Abstract
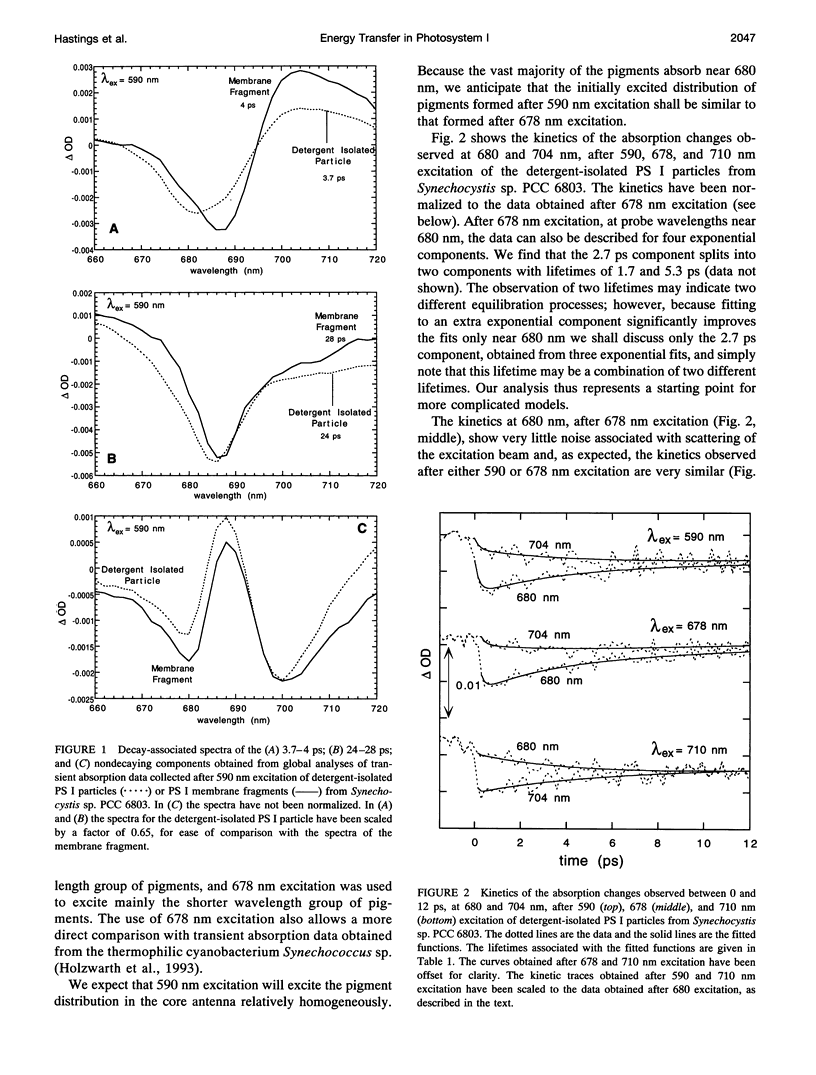
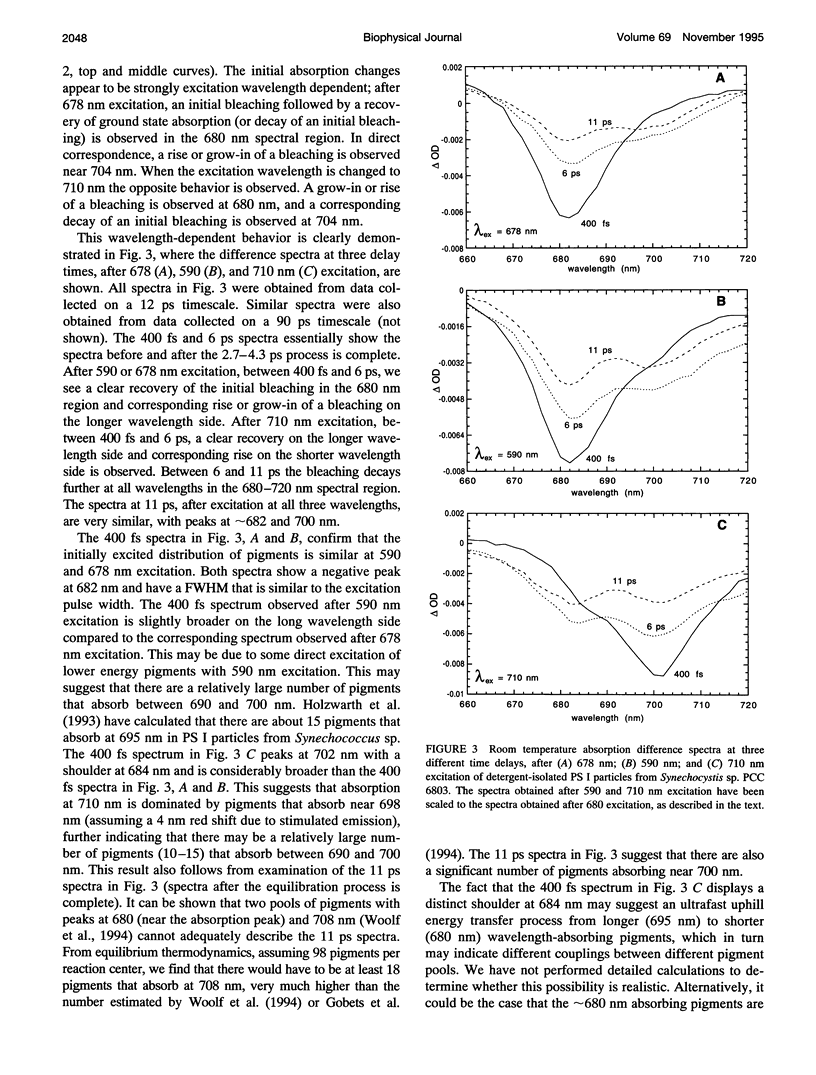
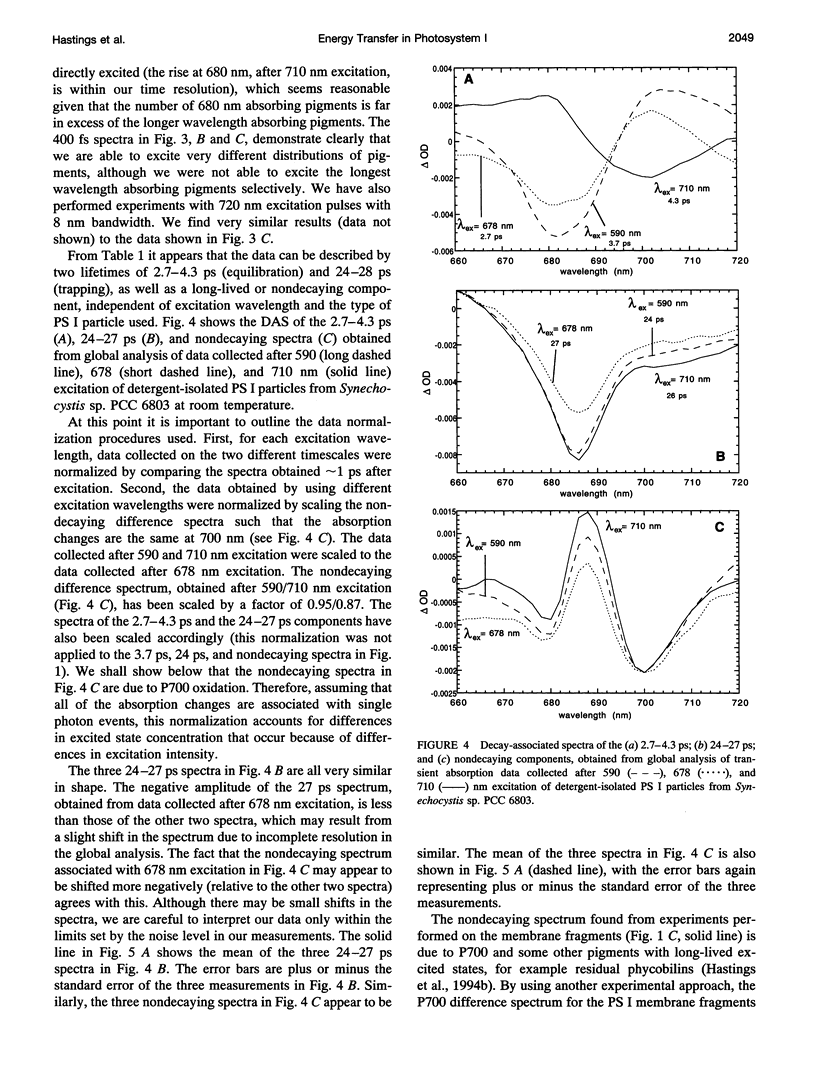
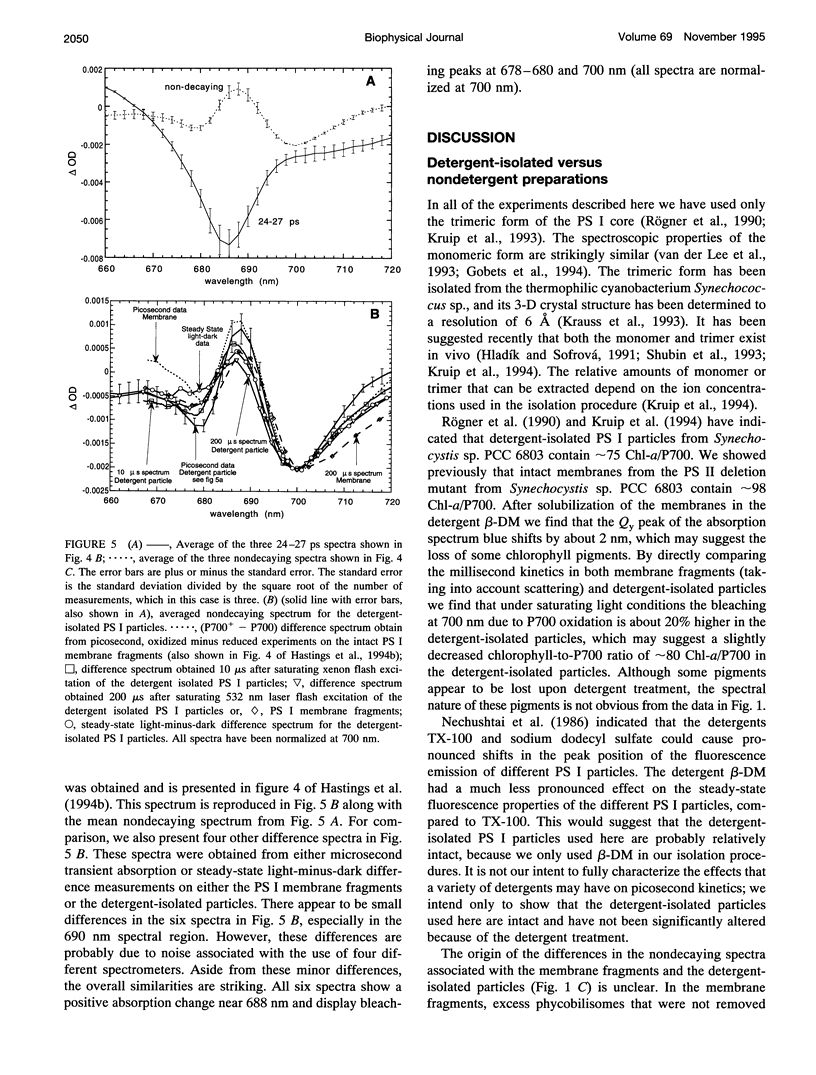
Femtosecond transient absorption spectroscopy has been used to investigate the energy transfer and trapping processes in both intact membranes and purified detergent-isolated particles from a photosystem II deletion mutant of the cyanobacterium Synechocystis sp. PCC 6803, which contains only the photosystem I reaction center. Processes with similar lifetimes and spectra are observed in both the membrane fragments and the detergent-isolated particles, suggesting little disruption of the core antenna resulting from the detergent treatment. For the detergent-isolated particles, three different excitation wavelengths were used to excite different distributions of pigments in the spectrally heterogeneous core antenna. Only two lifetimes of 2.7-4.3 ps and 24-28 ps, and a nondecaying component are required to describe all the data. The 24-28 ps component is associated with trapping. The trapping process gives rise to a nondecaying spectrum that is due to oxidation of the primary electron donor. The lifetimes and spectra associated with trapping and radical pair formation are independent of excitation wavelength, suggesting that trapping proceeds from an equilibrated excited state. The 2.7-4.3 ps component characterizes the evolution from the initially excited distribution of pigments to the equilibrated excited state distribution. The spectrum associated with the 2.7-4.3 ps component is therefore strongly excitation wavelength dependent. Comparison of the difference spectra associated with the spectrally equilibrated state and the radical pair state suggests that the pigments in the photosystem I core antenna display some degree of excitonic coupling.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durrant J. R., Hastings G., Joseph D. M., Barber J., Porter G., Klug D. R. Subpicosecond equilibration of excitation energy in isolated photosystem II reaction centers. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11632–11636. doi: 10.1073/pnas.89.23.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings G., Kleinherenbrink F. A., Lin S., Blankenship R. E. Time-resolved fluorescence and absorption spectroscopy of photosystem I. Biochemistry. 1994 Mar 22;33(11):3185–3192. doi: 10.1021/bi00177a007. [DOI] [PubMed] [Google Scholar]
- Hastings G., Kleinherenbrink F. A., Lin S., McHugh T. J., Blankenship R. E. Observation of the reduction and reoxidation of the primary electron acceptor in photosystem I. Biochemistry. 1994 Mar 22;33(11):3193–3200. doi: 10.1021/bi00177a008. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R., Schatz G., Brock H., Bittersmann E. Energy transfer and charge separation kinetics in photosystem I: Part 1: Picosecond transient absorption and fluorescence study of cyanobacterial photosystem I particles. Biophys J. 1993 Jun;64(6):1813–1826. doi: 10.1016/S0006-3495(93)81552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Jean J. M., Werst M. M., Chan C. K., Fleming G. R. Simulations of the temperature dependence of energy transfer in the PSI core antenna. Biophys J. 1992 Jul;63(1):259–273. doi: 10.1016/S0006-3495(92)81589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinherenbrink F. A., Chiou H. C., LoBrutto R., Blankenship R. E. Spectroscopic evidence for the presence of an iron-sulfur center similar to Fx of Photosystem I in Heliobacillus mobilis. Photosynth Res. 1994 Jul;41(1):115–123. doi: 10.1007/BF02184151. [DOI] [PubMed] [Google Scholar]
- Kleinherenbrink F. A., Hastings G., Wittmerhaus B. P., Blankenship R. E. Delayed fluorescence from Fe-S type photosynthetic reaction centers at low redox potential. Biochemistry. 1994 Mar 15;33(10):3096–3105. doi: 10.1021/bi00176a044. [DOI] [PubMed] [Google Scholar]
- Kruip J., Boekema E. J., Bald D., Boonstra A. F., Rögner M. Isolation and structural characterization of monomeric and trimeric photosystem I complexes (P700.FA/FB and P700.FX) from the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1993 Nov 5;268(31):23353–23360. [PubMed] [Google Scholar]
- Laible P. D., Zipfel W., Owens T. G. Excited state dynamics in chlorophyll-based antennae: the role of transfer equilibrium. Biophys J. 1994 Mar;66(3 Pt 1):844–860. doi: 10.1016/s0006-3495(94)80861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl U., Mockensturm-Wilson M., Trost J. T., Brune D. C., Blankenship R. E., Vermaas W. Single core polypeptide in the reaction center of the photosynthetic bacterium Heliobacillus mobilis: structural implications and relations to other photosystems. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7124–7128. doi: 10.1073/pnas.90.15.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Chiou H. C., Kleinherenbrink F. A., Blankenship R. E. Time-resolved spectroscopy of energy and electron transfer processes in the photosynthetic bacterium Heliobacillus mobilis. Biophys J. 1994 Feb;66(2 Pt 1):437–445. doi: 10.1016/s0006-3495(94)80794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M. Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman spectroscopy. Biochim Biophys Acta. 1977 Jun 9;460(3):408–430. doi: 10.1016/0005-2728(77)90081-0. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenhoff U., Haehnel W., Witt H., Herrmann R. G. Genes encoding eleven subunits of photosystem I from the thermophilic cyanobacterium Synechococcus sp. Gene. 1993 May 15;127(1):71–78. doi: 10.1016/0378-1119(93)90618-d. [DOI] [PubMed] [Google Scholar]
- Nitschke W., Sétif P., Liebl U., Feiler U., Rutherford A. W. Reaction center photochemistry of Heliobacterium chlorum. Biochemistry. 1990 Dec 18;29(50):11079–11088. doi: 10.1021/bi00502a010. [DOI] [PubMed] [Google Scholar]
- Novoderezhkin V. I., Razjivin A. P. Excitonic interactions in the light-harvesting antenna of photosynthetic purple bacteria and their influence on picosecond absorbance difference spectra. FEBS Lett. 1993 Sep 6;330(1):5–7. doi: 10.1016/0014-5793(93)80907-c. [DOI] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna structure and excitation dynamics in photosystem I. II. Studies with mutants of Chlamydomonas reinhardtii lacking photosystem II. Biophys J. 1989 Jul;56(1):95–106. doi: 10.1016/S0006-3495(89)82654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rögner M., Nixon P. J., Diner B. A. Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1990 Apr 15;265(11):6189–6196. [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Shubin V. V., Tsuprun V. L., Bezsmertnaya I. N., Karapetyan N. V. Trimeric forms of the photosystem I reaction center complex pre-exist in the membranes of the cyanobacterium Spirulina platensis. FEBS Lett. 1993 Nov 8;334(1):79–82. doi: 10.1016/0014-5793(93)81685-s. [DOI] [PubMed] [Google Scholar]
- Trinkunas G., Holzwarth A. R. Kinetic modeling of exciton migration in photosynthetic systems. 2. Simulations of excitation dynamics in two-dimensional photosystem I core antenna/reaction center complexes. Biophys J. 1994 Feb;66(2 Pt 1):415–429. doi: 10.1016/s0006-3495(94)80792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost J. T., Blankenship R. E. Isolation of a photoactive photosynthetic reaction center-core antenna complex from Heliobacillus mobilis. Biochemistry. 1989 Dec 26;28(26):9898–9904. doi: 10.1021/bi00452a003. [DOI] [PubMed] [Google Scholar]
- Xiao W., Lin S., Taguchi A. K., Woodbury N. W. Femtosecond pump-probe analysis of energy and electron transfer in photosynthetic membranes of Rhodobacter capsulatus. Biochemistry. 1994 Jul 12;33(27):8313–8322. doi: 10.1021/bi00193a019. [DOI] [PubMed] [Google Scholar]


