Abstract
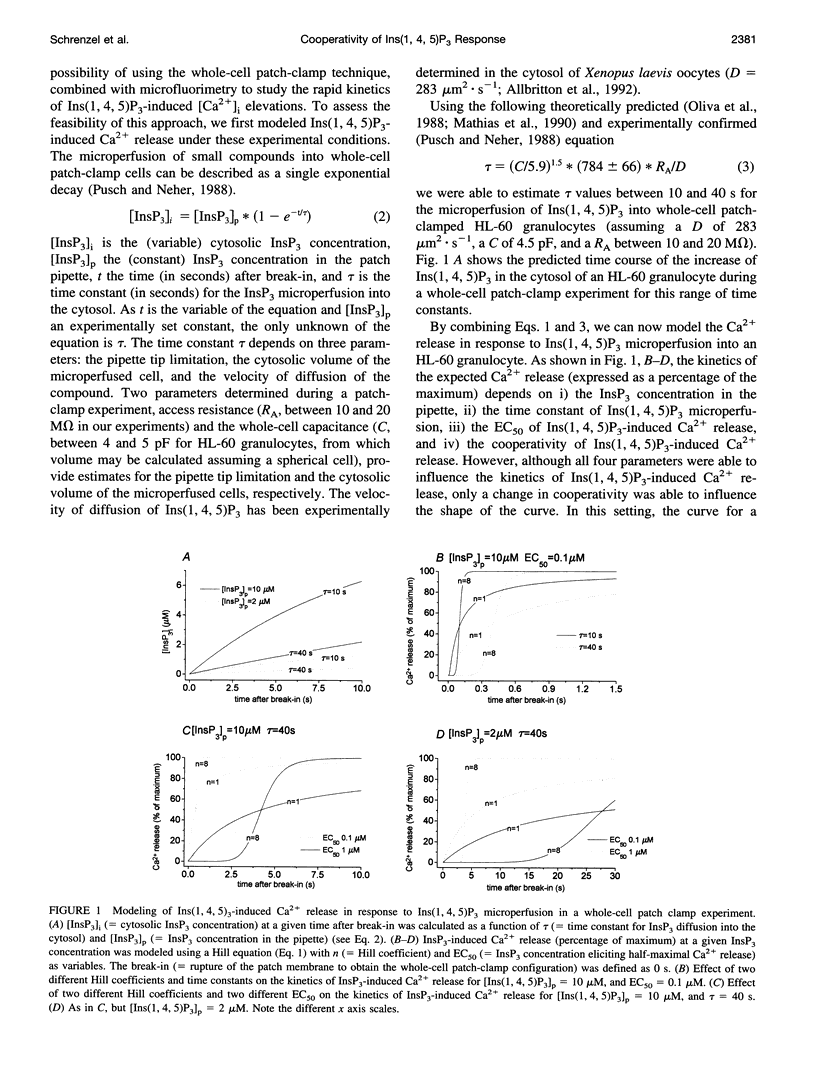
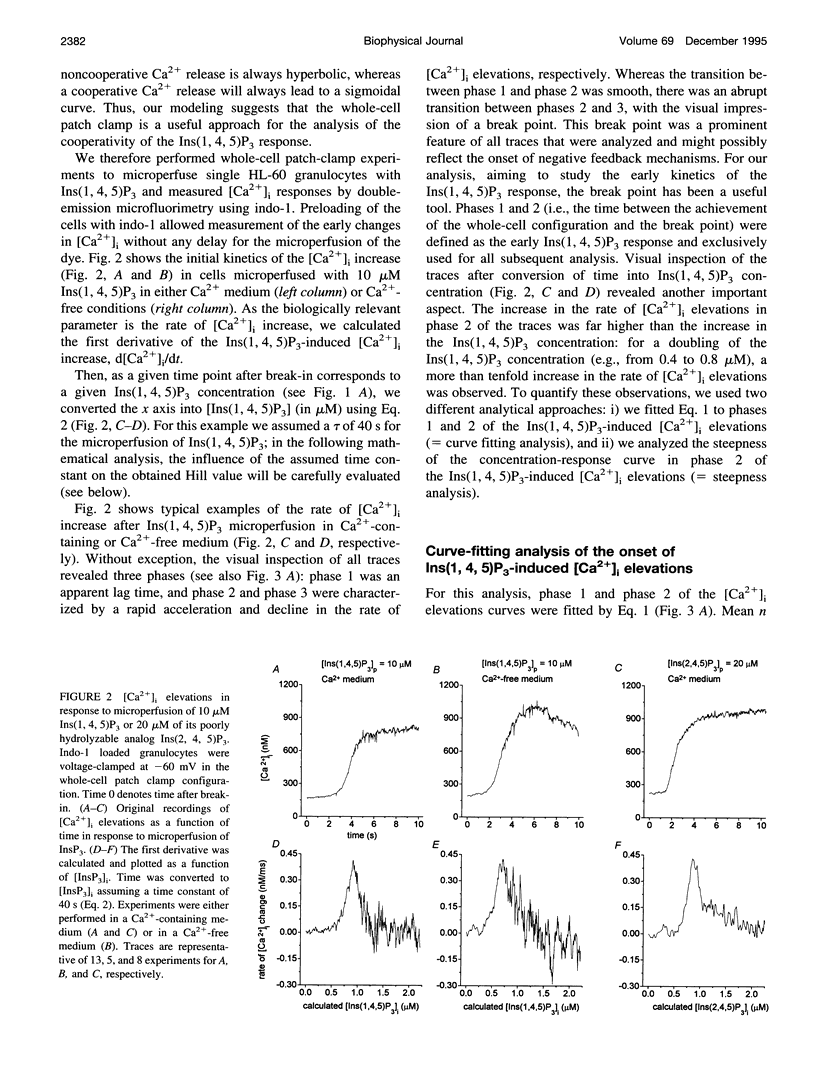
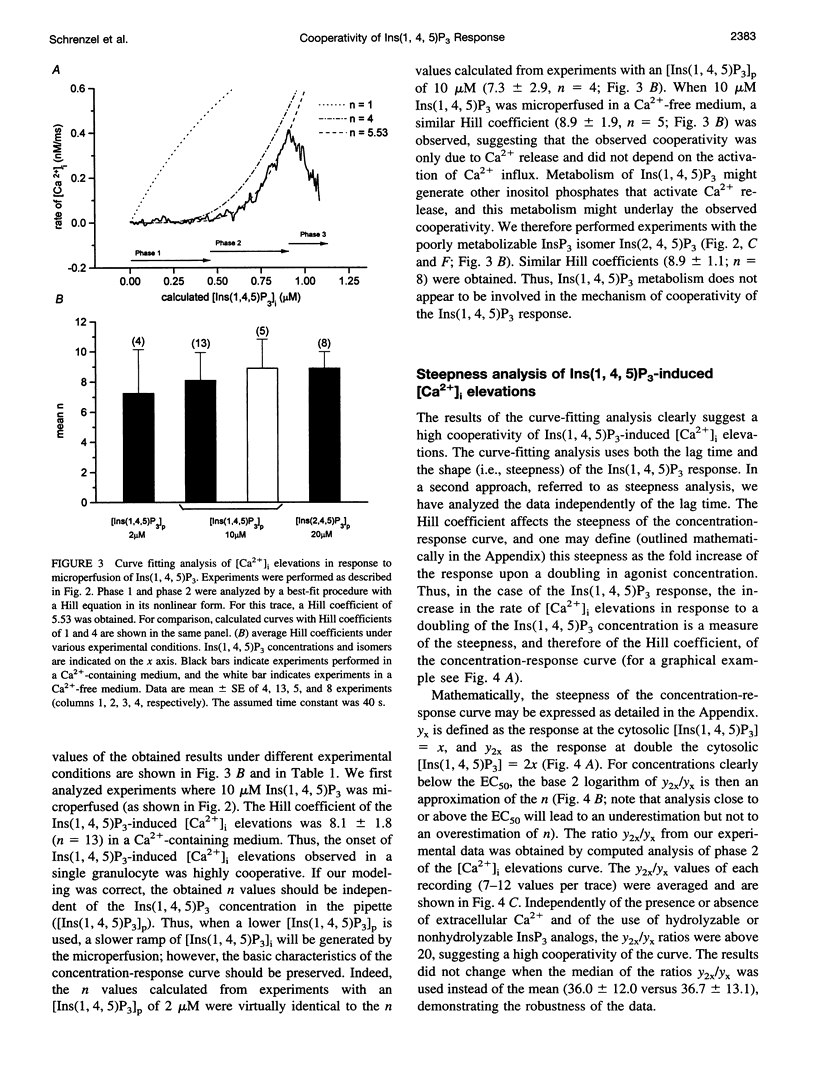
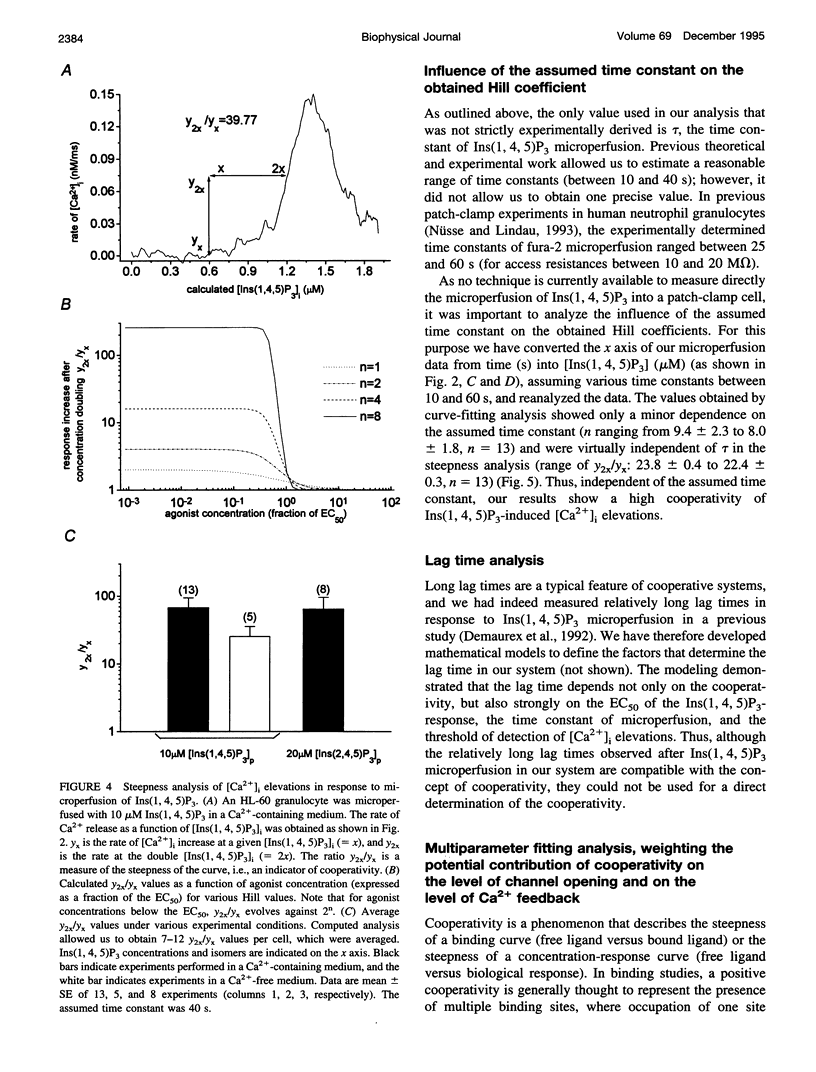
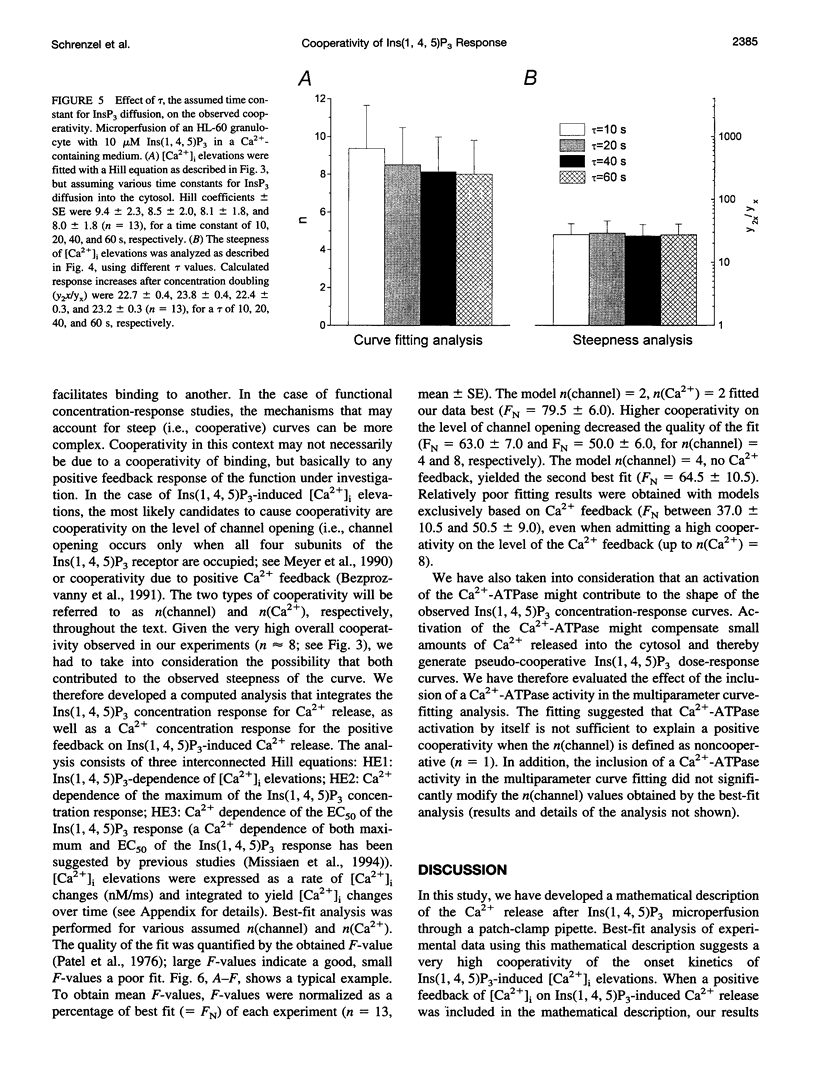
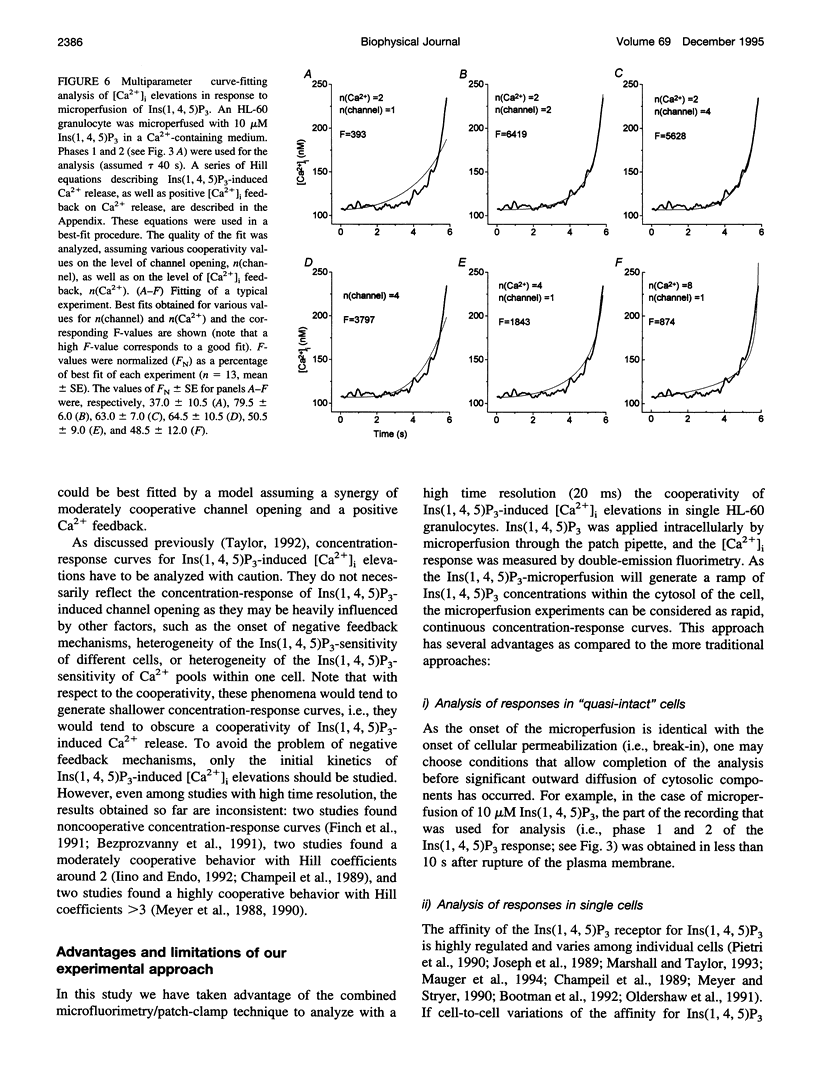
To study the initial kinetics of Ins(1,4,5)P3-induced [Ca2+]i elevations with a high time resolution and to avoid the problem of cell-to-cell heterogeneity, we have used the combined patch-clamp/microfluorimetry technique. The mathematical description of the microperfusion of Ins(1,4,5)P3 and the subsequent Ca2+ release consists of a monoexponential decay (cytosolic Ins(1,4,5)P3 concentration) and a Hill equation (Ins(1,4,5)P3 dose-response curve). Two additional Hill equations and an integration were necessary to include a putative dependence of Ins(1,4,5)P3-induced Ca2+ release on [Ca2+]i. Best-fitting analysis assuming [Ca2+]i-independent Ca2+ release yielded Hill coefficients between 4 and 12. The high cooperativity was also observed with the poorly metabolizable analog Ins(2,4,5)P3 and was independent of extracellular [Ca2+]. Best-fitting analysis including a positive [Ca2+]i feedback suggested a cooperativity on the level of Ins(1,4,5)P3-induced channel opening (n = 2) and an enhancement of Ins(1,4,5)P3-induced Ca2+ release by [Ca2+]i. In summary, the onset kinetics of Ins(1,4,5)P3-induced [Ca2+]i elevations in single HL-60 granulocytes showed a very high cooperativity, presumably because of a cooperativity on the level of channel opening and a positive Ca2+ feedback, but not because of Ca2+ influx or Ins(1,4,5)P3 metabolism. This high cooperativity, acting in concert with negative feedback mechanisms, might play an important role in the fine-tuning of the cellular Ca2+ signal.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Barlow R., Blake J. F. Hill coefficients and the logistic equation. Trends Pharmacol Sci. 1989 Nov;10(11):440–441. doi: 10.1016/S0165-6147(89)80006-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Taylor C. W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol. 1992 May;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeil P., Combettes L., Berthon B., Doucet E., Orlowski S., Claret M. Fast kinetics of calcium release induced by myo-inositol trisphosphate in permeabilized rat hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17665–17673. [PubMed] [Google Scholar]
- Combettes L., Hannaert-Merah Z., Coquil J. F., Rousseau C., Claret M., Swillens S., Champeil P. Rapid filtration studies of the effect of cytosolic Ca2+ on inositol 1,4,5-trisphosphate-induced 45Ca2+ release from cerebellar microsomes. J Biol Chem. 1994 Jul 1;269(26):17561–17571. [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Demaurex N., Schlegel W., Varnai P., Mayr G., Lew D. P., Krause K. H. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. J Clin Invest. 1992 Sep;90(3):830–839. doi: 10.1172/JCI115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre C. J., Lew D. P., Krause K. H. Rapid heparin-sensitive Ca2+ release following Ca(2+)-ATPase inhibition in intact HL-60 granulocytes. Evidence for Ins(1,4,5)P3-dependent Ca2+ cycling across the membrane of Ca2+ stores. Biochem J. 1994 Aug 15;302(Pt 1):155–162. doi: 10.1042/bj3020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henne V., Mayr G. W., Grabowski B., Koppitz B., Söling H. D. Semisynthetic derivatives of inositol 1,4,5-trisphosphate substituted at the 1-phosphate group. Effects on calcium release from permeabilized guinea-pig parotid acinar cells and comparison with binding to aldolase A. Eur J Biochem. 1988 May 16;174(1):95–101. doi: 10.1111/j.1432-1033.1988.tb14067.x. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Iino M., Tsukioka M. Feedback control of inositol trisphosphate signalling bycalcium. Mol Cell Endocrinol. 1994 Jan;98(2):141–146. doi: 10.1016/0303-7207(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Williamson J. R. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989 Feb 15;258(1):261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I., Payne R., Potter B. V., Hillman P. Facilitation of the responses to injections of inositol 1,4,5-trisphosphate analogs in Limulus ventral photoreceptors. Biophys J. 1994 Sep;67(3):1161–1172. doi: 10.1016/S0006-3495(94)80584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Marshall I. C., Taylor C. W. Biphasic effects of cytosolic Ca2+ on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J Biol Chem. 1993 Jun 25;268(18):13214–13220. [PubMed] [Google Scholar]
- Mathias R. T., Cohen I. S., Oliva C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys J. 1990 Sep;58(3):759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger J. P., Lièvremont J. P., Piétri-Rouxel F., Hilly M., Coquil J. F. The inositol 1,4,5-trisphosphate receptor: kinetic properties and regulation. Mol Cell Endocrinol. 1994 Jan;98(2):133–139. doi: 10.1016/0303-7207(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Parys J. B., Casteels R. Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem. 1994 Mar 11;269(10):7238–7242. [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Liver inositol, 1,4,5-trisphosphate-binding sites are the Ca2(+)-mobilizing receptors. Biochem J. 1990 Aug 15;270(1):227–232. doi: 10.1042/bj2700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M. The calcium signal in human neutrophils and its relation to exocytosis investigated by patch-clamp capacitance and Fura-2 measurements. Cell Calcium. 1993 Apr;14(4):255–269. doi: 10.1016/0143-4160(93)90047-a. [DOI] [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri F., Hilly M., Mauger J. P. Calcium mediates the interconversion between two states of the liver inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Oct 15;265(29):17478–17485. [PubMed] [Google Scholar]
- Pittet D., Schlegel W., Lew D. P., Monod A., Mayr G. W. Mass changes in inositol tetrakis- and pentakisphosphate isomers induced by chemotactic peptide stimulation in HL-60 cells. J Biol Chem. 1989 Nov 5;264(31):18489–18493. [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. Kinetics of inositol 1,4,5-trisphosphate-stimulated Ca2+ mobilization. Adv Second Messenger Phosphoprotein Res. 1992;26:109–142. [PubMed] [Google Scholar]
- Van Delden C., Favre C., Spät A., Cerny E., Krause K. H., Lew D. P. Purification of an inositol 1,4,5-trisphosphate-binding calreticulin-containing intracellular compartment of HL-60 cells. Biochem J. 1992 Feb 1;281(Pt 3):651–656. doi: 10.1042/bj2810651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Delden C., Foti M., Lew D. P., Krause K. H. Ca2+ and Mg2+ regulation of inositol 1,4,5-triphosphate binding in myeloid cells. J Biol Chem. 1993 Jun 15;268(17):12443–12448. [PubMed] [Google Scholar]
- Varnai P., Demaurex N., Jaconi M., Schlegel W., Lew D. P., Krause K. H. Highly co-operative Ca2+ activation of intermediate-conductance K+ channels in granulocytes from a human cell line. J Physiol. 1993 Dec;472:373–390. doi: 10.1113/jphysiol.1993.sp019952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Snyder S. H. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989 Jan;9(1):339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., Deranleau D. A., Baggiolini M. Calcium fluxes and calcium buffering in human neutrophils. J Biol Chem. 1986 Aug 5;261(22):10163–10168. [PubMed] [Google Scholar]



