Abstract
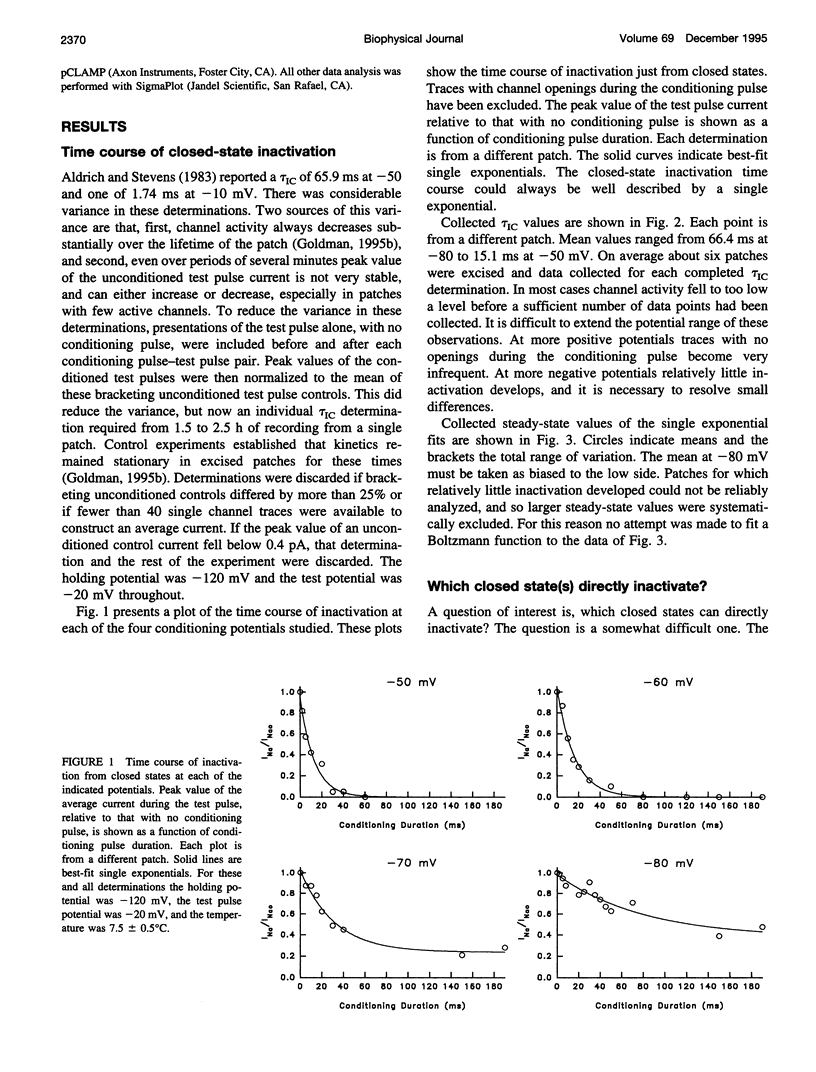
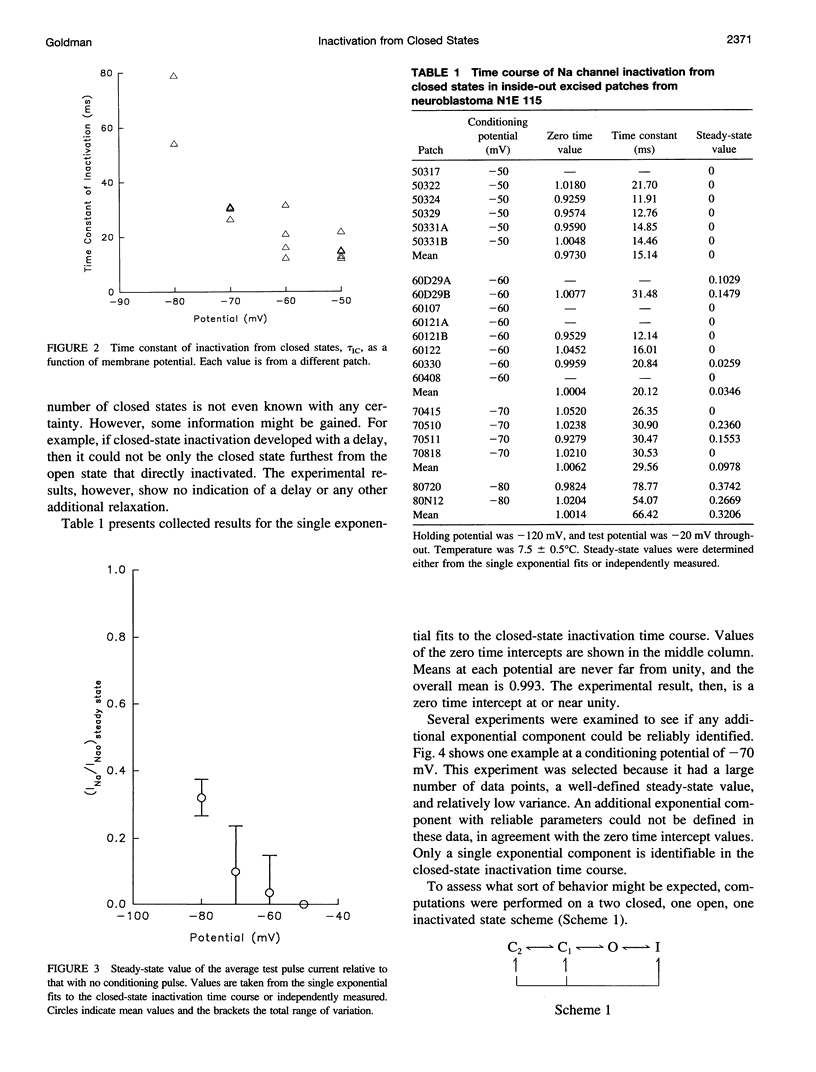
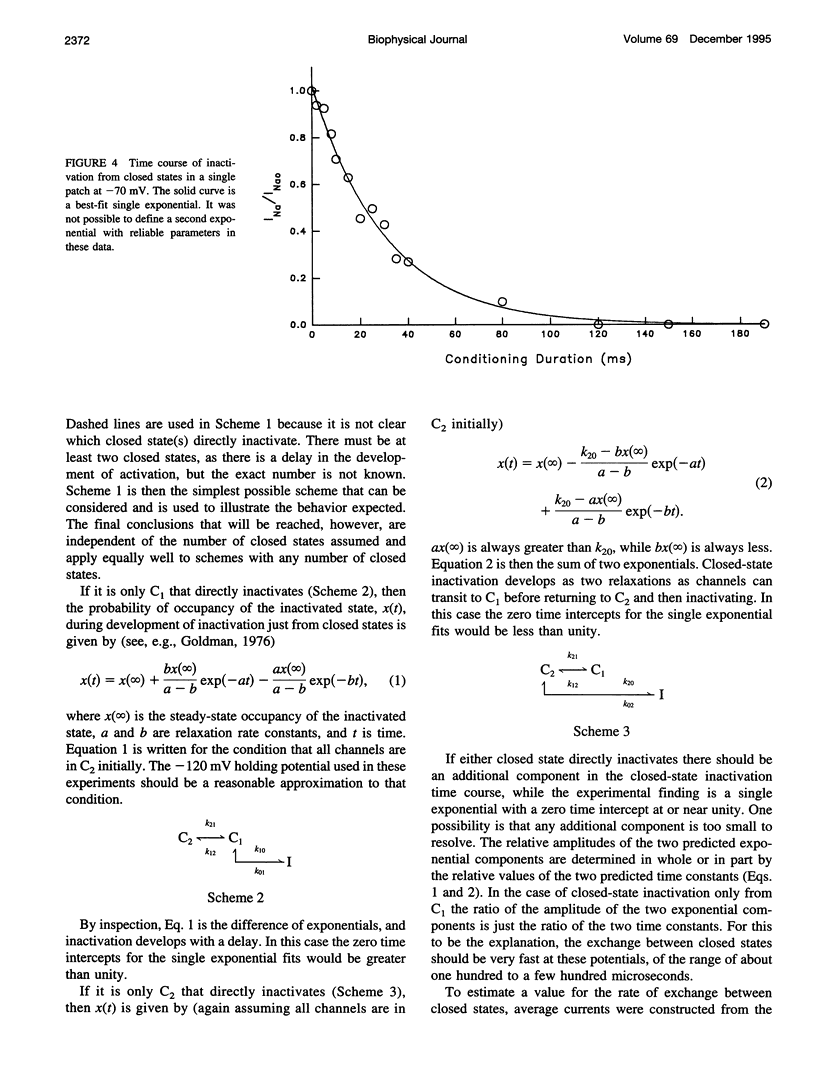
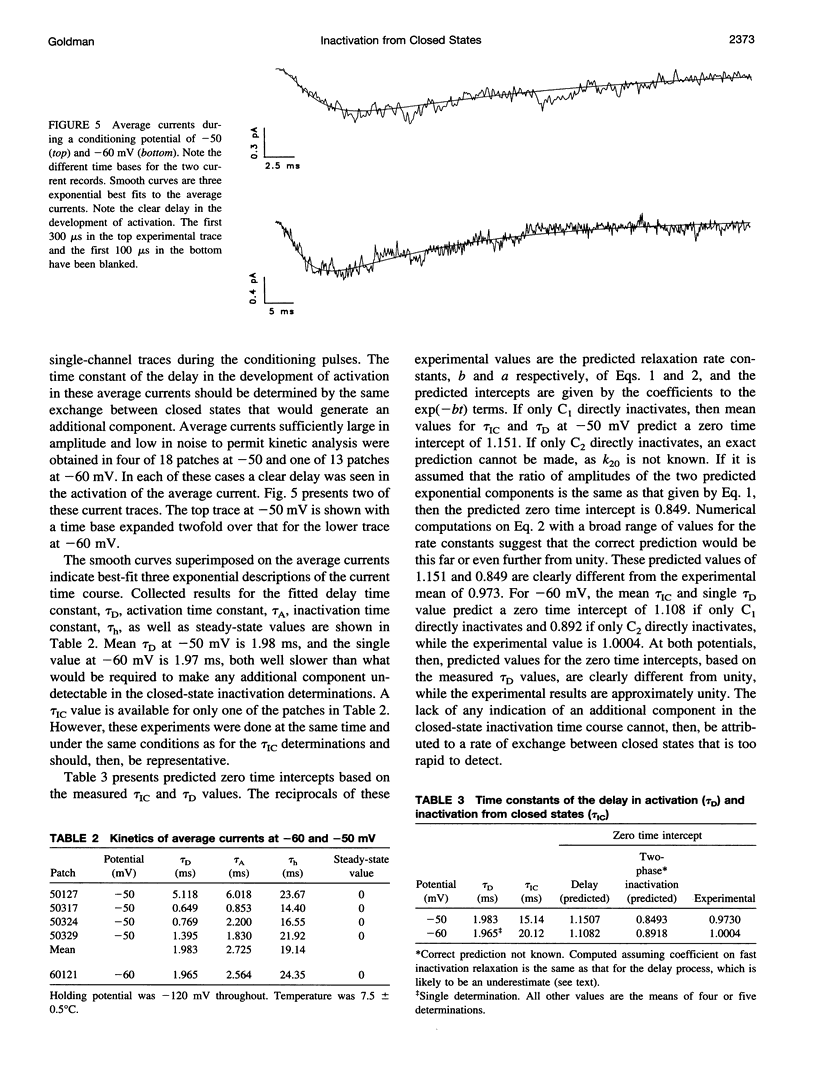
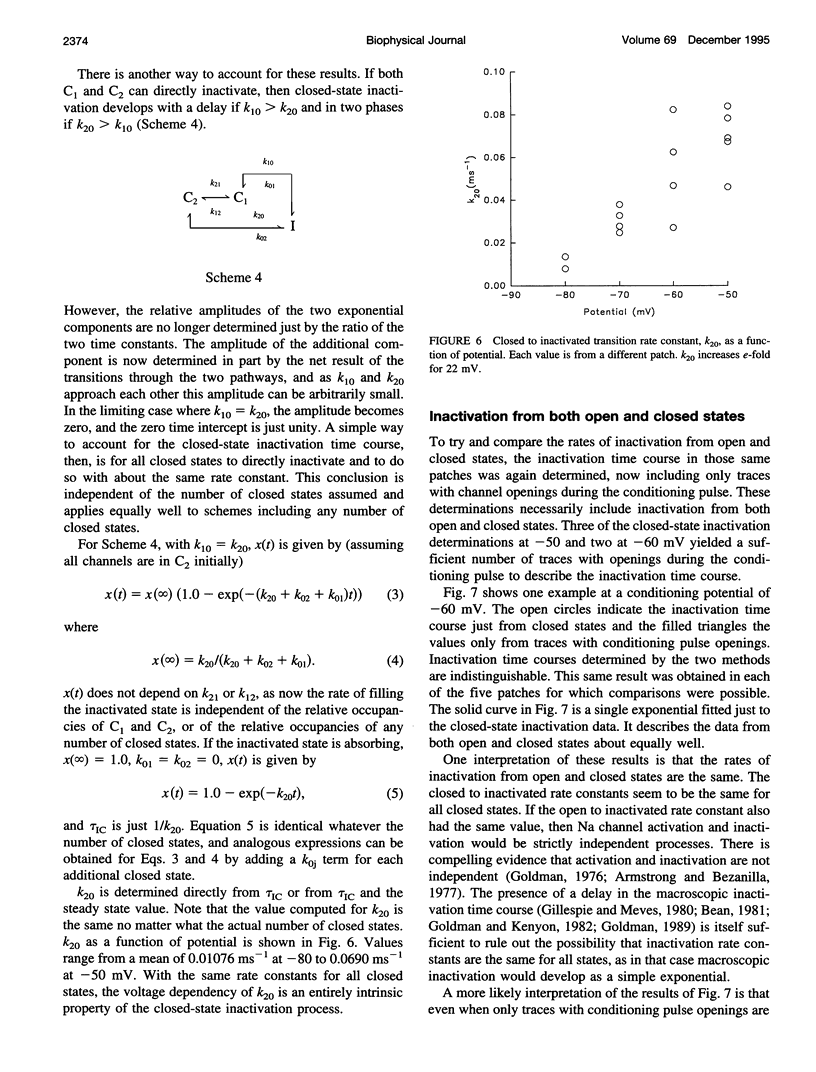
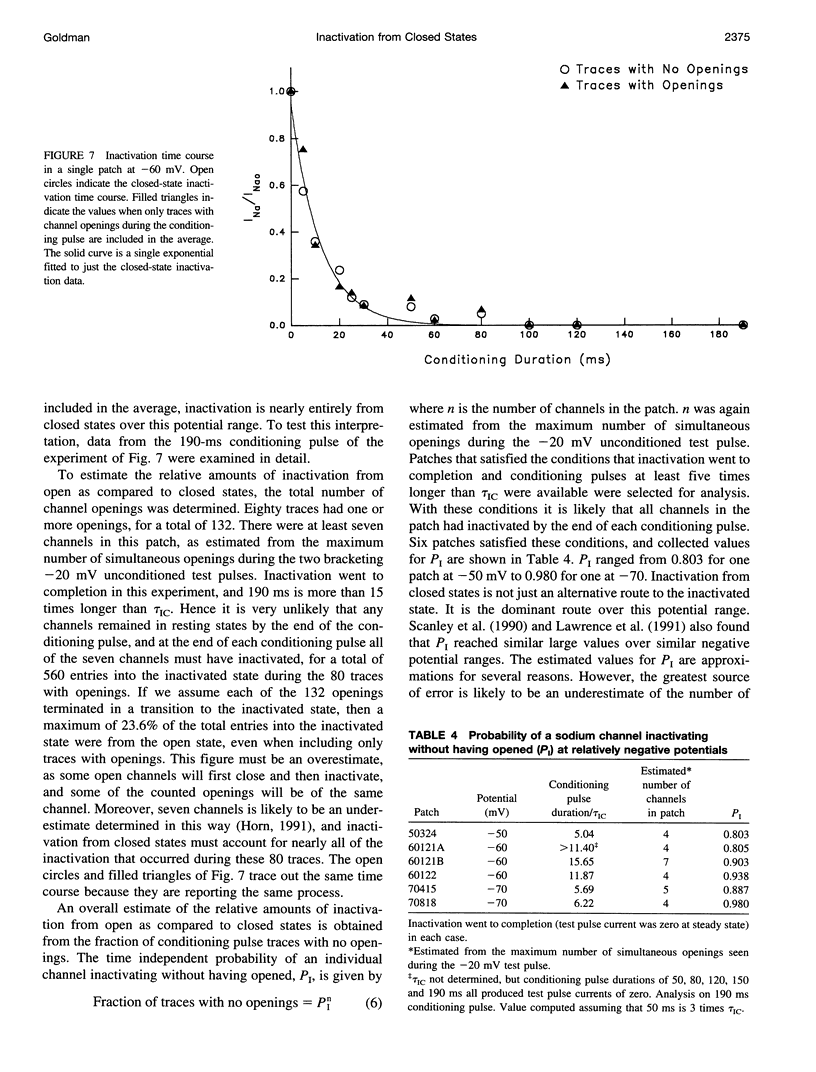
The time course of Na channel inactivation from closed states was determined on inside-out excised patches from neuroblastoma N1E 115. Closed-state inactivation develops as a single exponential with mean time constants of 66.4 ms at -80 mV, 29.6 ms at -70 mV, 20.1 ms at -60 mV, and 15.1 ms at -50 mV. Corresponding mean steady-state values of the fitted exponentials were 0.321, 0.098, 0.035, and 0. Closed-state inactivation, in general, should develop either with a delay or as more than one exponential, depending on which closed state(s) directly inactivate. The absence of additional components cannot be attributed to a rate of exchange between closed states too rapid to detect. The time course is simply accounted for if all closed states directly inactivate and do so with the same rate constant for each closed state, suggesting that those conformational changes constituting the transitions between closed states have little effect on the structural components involved in inactivation. Closed to inactivated rate constants ranged from a mean of 0.0108 ms-1 at -80 mV to 0.0690 ms-1 at -50 mV. This voltage dependency is entirely intrinsic to closed-state inactivation with closed to inactivated rate constants similar for all closed states. Over the potential range studied nearly all the inactivation is from closed states.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Stevens C. F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):147–153. doi: 10.1101/sqb.1983.048.01.017. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. S., Korenberg M. J. A nonlinear cascade model for action potential encoding in an insect sensory neuron. Biophys J. 1989 Apr;55(4):655–661. doi: 10.1016/S0006-3495(89)82863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Kenyon J. L. Delays in inactivation development and activation kinetics in myxicola giant axons. J Gen Physiol. 1982 Jul;80(1):83–102. doi: 10.1085/jgp.80.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Kinetics of channel gating in excitable membranes. Q Rev Biophys. 1976 Nov;9(4):491–526. doi: 10.1017/s0033583500002651. [DOI] [PubMed] [Google Scholar]
- Goldman L. Sodium channel opening as a precursor to inactivation. A route to the inactivated state. Eur Biophys J. 1989;16(6):321–325. doi: 10.1007/BF00257880. [DOI] [PubMed] [Google Scholar]
- Horn R. Estimating the number of channels in patch recordings. Biophys J. 1991 Aug;60(2):433–439. doi: 10.1016/S0006-3495(91)82069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J., Stevens C. F. Sodium channels need not open before they inactivate. Nature. 1981 Jun 4;291(5814):426–427. doi: 10.1038/291426a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Bean B. P. Na+ channels must deactivate to recover from inactivation. Neuron. 1994 Apr;12(4):819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Lawrence J. H., Yue D. T., Rose W. C., Marban E. Sodium channel inactivation from resting states in guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:629–650. doi: 10.1113/jphysiol.1991.sp018855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanley B. E., Hanck D. A., Chay T., Fozzard H. A. Kinetic analysis of single sodium channels from canine cardiac Purkinje cells. J Gen Physiol. 1990 Mar;95(3):411–437. doi: 10.1085/jgp.95.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


