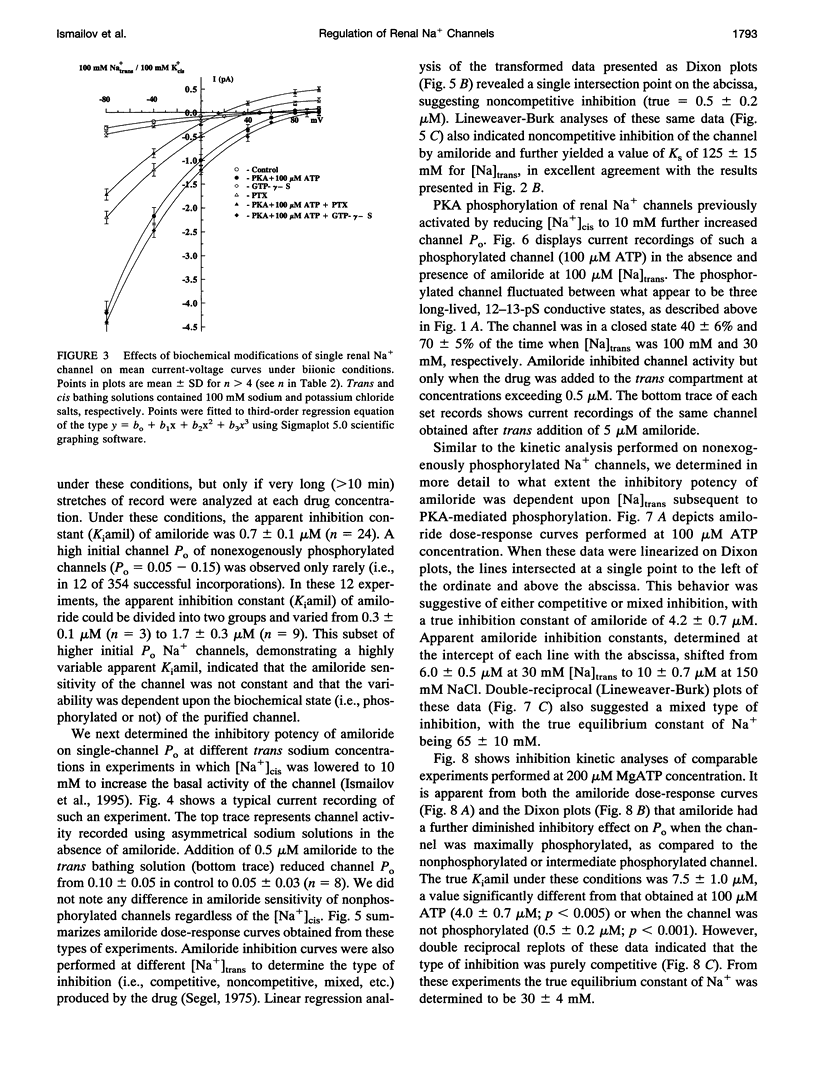
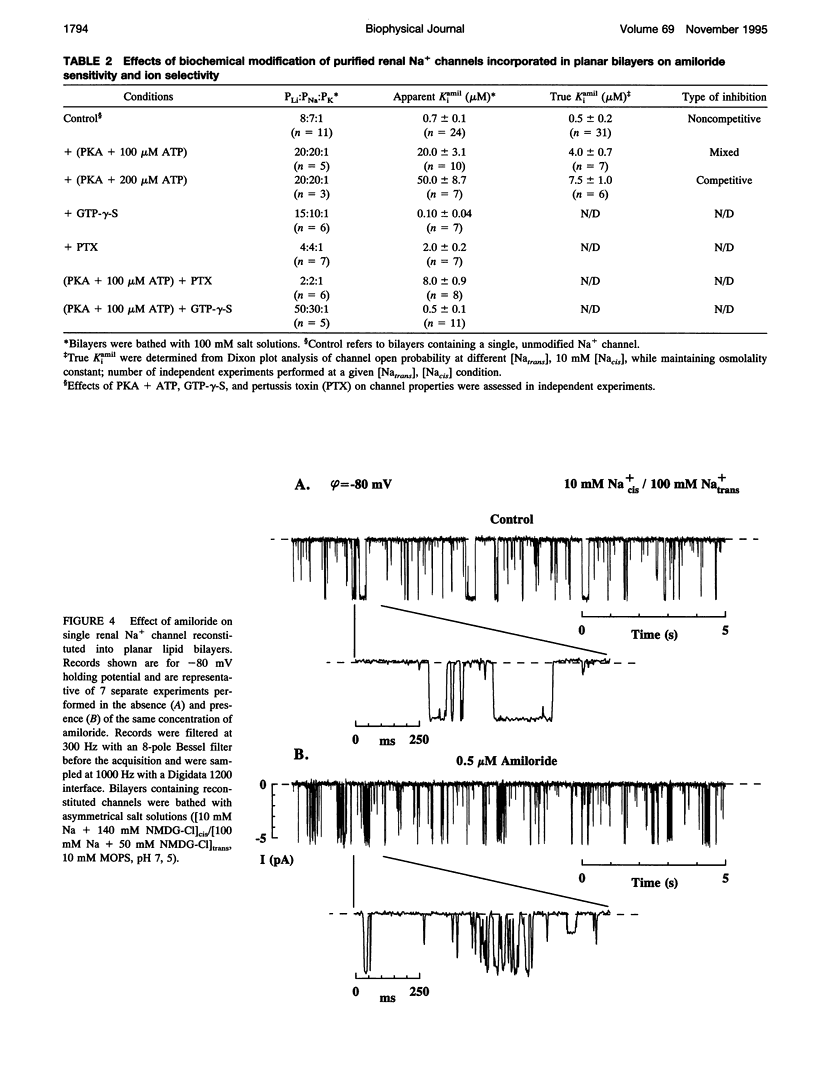
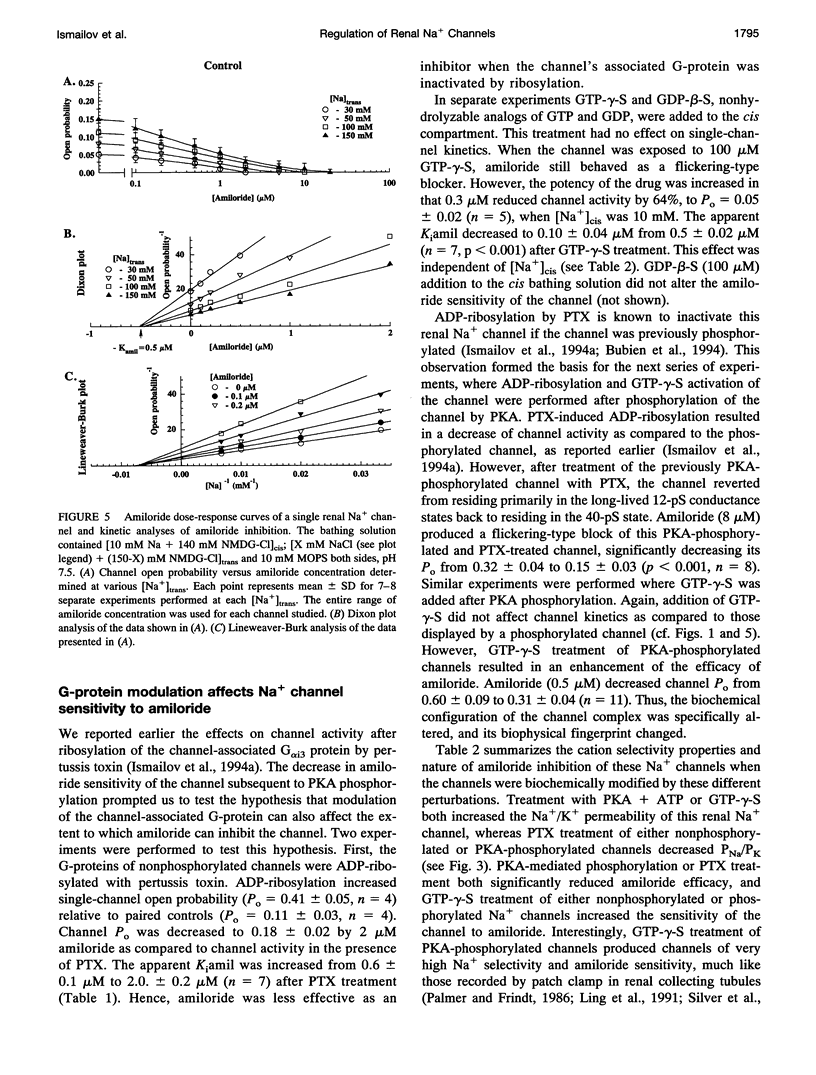
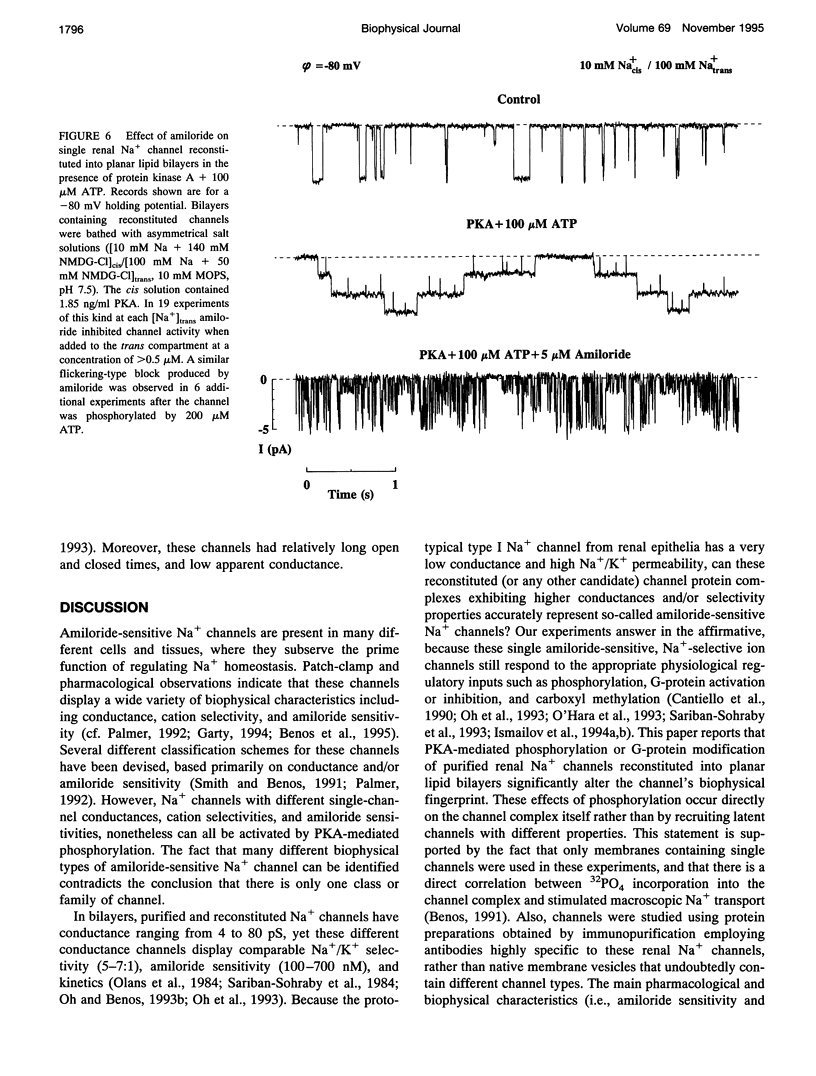
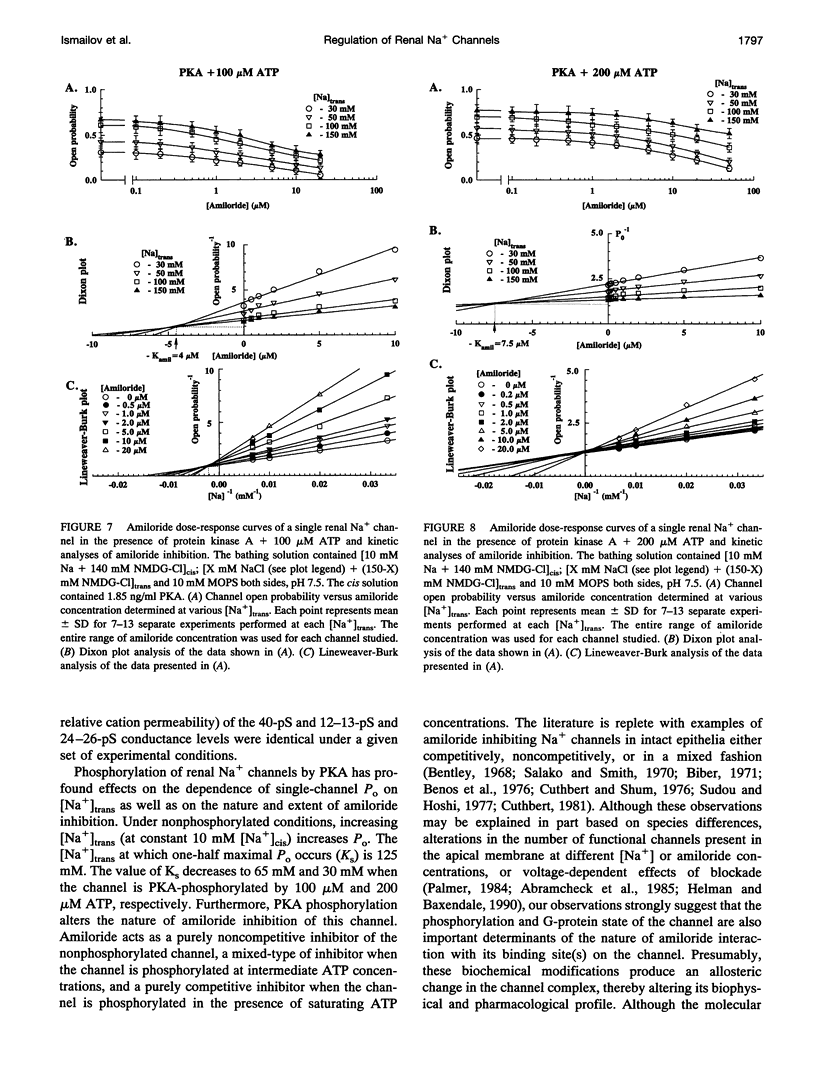
Purified bovine renal papillary Na+ channels, when reconstituted into planar lipid bilayers, reside in three conductance states: a 40-pS main state, and two subconductive states (12-13 pS and 24-26 pS). The activity of these channels is regulated by phosphorylation and by G-proteins. Protein kinase A (PKA)-induced phosphorylation increased channel activity by increasing the open state time constants from 160 +/- 30 (main conductance), and 15 +/- 5 ms (both lower conductances), respectively, to 365 +/- 30 ms for all of them. PKA phosphorylation also altered the closed time of the channel from 250 +/- 30 ms to 200 +/- 35 ms, thus shifting the channel into a lower-conductance, long open time mode. PKA phosphorylation increased the PNa:PK of the channel from 7:1 to 20:1, and shifted the amiloride inhibition curve to the right (apparent K(i)amil from 0.7 to 20 microM). Pertussis toxin-induced ADP-ribosylation of either phosphorylated of either phosphorylated or nonphosphorylated channels decreased the PNa:PK to 2:1 and 4:1, respectively, and altered K(i)amil to 8 and 2 microM for phosphorylated and nonphosphorylated channels, respectively. GTP-gamma-S treatment of either phosphorylated or nonphosphorylated channels resulted in an increase of PNa:PK to 30:1 and 10:1, respectively, and produced a leftward shift in the amiloride dose-response curve, altering K(i)amil to 0.5 and 0.1 microM, respectively. These results suggest that amiloride-sensitive renal Na+ channel biophysical characteristics are not static, but depend upon the biochemical state of the channel protein and/or its associated G-protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck F. J., Van Driessche W., Helman S. I. Autoregulation of apical membrane Na+ permeability of tight epithelia. Noise analysis with amiloride and CGS 4270. J Gen Physiol. 1985 Apr;85(4):555–582. doi: 10.1085/jgp.85.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Koeppe R. E., 2nd Molecular determinants of channel function. Physiol Rev. 1992 Oct;72(4 Suppl):S89–158. doi: 10.1152/physrev.1992.72.suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- Ausiello D. A., Stow J. L., Cantiello H. F., de Almeida J. B., Benos D. J. Purified epithelial Na+ channel complex contains the pertussis toxin-sensitive G alpha i-3 protein. J Biol Chem. 1992 Mar 5;267(7):4759–4765. [PubMed] [Google Scholar]
- Benos D. J., Awayda M. S., Ismailov I. I., Johnson J. P. Structure and function of amiloride-sensitive Na+ channels. J Membr Biol. 1995 Jan;143(1):1–18. doi: 10.1007/BF00232519. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Cunningham S., Baker R. R., Beason K. B., Oh Y., Smith P. R. Molecular characteristics of amiloride-sensitive sodium channels. Rev Physiol Biochem Pharmacol. 1992;120:31–113. doi: 10.1007/BFb0036122. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Saccomani G., Sariban-Sohraby S. The epithelial sodium channel. Subunit number and location of the amiloride binding site. J Biol Chem. 1987 Aug 5;262(22):10613–10618. [PubMed] [Google Scholar]
- Benos D. J., Simon S. A., Mandel L. J., Cala P. M. Effect of amiloride and some of its analogues of cation transport in isolated frog skin and thin lipid membranes. J Gen Physiol. 1976 Jul;68(1):43–63. doi: 10.1085/jgp.68.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U. Effect of changes in transepithelial transport on the uptake of sodium across the outer surface of the frog skin. J Gen Physiol. 1971 Aug;58(2):131–144. doi: 10.1085/jgp.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubien J. K., Jope R. S., Warnock D. G. G-proteins modulate amiloride-sensitive sodium channels. J Biol Chem. 1994 Jul 8;269(27):17780–17783. [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Rossier B. C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993 Feb 4;361(6411):467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Codina J., Birnbaumer L., Ausiello D. A. G alpha i-3 regulates epithelial Na+ channels by activation of phospholipase A2 and lipoxygenase pathways. J Biol Chem. 1990 Dec 15;265(35):21624–21628. [PubMed] [Google Scholar]
- Coronado R., Rosenberg R. L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980 Oct;76(4):425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Characteristics of the entry process for sodium in transporting epithelia as revealed with amiloride. J Physiol. 1976 Mar;255(3):587–604. doi: 10.1113/jphysiol.1976.sp011297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W. Sodium entry step in transporting epithelia: results of ligand-binding studies. Soc Gen Physiol Ser. 1981;36:181–195. [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994 May;8(8):522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. Na+ selective channels in the apical membrane of rabbit late proximal tubules (pars recta). Pflugers Arch. 1986 Feb;406(2):198–203. doi: 10.1007/BF00586683. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Baxendale L. M. Blocker-related changes of channel density. Analysis of a three-state model for apical Na channels of frog skin. J Gen Physiol. 1990 Apr;95(4):647–678. doi: 10.1085/jgp.95.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailov I. I., McDuffie J. H., Benos D. J. Protein kinase A phosphorylation and G protein regulation of purified renal Na+ channels in planar bilayer membranes. J Biol Chem. 1994 Apr 8;269(14):10235–10241. [PubMed] [Google Scholar]
- Ismailov I. I., McDuffie J. H., Sariban-Sohraby S., Johnson J. P., Benos D. J. Carboxyl methylation activates purified renal amiloride-sensitive Na+ channels in planar lipid bilayers. J Biol Chem. 1994 Sep 2;269(35):22193–22197. [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Hinton C. F., Eaton D. C. Amiloride-sensitive sodium channels in rabbit cortical collecting tubule primary cultures. Am J Physiol. 1991 Dec;261(6 Pt 2):F933–F944. doi: 10.1152/ajprenal.1991.261.6.F933. [DOI] [PubMed] [Google Scholar]
- Oh Y., Benos D. J. Rapid purification of an amiloride-sensitive Na+ channel from bovine kidney and its functional reconstitution. Protein Expr Purif. 1993 Aug;4(4):312–319. doi: 10.1006/prep.1993.1040. [DOI] [PubMed] [Google Scholar]
- Oh Y., Benos D. J. Single-channel characteristics of a purified bovine renal amiloride-sensitive Na+ channel in planar lipid bilayers. Am J Physiol. 1993 Jun;264(6 Pt 1):C1489–C1499. doi: 10.1152/ajpcell.1993.264.6.C1489. [DOI] [PubMed] [Google Scholar]
- Oh Y., Smith P. R., Bradford A. L., Keeton D., Benos D. J. Regulation by phosphorylation of purified epithelial Na+ channels in planar lipid bilayers. Am J Physiol. 1993 Jul;265(1 Pt 1):C85–C91. doi: 10.1152/ajpcell.1993.265.1.C85. [DOI] [PubMed] [Google Scholar]
- Ohara A., Matsunaga H., Eaton D. C. G protein activation inhibits amiloride-blockable highly selective sodium channels in A6 cells. Am J Physiol. 1993 Feb;264(2 Pt 1):C352–C360. doi: 10.1152/ajpcell.1993.264.2.C352. [DOI] [PubMed] [Google Scholar]
- Olans L., Sariban-Sohraby S., Benos D. J. Saturation behavior of single, amiloride-sensitive Na+ channels in planar lipid bilayers. Biophys J. 1984 Dec;46(6):831–835. doi: 10.1016/S0006-3495(84)84082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G. Epithelial Na channels: function and diversity. Annu Rev Physiol. 1992;54:51–66. doi: 10.1146/annurev.ph.54.030192.000411. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G. Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membr Biol. 1984;80(2):153–165. doi: 10.1007/BF01868771. [DOI] [PubMed] [Google Scholar]
- Salako L. A., Smith A. J. Changes in sodium pool and kinetics of sodium transport in frog skin produced by amiloride. Br J Pharmacol. 1970 May;39(1):99–109. doi: 10.1111/j.1476-5381.1970.tb09559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Fisher R. S., Abramow M. Aldosterone-induced and GTP-stimulated methylation of a 90-kDa polypeptide in the apical membrane of A6 epithelia. J Biol Chem. 1993 Dec 15;268(35):26613–26617. [PubMed] [Google Scholar]
- Sariban-Sohraby S., Latorre R., Burg M., Olans L., Benos D. Amiloride-sensitive epithelial Na+ channels reconstituted into planar lipid bilayer membranes. Nature. 1984 Mar 1;308(5954):80–82. doi: 10.1038/308080a0. [DOI] [PubMed] [Google Scholar]
- Senyk O., Ismailov I., Bradford A. L., Baker R. R., Matalon S., Benos D. J. Reconstitution of immunopurified alveolar type II cell Na+ channel protein into planar lipid bilayers. Am J Physiol. 1995 May;268(5 Pt 1):C1148–C1156. doi: 10.1152/ajpcell.1995.268.5.C1148. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Frindt G., Windhager E. E., Palmer L. G. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am J Physiol. 1993 Mar;264(3 Pt 2):F557–F564. doi: 10.1152/ajprenal.1993.264.3.F557. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Benos D. J. Epithelial Na+ channels. Annu Rev Physiol. 1991;53:509–530. doi: 10.1146/annurev.ph.53.030191.002453. [DOI] [PubMed] [Google Scholar]
- Sudou K., Hoshi T. Mode of action of amiloride in toad urinary bladder. An electrophysiological study of the drug action on sodium permeability of the mucosal border. J Membr Biol. 1977 Apr 7;32(1-2):115–132. doi: 10.1007/BF01905212. [DOI] [PubMed] [Google Scholar]
- Tien H. T., Diana A. L. Some physical properties of bimolecular lipid membranes produced from new lipid solutions. Nature. 1967 Sep 9;215(5106):1199–1200. doi: 10.1038/2151199a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Anderson M. P., Rich D. P., Berger H. A., Denning G. M., Ostedgaard L. S., Sheppard D. N., Cheng S. H., Gregory R. J., Smith A. E. Cystic fibrosis transmembrane conductance regulator: a chloride channel with novel regulation. Neuron. 1992 May;8(5):821–829. doi: 10.1016/0896-6273(92)90196-k. [DOI] [PubMed] [Google Scholar]
- West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991 Nov 8;254(5033):866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- Wilson G. F., Kaczmarek L. K. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993 Dec 2;366(6454):433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]



