Abstract
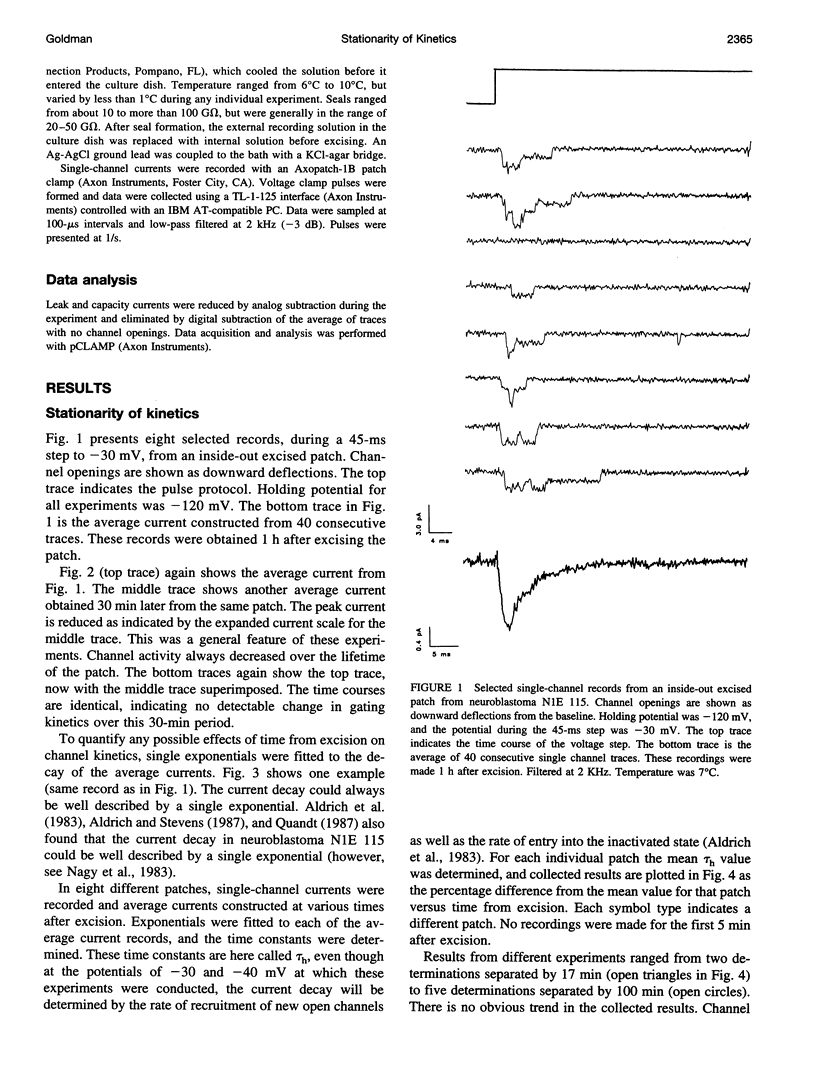
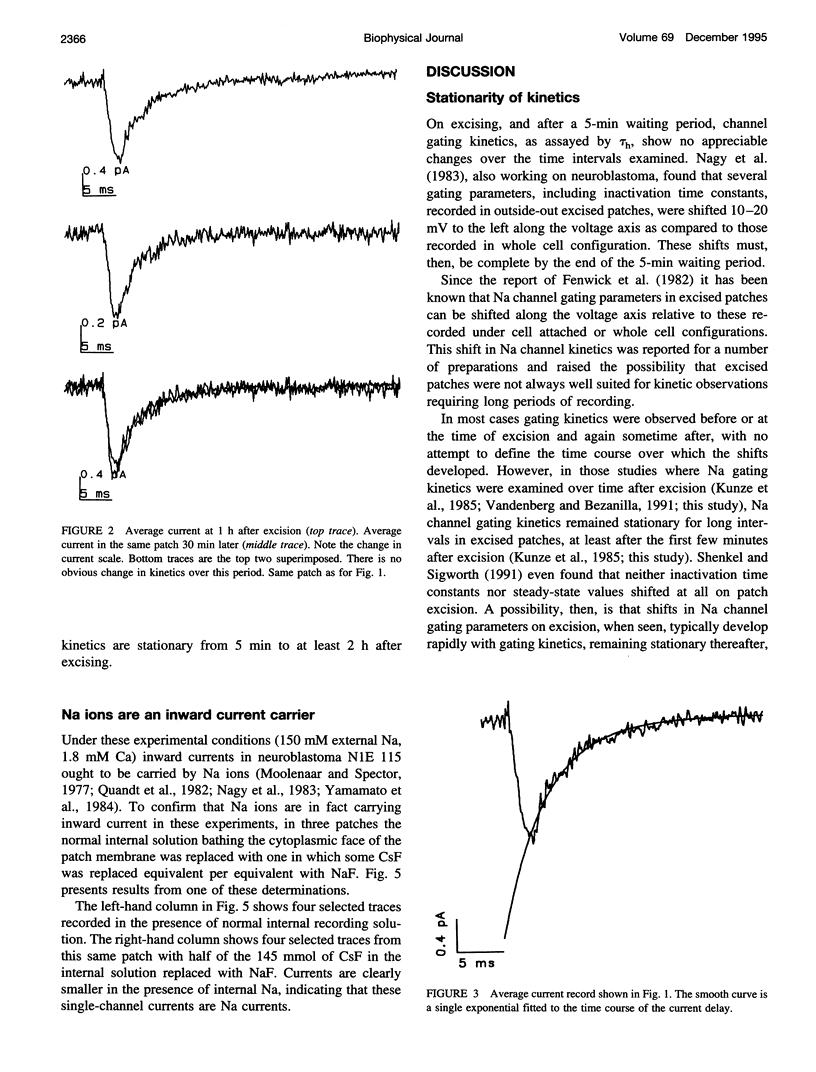
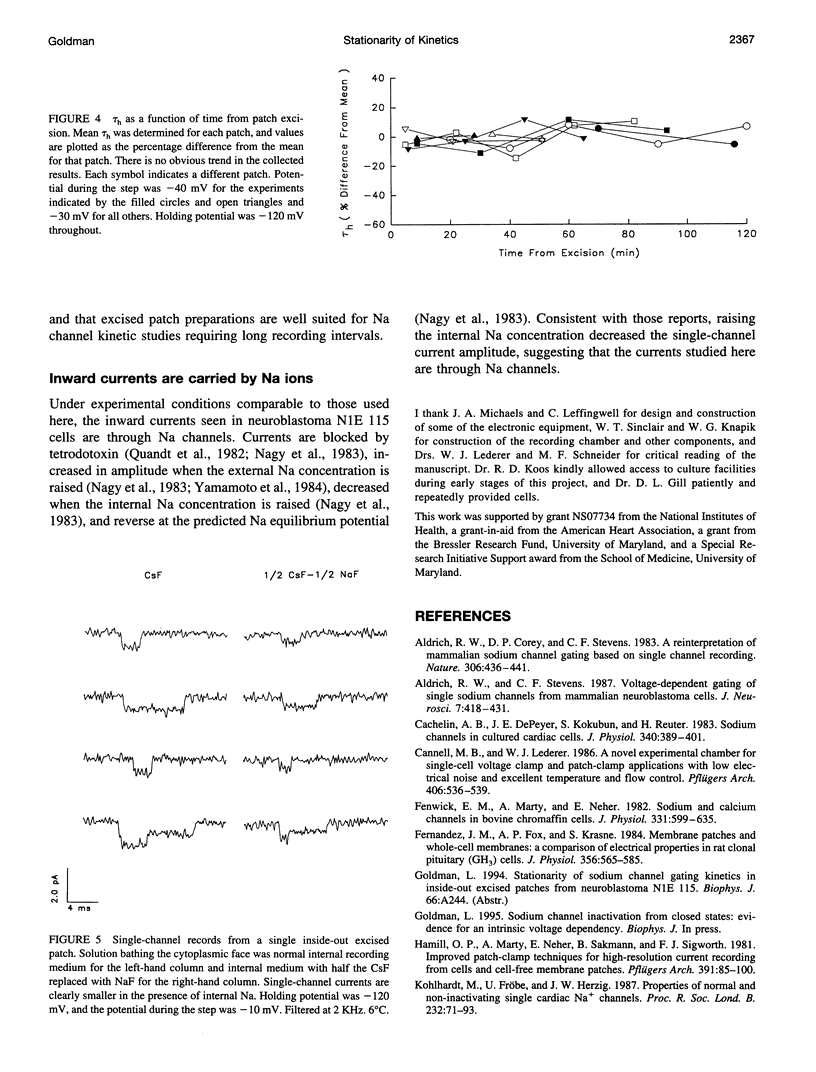
Na channel gating parameters in a number of preparations are translated along the voltage axis in excised patches compared to cell attached or whole cell recording. The aim of this study is to determine whether these changes in gating behavior continue over an extended period or, rather, develop rapidly on excision with stationary kinetics thereafter. Average currents were constructed from single-channel records from neuroblastoma N1E 115 at various times after excision, excluding the first 5 min, in eight inside-out excised patches. Single exponentials were fitted to the current decay of the average records, and the mean time constant for each patch was determined. Values were plotted as the percentage difference from these means for each patch against time from excision. Collected results show no obvious trend in values from 5 min to 2 h. Kinetics are stationary, and shifts in Na channel gating parameters along the voltage axis seen in excised as compared to whole cell configuration in neuroblastoma must be complete by the first few minutes after excision. Raising the internal Na concentration reduced the single channel current amplitude, confirming that these are Na channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Fröbe U., Herzig J. W. Properties of normal and non-inactivating single cardiac Na+ channels. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):71–93. doi: 10.1098/rspb.1987.0062. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Membrane currents examined under voltage clamp in cultured neuroblastoma cells. Science. 1977 Apr 15;196(4287):331–333. doi: 10.1126/science.557842. [DOI] [PubMed] [Google Scholar]
- Nagy K., Kiss T., Hof D. Single Na channels in mouse neuroblastoma cell membrane. Indications for two open states. Pflugers Arch. 1983 Dec;399(4):302–308. doi: 10.1007/BF00652757. [DOI] [PubMed] [Google Scholar]
- Quandt F. N. Burst kinetics of sodium channels which lack fast inactivation in mouse neuroblastoma cells. J Physiol. 1987 Nov;392:563–585. doi: 10.1113/jphysiol.1987.sp016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkel S., Sigworth F. J. Patch recordings from the electrocytes of Electrophorus electricus. Na currents and PNa/PK variability. J Gen Physiol. 1991 May;97(5):1013–1041. doi: 10.1085/jgp.97.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Bezanilla F. A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J. 1991 Dec;60(6):1511–1533. doi: 10.1016/S0006-3495(91)82186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


