Abstract
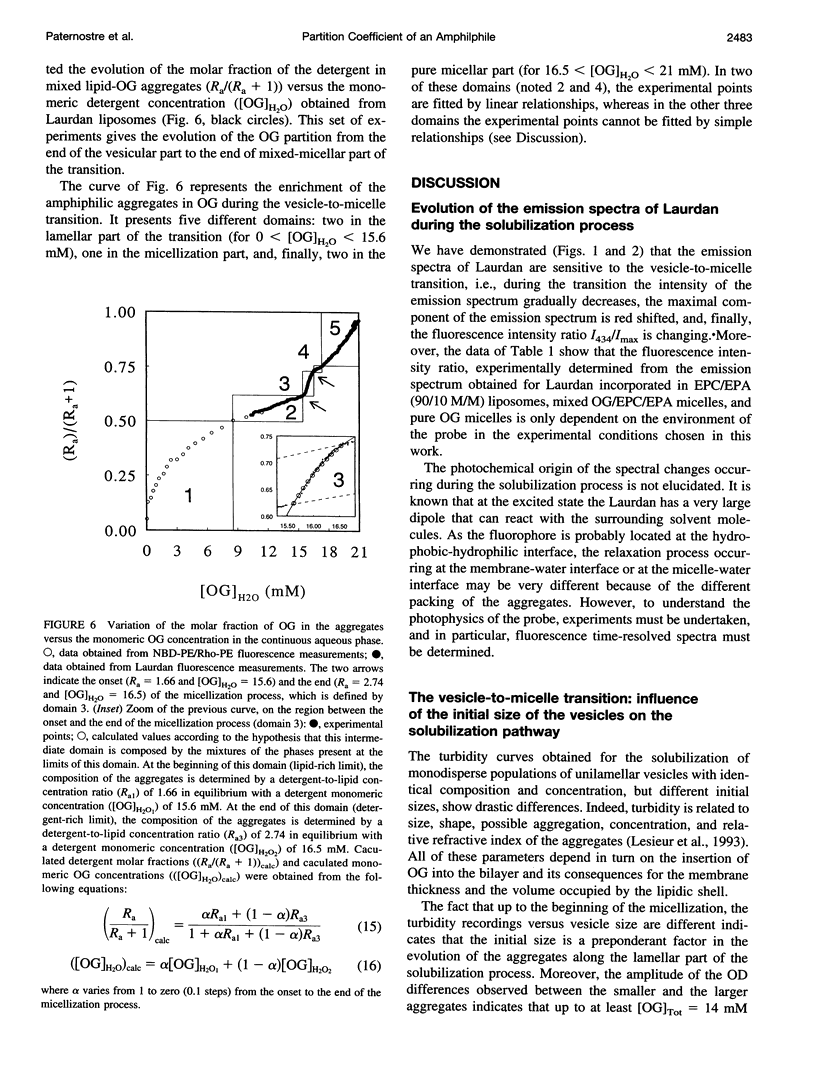
The mechanism of the solubilization of egg phosphatidylcholine containing 10% (M/M) of egg phosphatidic acid unilamellar vesicles by the nonionic detergent, octyl beta-D-glucopyranoside, has been investigated at both molecular and supramolecular levels by using fluorescence and turbidity measurements. In the lamellar region of the transition, the solubilization process has been shown to be first a function of the initial size before reaching an equilibrium aggregation state at the end of this region (the onset of the micellization process). The analysis during the solubilization process of the evolution of both the fluorescence energy transfer between N-(7-nitro-2,1,3-benzoxadiazol-4-yl)-phosphatidylethanolamine (NBD-PE) and N-(lissamine rhodamine B sulfonyl)-phosphatidylethanolamine (Rho-PE) and the fluorescence of 6-dodecanoyl-2-dimethylaminoaphtalene (Laurdan) has allowed us to determine the evolution of the detergent partitioning between the aqueous and the lipidic phases, i.e., the evolution of the molar fraction of OG in the aggregates (XOG/Lip) with its monomeric detergent concentration in equilibrium ([OG]H2O), throughout the vesicle-to-micelle transition without isolating the aqueous medium from the aggregates. The curve described by XOG/Lip versus [OG]H2O shows that the partition coefficient of OG is changing throughout the solubilization process. From this curve, which tends to a value of 1/(critical micellar concentration), five different domains have been delimited: two in the lamellar part of the transition (for 0 < [OG]H2O < 15.6 mM), one in the micellization part, and finally two in the pure micellar region (for 16.5 < [OG]H2O < 21 mM). The first domain in the lamellar part of the transition is characterized by a continuous variation of the partition coefficient. In the second domain, a linear relation relates XOG/Lip and [OG]H2O, indicating the existence of a biphasic domain for which the detergent presents a constant partition coefficient of 18.2 M-1. From the onset to the end of the solubilization process (domain 3), the evolution of (XOG/Lip) with [OG]H2O can be fitted by a model corresponding to the coexistence of detergent-saturated lamellar phase with lipid-saturated mixed micelles, both in equilibrium with an aqueous phase, i.e., a three-phase domain. The micellar region is characterized first by a small two-phase domain (domain 4) with a constant partition coefficient of 21 M-1, followed by a one-phase mixed-micellar domain for which XOG/Lip no longer linearly depends on [OG]H2O. The results are discussed in terms of a phase diagram.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Capes S., Wong P. T. Effects of hydrostatic pressure on the location of PRODAN in lipid bilayers: a FT-IR study. Biochemistry. 1989 Oct 17;28(21):8358–8363. doi: 10.1021/bi00447a014. [DOI] [PubMed] [Google Scholar]
- Chong P. L. Effects of hydrostatic pressure on the location of PRODAN in lipid bilayers and cellular membranes. Biochemistry. 1988 Jan 12;27(1):399–404. doi: 10.1021/bi00401a060. [DOI] [PubMed] [Google Scholar]
- Christie J. D., Rakusan T. A., Martinez M. A., Lucia H. L., Rajaraman S., Edwards S. B., Hayden C. K., Jr Hydranencephaly caused by congenital infection with herpes simplex virus. Pediatr Infect Dis. 1986 Jul-Aug;5(4):473–478. doi: 10.1097/00006454-198607000-00020. [DOI] [PubMed] [Google Scholar]
- Conrad M. J., Singer S. J. The solubility of amphipathic molecules in biological membranes and lipid bilayers and its implications for membrane structure. Biochemistry. 1981 Feb 17;20(4):808–818. doi: 10.1021/bi00507a024. [DOI] [PubMed] [Google Scholar]
- Eidelman O., Blumenthal R., Walter A. Composition of octyl glucoside-phosphatidylcholine mixed micelles. Biochemistry. 1988 Apr 19;27(8):2839–2846. doi: 10.1021/bi00408a027. [DOI] [PubMed] [Google Scholar]
- Jackson M. L., Schmidt C. F., Lichtenberg D., Litman B. J., Albert A. D. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry. 1982 Sep 14;21(19):4576–4582. doi: 10.1021/bi00262a010. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U., le Maire M., Nöel J. P., Gulik-Krzywicki T., Møller J. V. Transitional steps in the solubilization of protein-containing membranes and liposomes by nonionic detergent. Biochemistry. 1993 Feb 16;32(6):1648–1656. doi: 10.1021/bi00057a032. [DOI] [PubMed] [Google Scholar]
- Lasch J., Hoffmann J., Omelyanenko W. G., Klibanov A. A., Torchilin V. P., Binder H., Gawrisch K. Interaction of Triton X-100 and octyl glucoside with liposomal membranes at sublytic and lytic concentrations. Spectroscopic studies. Biochim Biophys Acta. 1990 Feb 28;1022(2):171–180. doi: 10.1016/0005-2736(90)90111-z. [DOI] [PubMed] [Google Scholar]
- Lesieur S., Grabielle-Madelmont C., Paternostre M. T., Ollivon M. Size analysis and stability study of lipid vesicles by high-performance gel exclusion chromatography, turbidity, and dynamic light scattering. Anal Biochem. 1991 Feb 1;192(2):334–343. doi: 10.1016/0003-2697(91)90545-5. [DOI] [PubMed] [Google Scholar]
- Levy D., Gulik A., Seigneuret M., Rigaud J. L. Phospholipid vesicle solubilization and reconstitution by detergents. Symmetrical analysis of the two processes using octaethylene glycol mono-n-dodecyl ether. Biochemistry. 1990 Oct 9;29(40):9480–9488. doi: 10.1021/bi00492a022. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Barenholz Y. Liposomes: preparation, characterization, and preservation. Methods Biochem Anal. 1988;33:337–462. doi: 10.1002/9780470110546.ch7. [DOI] [PubMed] [Google Scholar]
- Livesey A. K., Brochon J. C. Analyzing the distribution of decay constants in pulse-fluorimetry using the maximum entropy method. Biophys J. 1987 Nov;52(5):693–706. doi: 10.1016/S0006-3495(87)83264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor R. B., Weber G. Estimation of the polarity of the protein interior by optical spectroscopy. Nature. 1986 Jan 2;319(6048):70–73. doi: 10.1038/319070a0. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966 Jun;5(6):1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- Meyer O., Ollivon M., Paternostre M. T. Solubilization steps of dark-adapted purple membrane by Triton X-100. A spectroscopic study. FEBS Lett. 1992 Jul 6;305(3):249–253. doi: 10.1016/0014-5793(92)80679-b. [DOI] [PubMed] [Google Scholar]
- Ollivon M., Eidelman O., Blumenthal R., Walter A. Micelle-vesicle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry. 1988 Mar 8;27(5):1695–1703. doi: 10.1021/bi00405a047. [DOI] [PubMed] [Google Scholar]
- Ollivon M., Walter A., Blumenthal R. Sizing and separation of liposomes, biological vesicles, and viruses by high-performance liquid chromatography. Anal Biochem. 1986 Feb 1;152(2):262–274. doi: 10.1016/0003-2697(86)90408-2. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Conti F., Gratton E. Time-resolved fluorescence emission spectra of Laurdan in phospholipid vesicles by multifrequency phase and modulation fluorometry. Cell Mol Biol. 1986;32(1):103–108. [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990 Jun;57(6):1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J. 1994 Jan;66(1):120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternostre M. T., Roux M., Rigaud J. L. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by triton X-100, octyl glucoside, and sodium cholate. Biochemistry. 1988 Apr 19;27(8):2668–2677. doi: 10.1021/bi00408a006. [DOI] [PubMed] [Google Scholar]
- Rigaud J. L., Paternostre M. T., Bluzat A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry. 1988 Apr 19;27(8):2677–2688. doi: 10.1021/bi00408a007. [DOI] [PubMed] [Google Scholar]
- Schubert R., Schmidt K. H. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 1988 Nov 29;27(24):8787–8794. doi: 10.1021/bi00424a015. [DOI] [PubMed] [Google Scholar]
- Seras M., Ollivon M., Edwards K., Lesieur S. Reconstitution of non-ionic monoalkyl amphiphile-cholesterol vesicles by dilution of lipids-octylglucoside mixed micelles. Chem Phys Lipids. 1993 Nov;66(1-2):93–109. doi: 10.1016/0009-3084(93)90035-2. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M. Partition behavior of a nonionic detergent, octyl glucoside, between membrane and water phases, and its effect on membrane permeability. Biochemistry. 1989 Jun 27;28(13):5631–5634. doi: 10.1021/bi00439a044. [DOI] [PubMed] [Google Scholar]
- Urbaneja M. A., Alonso A., Gonzalez-Mañas J. M., Goñi F. M., Partearroyo M. A., Tribout M., Paredes S. Detergent solubilization of phospholipid vesicle. Effect of electric charge. Biochem J. 1990 Sep 1;270(2):305–308. doi: 10.1042/bj2700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson P. K., Talmon Y., Walter A. Vesicle-micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. Biophys J. 1989 Oct;56(4):669–681. doi: 10.1016/S0006-3495(89)82714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A. Membrane solubilization with and reconstitution from surfactant solutions: a comparison of phosphatidylserine and phosphatidylcholine interactions with octyl glucoside. Mol Cell Biochem. 1990 Dec 20;99(2):117–123. doi: 10.1007/BF00230341. [DOI] [PubMed] [Google Scholar]
- Weber G., Farris F. J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979 Jul 10;18(14):3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- da Graça Miguel M., Eidelman O., Ollivon M., Walter A. Temperature dependence of the vesicle-micelle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry. 1989 Oct 31;28(22):8921–8928. doi: 10.1021/bi00448a035. [DOI] [PubMed] [Google Scholar]
- de Foresta B., Merah Z., le Maire M., Champeil P. How to evaluate the distribution of an "invisible" amphiphile between biological membranes and water. Anal Biochem. 1990 Aug 15;189(1):59–67. doi: 10.1016/0003-2697(90)90044-a. [DOI] [PubMed] [Google Scholar]
- del Río E., González-Mañas J. M., Gurtubay J. I., Goñi F. M. On the mechanism of bacteriorhodopsin solubilization by surfactants. Arch Biochem Biophys. 1991 Dec;291(2):300–306. doi: 10.1016/0003-9861(91)90138-9. [DOI] [PubMed] [Google Scholar]
- le Maire M., Møller J. V., Champeil P. Binding of a nonionic detergent to membranes: flip-flop rate and location on the bilayer. Biochemistry. 1987 Jul 28;26(15):4803–4810. doi: 10.1021/bi00389a030. [DOI] [PubMed] [Google Scholar]


