Abstract
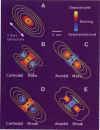
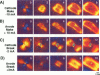
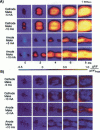
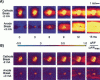
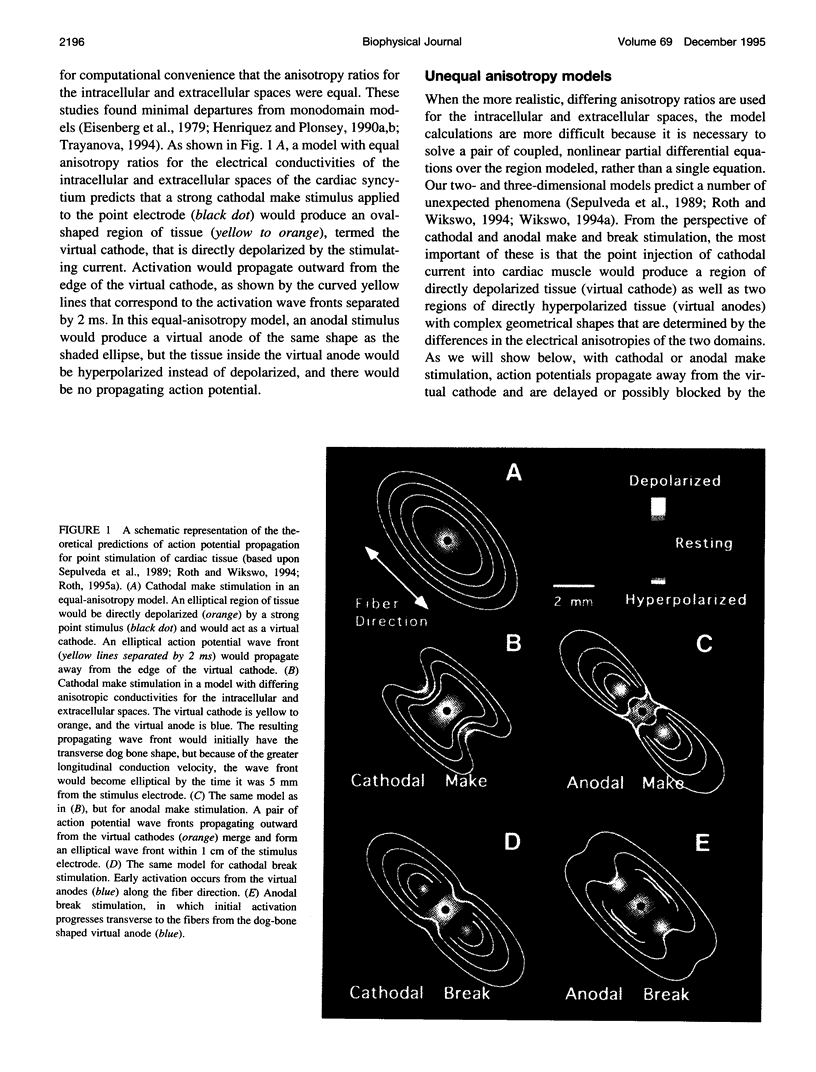
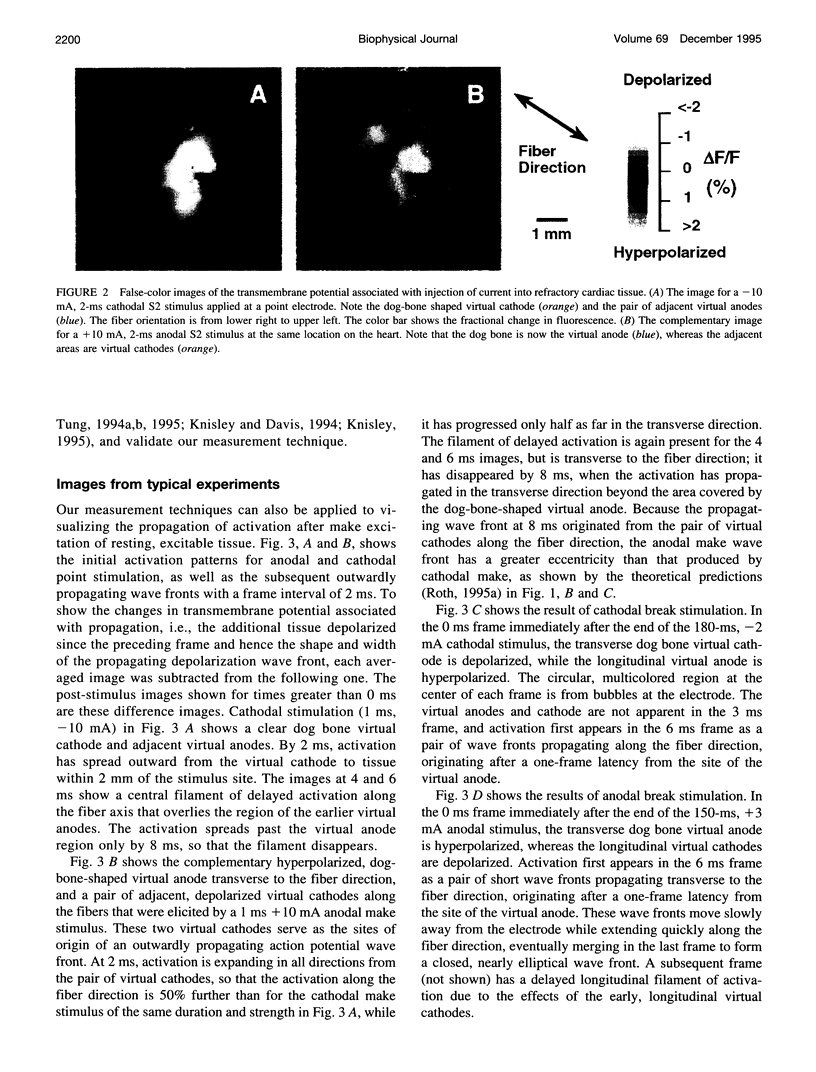
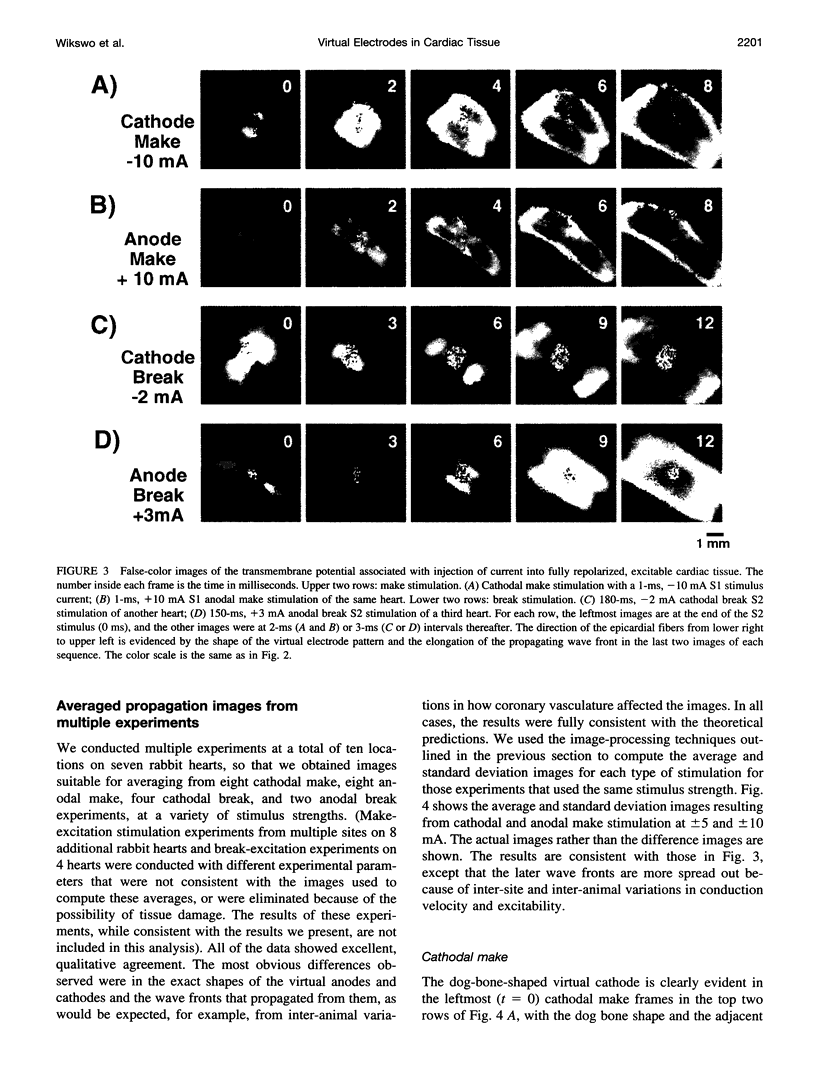
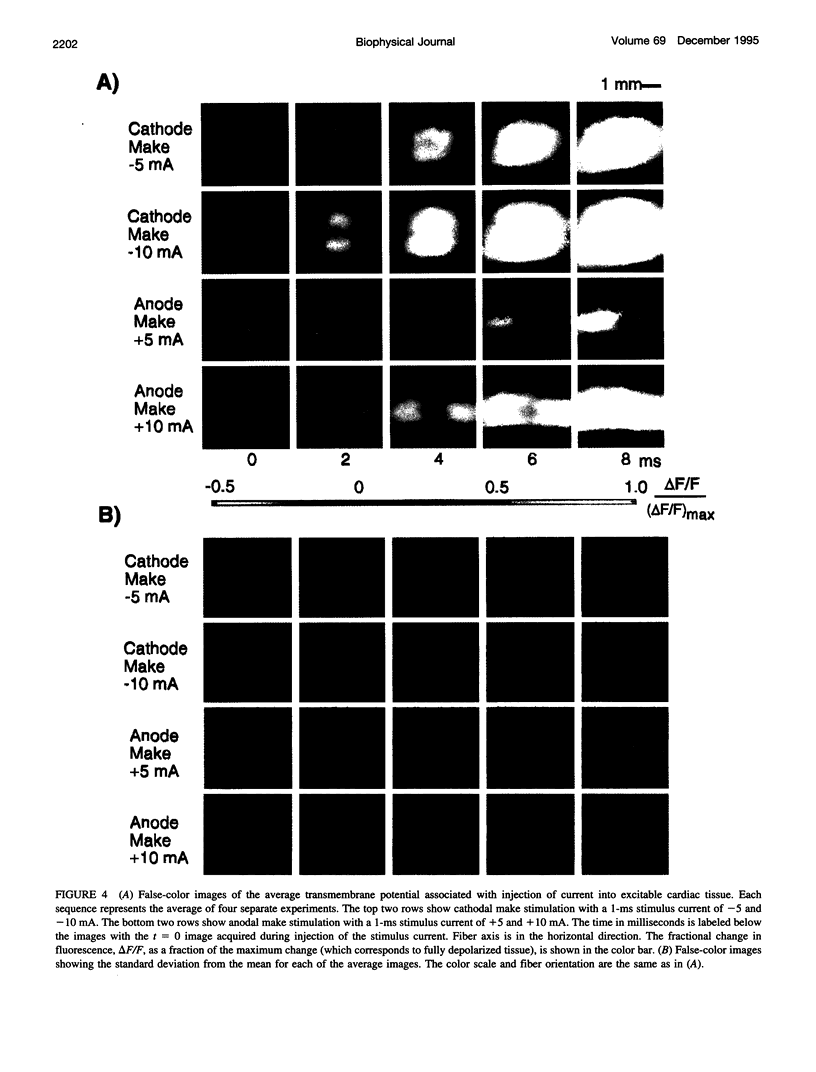
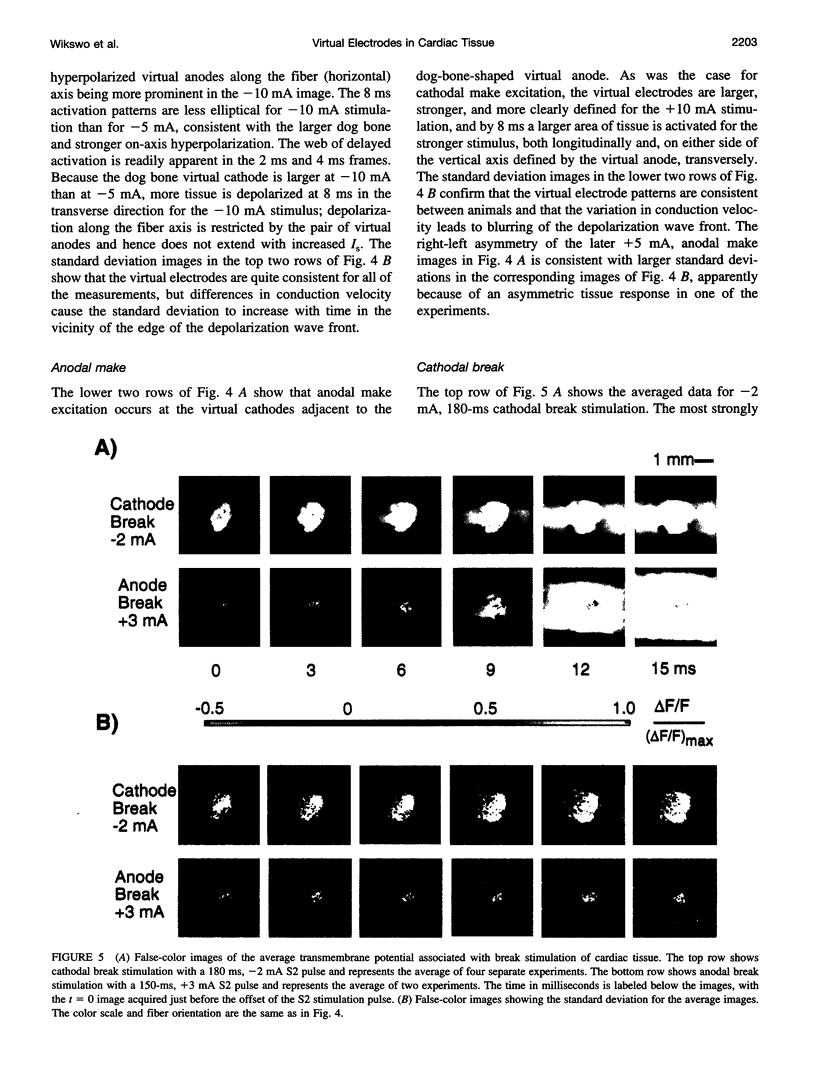
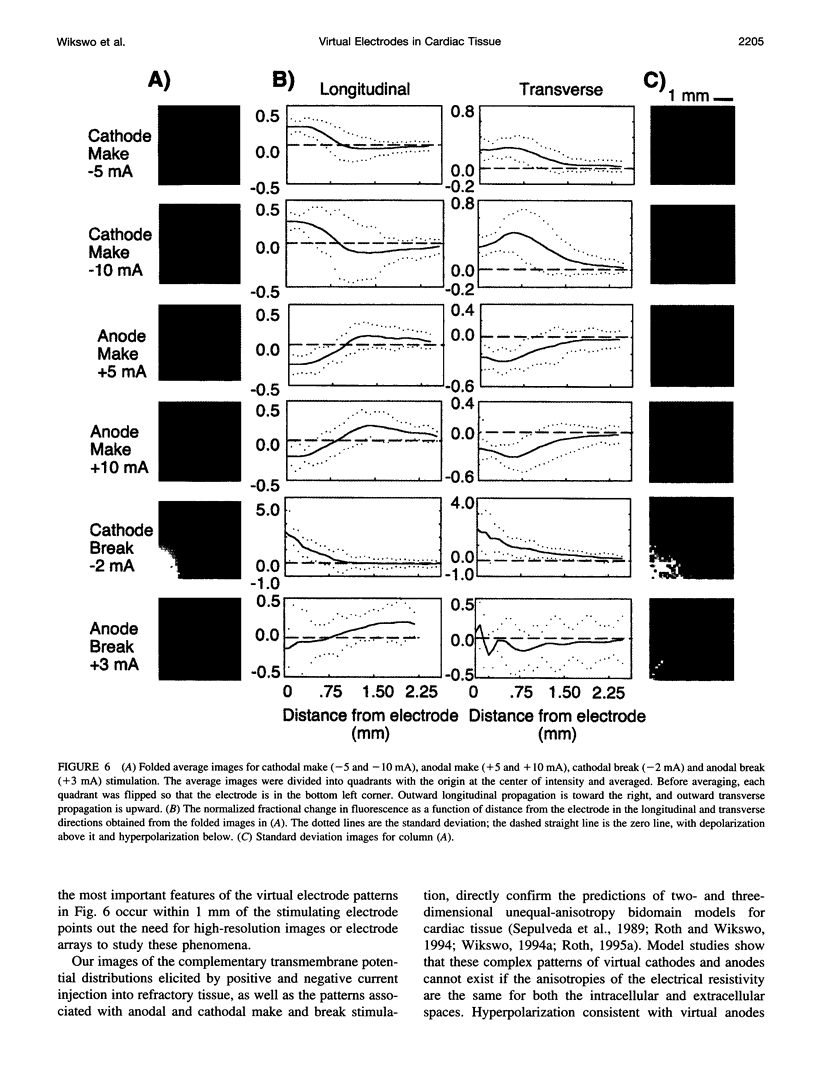
Traditional cable analyses cannot explain complex patterns of excitation in cardiac tissue with unipolar, extracellular anodal, or cathodal stimuli. Epifluorescence imaging of the transmembrane potential during and after stimulation of both refractory and excitable tissue shows distinctive regions of simultaneous depolarization and hyperpolarization during stimulation that act as virtual cathodes and anodes. The results confirm bidomain model predictions that the onset (make) of a stimulus induces propagation from the virtual cathode, whereas stimulus termination (break) induces it from the virtual anode. In make stimulation, the virtual anode can delay activation of the underlying tissue, whereas in break stimulation this occurs under the virtual cathode. Thus make and break stimulations in cardiac tissue have a common mechanism that is the result of differences in the electrical anisotropy of the intracellular and extracellular spaces and provides clear proof of the validity of the bidomain model.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barach J. P., Wikswo J. P., Jr A numerical reconstruction of the effects of late stimulation on a cardiac ventricular action potential. Comput Biomed Res. 1992 Jun;25(3):212–217. doi: 10.1016/0010-4809(92)90039-d. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaster T. A., Davis C. C., Krauthamer V. Excitation and detection of action potential-induced fluorescence changes through a single monomode optical fiber. Biochim Biophys Acta. 1991 Jan 10;1091(1):9–14. doi: 10.1016/0167-4889(91)90214-i. [DOI] [PubMed] [Google Scholar]
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Corbin L. V., 2nd, Scher A. M. The canine heart as an electrocardiographic generator. Dependence on cardiac cell orientation. Circ Res. 1977 Jul;41(1):58–67. doi: 10.1161/01.res.41.1.58. [DOI] [PubMed] [Google Scholar]
- Davidenko J. M., Pertsov A. V., Salomonsz R., Baxter W., Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992 Jan 23;355(6358):349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- Dekker E. Direct current make and break thresholds for pacemaker electrodes on the canine ventricle. Circ Res. 1970 Nov;27(5):811–823. doi: 10.1161/01.res.27.5.811. [DOI] [PubMed] [Google Scholar]
- Dillon S., Morad M. A new laser scanning system for measuring action potential propagation in the heart. Science. 1981 Oct 23;214(4519):453–456. doi: 10.1126/science.6974891. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Barcilon V., Mathias R. T. Electrical properties of spherical syncytia. Biophys J. 1979 Jan;25(1):151–180. doi: 10.1016/S0006-3495(79)85283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geselowitz D. B., Miller W. T., 3rd A bidomain model for anisotropic cardiac muscle. Ann Biomed Eng. 1983;11(3-4):191–206. doi: 10.1007/BF02363286. [DOI] [PubMed] [Google Scholar]
- Henriquez C. S., Plonsey R. Simulation of propagation along a cylindrical bundle of cardiac tissue--I: Mathematical formulation. IEEE Trans Biomed Eng. 1990 Sep;37(9):850–860. doi: 10.1109/10.58596. [DOI] [PubMed] [Google Scholar]
- Henriquez C. S., Plonsey R. Simulation of propagation along a cylindrical bundle of cardiac tissue--II: Results of simulation. IEEE Trans Biomed Eng. 1990 Sep;37(9):861–875. doi: 10.1109/10.58597. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Courtney K. R. Design of a multi-point laser scanned optical monitor of cardiac action potential propagation: application to microreentry in guinea pig atrium. Ann Biomed Eng. 1987;15(6):567–577. doi: 10.1007/BF02364249. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Blitchington T. F., Hill B. C., Grant A. O., Smith W. M., Pilkington T. C., Ideker R. E. Optical measurements of transmembrane potential changes during electric field stimulation of ventricular cells. Circ Res. 1993 Feb;72(2):255–270. doi: 10.1161/01.res.72.2.255. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Hill B. C., Ideker R. E. Virtual electrode effects in myocardial fibers. Biophys J. 1994 Mar;66(3 Pt 1):719–728. doi: 10.1016/s0006-3495(94)80846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cabo C., Salomonsz R., Delmar M., Davidenko J., Jalife J. Effects of diacetyl monoxime on the electrical properties of sheep and guinea pig ventricular muscle. Cardiovasc Res. 1993 Nov;27(11):1991–1997. doi: 10.1093/cvr/27.11.1991. [DOI] [PubMed] [Google Scholar]
- Muler A. L., Markin V. S. Elektricheskie svoistva anizotropnykh nervno-myshechnykh sintsitiev. I. Raspredelenie élektrotonicheskogo potentsiala. Biofizika. 1977 Mar-Apr;22(2):307–312. [PubMed] [Google Scholar]
- Muler A. L., Markin V. S. Elektricheskie svoistva anizotropnykh nervno-myshechnykh sintsitiev. II. Rasprostranenie ploskogo fronta vozbuzhdeniia. Biofizika. 1977 May-Jun;22(3):518–522. [PubMed] [Google Scholar]
- Muler A. L., Markin V. S. Elektricheskie svoistva anizotropnykh nervno-myshechnykh sintsitiev. III. Statsionarnaia forma fronta vozbuzhdeniia. Biofizika. 1977 Jul-Aug;22(4):671–675. [PubMed] [Google Scholar]
- Neunlist M., Tung L. Optical recordings of ventricular excitability of frog heart by an extracellular stimulating point electrode. Pacing Clin Electrophysiol. 1994 Oct;17(10):1641–1654. doi: 10.1111/j.1540-8159.1994.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Tung L. Spatial distribution of cardiac transmembrane potentials around an extracellular electrode: dependence on fiber orientation. Biophys J. 1995 Jun;68(6):2310–2322. doi: 10.1016/S0006-3495(95)80413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M., Zou S. Z., Tung L. Design and use of an "optrode" for optical recordings of cardiac action potentials. Pflugers Arch. 1992 Apr;420(5-6):611–617. doi: 10.1007/BF00374641. [DOI] [PubMed] [Google Scholar]
- Pertsov A. M., Davidenko J. M., Salomonsz R., Baxter W. T., Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res. 1993 Mar;72(3):631–650. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Current flow patterns in two-dimensional anisotropic bisyncytia with normal and extreme conductivities. Biophys J. 1984 Mar;45(3):557–571. doi: 10.1016/S0006-3495(84)84193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Effect of microscopic and macroscopic discontinuities on the response of cardiac tissue to defibrillating (stimulating) currents. Med Biol Eng Comput. 1986 Mar;24(2):130–136. doi: 10.1007/BF02443925. [DOI] [PubMed] [Google Scholar]
- RUSH S., ABILDSKOV J. A., McFEER Resistivity of body tissues at low frequencies. Circ Res. 1963 Jan;12:40–50. doi: 10.1161/01.res.12.1.40. [DOI] [PubMed] [Google Scholar]
- Roth B. J., Wikswo J. P., Jr A bidomain model for the extracellular potential and magnetic field of cardiac tissue. IEEE Trans Biomed Eng. 1986 Apr;33(4):467–469. doi: 10.1109/TBME.1986.325804. [DOI] [PubMed] [Google Scholar]
- Roth B. J., Wikswo J. P., Jr Electrical stimulation of cardiac tissue: a bidomain model with active membrane properties. IEEE Trans Biomed Eng. 1994 Mar;41(3):232–240. doi: 10.1109/10.284941. [DOI] [PubMed] [Google Scholar]
- Sepulveda N. G., Roth B. J., Wikswo J. P., Jr Current injection into a two-dimensional anisotropic bidomain. Biophys J. 1989 May;55(5):987–999. doi: 10.1016/S0006-3495(89)82897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda N. G., Wikswo J. P., Jr Electric and magnetic fields from two-dimensional anisotropic bisyncytia. Biophys J. 1987 Apr;51(4):557–568. doi: 10.1016/S0006-3495(87)83381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova N. A bidomain model for ring stimulation of a cardiac strand. IEEE Trans Biomed Eng. 1994 Apr;41(4):393–397. doi: 10.1109/10.284970. [DOI] [PubMed] [Google Scholar]
- Wikswo J. P., Jr The complexities of cardiac cables: virtual electrode effects. Biophys J. 1994 Mar;66(3 Pt 1):551–553. doi: 10.1016/s0006-3495(94)80832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo J. P., Jr, Wisialowski T. A., Altemeier W. A., Balser J. R., Kopelman H. A., Roden D. M. Virtual cathode effects during stimulation of cardiac muscle. Two-dimensional in vivo experiments. Circ Res. 1991 Feb;68(2):513–530. doi: 10.1161/01.res.68.2.513. [DOI] [PubMed] [Google Scholar]