Abstract
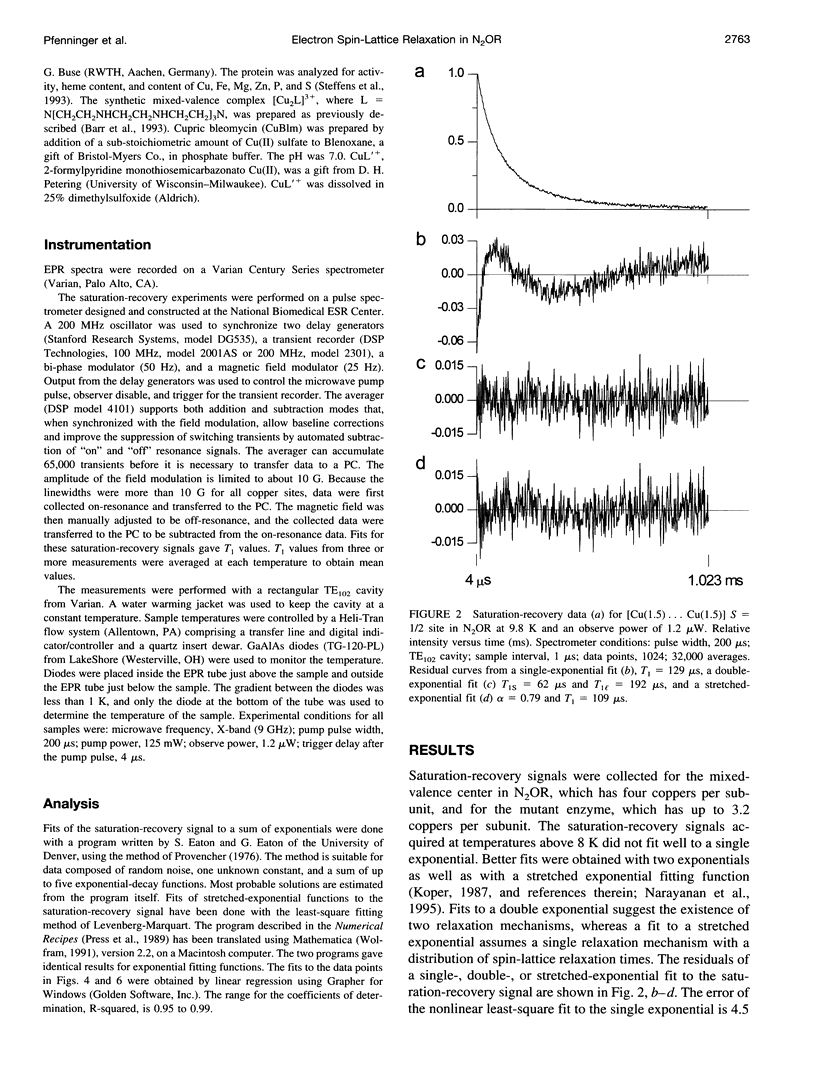
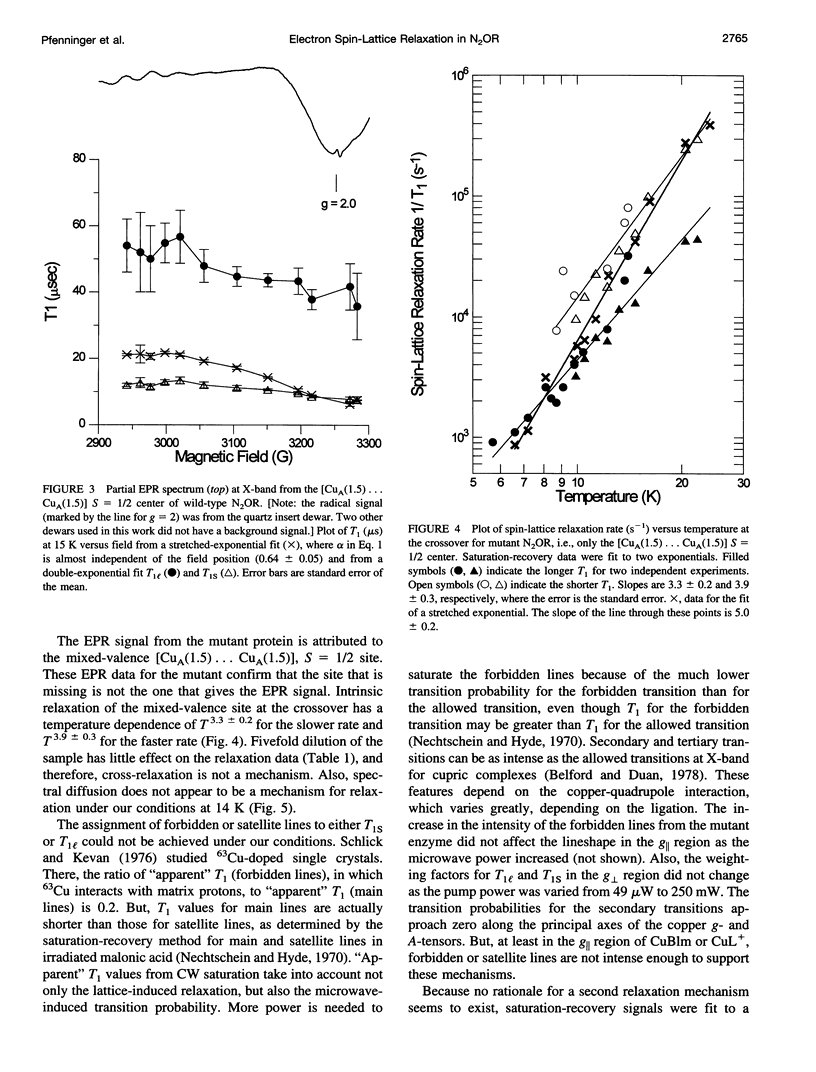
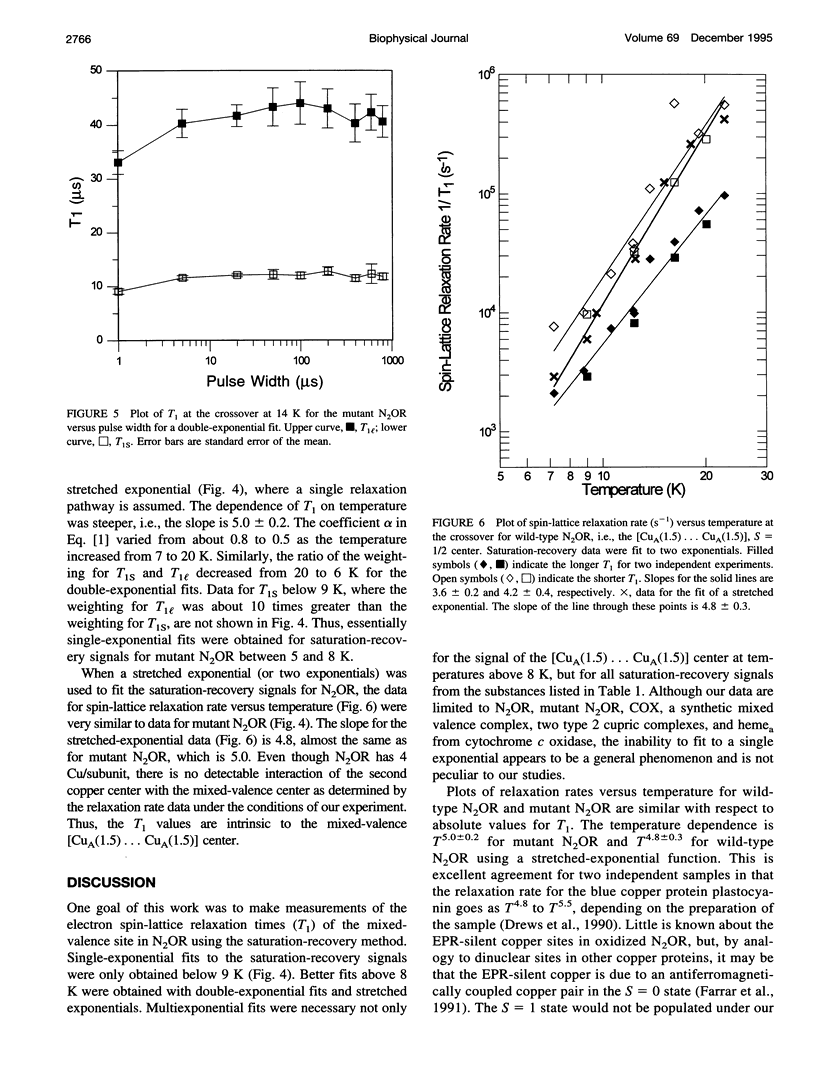
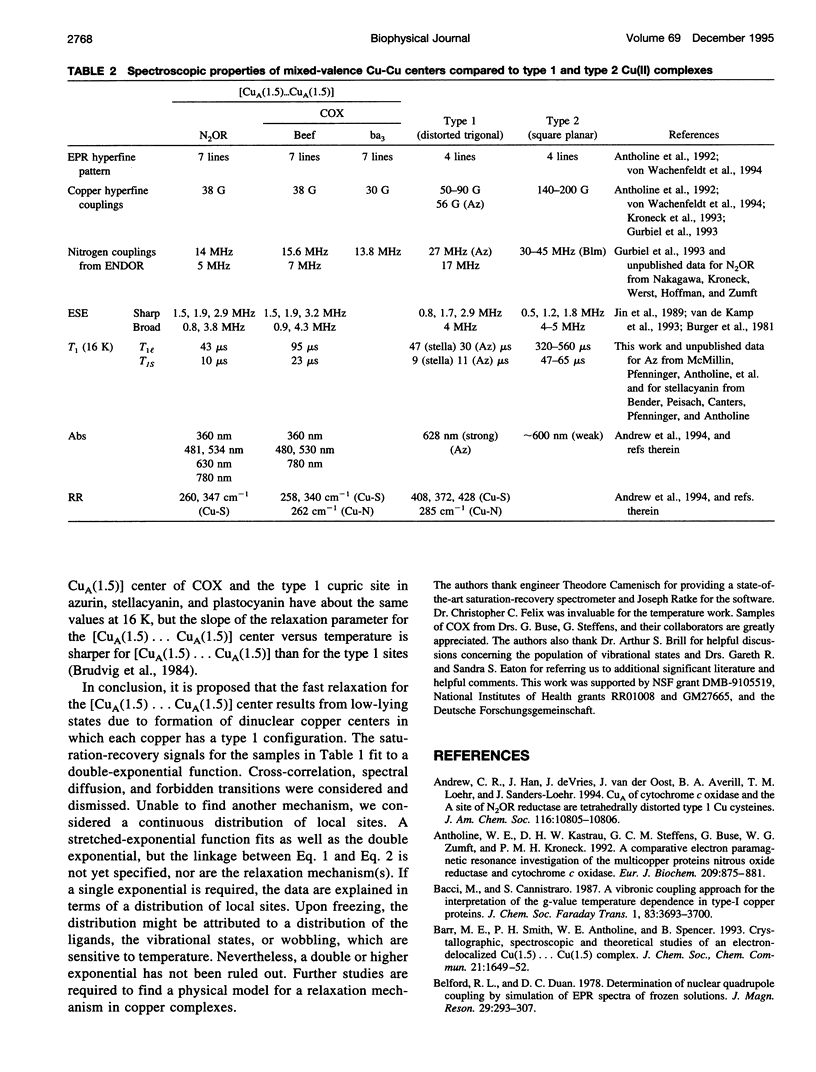
Relaxation times have been obtained with time-domain EPR for the dinuclear mixed valence [CuA(1.5) ... CuA(1.5)[ S = 1/2 center in nitrous oxide reductase, N2OR, from Pseudomonas stutzeri, in the TN5 mutant defective in copper chromophore biosynthesis, in a synthetic mixed valence complex, and in type 1 and 2 copper complexes. Data confirmed that the intrinsic electron spin-lattice relaxation time, T1, for N2OR in the temperature range of 6-25 K is unusually short for copper centers. At best, a twofold increase of T1 from g perpendicular to g parallel was measured. Optimized fits of the saturation-recovery data were obtained using both double-exponential and stretched-exponential functions. The temperature dependence of the spin-lattice relaxation rate of mutant N2OR is about T5.0 with the stretched-exponential model or T3.3 and T3.9 for the model using the sum of two exponentials. These T1s are intrinsic to the mixed valence [CuA(1.5) ... CuA(1.5)] center, and no interaction of the second copper center in wild-type N2OR with the [CuA(1.5) ... CuA(1.5)] center has been observed. The T1 of the mixed valence center of N2OR is not only shorter than for monomeric square planar Cu(II) complexes, but also shorter than for a synthetic mixed valence complex, Cu2(N[CH2CH2NHCH2CH2NHCH2CH2]3N). The short T1 is attributed to the vibrational modes of type 1 copper and/or the metal-metal interaction in [CuA(1.5) ... CuA(1.5)].
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antholine W. E., Kastrau D. H., Steffens G. C., Buse G., Zumft W. G., Kroneck P. M. A comparative EPR investigation of the multicopper proteins nitrous-oxide reductase and cytochrome c oxidase. Eur J Biochem. 1992 Nov 1;209(3):875–881. doi: 10.1111/j.1432-1033.1992.tb17360.x. [DOI] [PubMed] [Google Scholar]
- Blackburn N. J., Barr M. E., Woodruff W. H., van der Oost J., de Vries S. Metal-metal bonding in biology: EXAFS evidence for a 2.5 A copper-copper bond in the CuA center of cytochrome oxidase. Biochemistry. 1994 Aug 30;33(34):10401–10407. doi: 10.1021/bi00200a022. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Blair D. F., Chan S. I. Electron spin relaxation of CuA and cytochrome a in cytochrome c oxidase. Comparison to heme, copper, and sulfur radical complexes. J Biol Chem. 1984 Sep 10;259(17):11001–11009. [PubMed] [Google Scholar]
- Burger R. M., Adler A. D., Horwitz S. B., Mims W. B., Peisach J. Demonstration of nitrogen coordination in metal--bleomycin complexes by electron spin--echo envelope spectroscopy. Biochemistry. 1981 Mar 17;20(6):1701–1704. doi: 10.1021/bi00509a045. [DOI] [PubMed] [Google Scholar]
- Buse G., Steffens G. C. Cytochrome c oxidase in Paracoccus denitrificans. Protein, chemical, structural, and evolutionary aspects. J Bioenerg Biomembr. 1991 Apr;23(2):269–289. doi: 10.1007/BF00762222. [DOI] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Drews A. R., Thayer B. D., Stapleton H. J., Wagner G. C., Giugliarelli G., Cannistraro S. Electron spin relaxation measurements on the blue-copper protein plastocyanin: Deviations from a power law temperature dependence. Biophys J. 1990 Jan;57(1):157–162. doi: 10.1016/S0006-3495(90)82517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. A., Thomson A. J., Cheesman M. R., Dooley D. M., Zumft W. G. A model of the copper centres of nitrous oxide reductase (Pseudomonas stutzeri). Evidence from optical, EPR and MCD spectroscopy. FEBS Lett. 1991 Dec 2;294(1-2):11–15. doi: 10.1016/0014-5793(91)81331-2. [DOI] [PubMed] [Google Scholar]
- Goodman G., Leigh J. S., Jr Distance between the visible copper and cytochrome a in bovine heart cytochrome oxidase. Biochemistry. 1985 Apr 23;24(9):2310–2317. doi: 10.1021/bi00330a028. [DOI] [PubMed] [Google Scholar]
- Hansen A. P., Britt R. D., Klein M. P., Bender C. J., Babcock G. T. ENDOR and ESEEM studies of cytochrome c oxidase: evidence for exchangeable protons at the CuA site. Biochemistry. 1993 Dec 14;32(49):13718–13724. doi: 10.1021/bi00212a042. [DOI] [PubMed] [Google Scholar]
- Iwata S., Ostermeier C., Ludwig B., Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995 Aug 24;376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Kelly M., Lappalainen P., Talbo G., Haltia T., van der Oost J., Saraste M. Two cysteines, two histidines, and one methionine are ligands of a binuclear purple copper center. J Biol Chem. 1993 Aug 5;268(22):16781–16787. [PubMed] [Google Scholar]
- Kroneck P. M., Antholine W. E., Kastrau D. H., Buse G., Steffens G. C., Zumft W. G. Multifrequency EPR evidence for a bimetallic center at the CuA site in cytochrome c oxidase. FEBS Lett. 1990 Jul 30;268(1):274–276. doi: 10.1016/0014-5793(90)81026-k. [DOI] [PubMed] [Google Scholar]
- Mchaourab H. S., Pfenninger S., Antholine W. E., Felix C. C., Hyde J. S., Kroneck P. M. Multiquantum EPR of the mixed valence copper site in nitrous oxide reductase. Biophys J. 1993 May;64(5):1576–1579. doi: 10.1016/S0006-3495(93)81527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riester J., Zumft W. G., Kroneck P. M. Nitrous oxide reductase from Pseudomonas stutzeri. Redox properties and spectroscopic characterization of different forms of the multicopper enzyme. Eur J Biochem. 1989 Jan 2;178(3):751–762. doi: 10.1111/j.1432-1033.1989.tb14506.x. [DOI] [PubMed] [Google Scholar]
- Scholes C. P., Janakiraman R., Taylor H., King T. E. Temperature dependence of the electron spin-lattice relaxation rate from pulsed EPR of CUA and heme a in cytochrome c oxidase. Biophys J. 1984 May;45(5):1027–1030. doi: 10.1016/S0006-3495(84)84248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. C., Biewald R., Buse G. Cytochrome c oxidase is a three-copper, two-heme-A protein. Eur J Biochem. 1987 Apr 15;164(2):295–300. doi: 10.1111/j.1432-1033.1987.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Steffens G. C., Soulimane T., Wolff G., Buse G. Stoichiometry and redox behaviour of metals in cytochrome-c oxidase. Eur J Biochem. 1993 May 1;213(3):1149–1157. doi: 10.1111/j.1432-1033.1993.tb17865.x. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995 Aug 25;269(5227):1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- Van de Kamp M., Canters G. W., Andrew C. R., Sanders-Loehr J., Bender C. J., Peisach J. Effect of lysine ionization on the structure and electrochemical behaviour of the Met44-->Lys mutant of the blue-copper protein azurin from Pseudomonas aeruginosa. Eur J Biochem. 1993 Nov 15;218(1):229–238. doi: 10.1111/j.1432-1033.1993.tb18369.x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Dreusch A., Löchelt S., Cuypers H., Friedrich B., Schneider B. Derived amino acid sequences of the nosZ gene (respiratory N2O reductase) from Alcaligenes eutrophus, Pseudomonas aeruginosa and Pseudomonas stutzeri reveal potential copper-binding residues. Implications for the CuA site of N2O reductase and cytochrome-c oxidase. Eur J Biochem. 1992 Aug 15;208(1):31–40. doi: 10.1111/j.1432-1033.1992.tb17156.x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Viebrock-Sambale A., Braun C. Nitrous oxide reductase from denitrifying Pseudomonas stutzeri. Genes for copper-processing and properties of the deduced products, including a new member of the family of ATP/GTP-binding proteins. Eur J Biochem. 1990 Sep 24;192(3):591–599. doi: 10.1111/j.1432-1033.1990.tb19265.x. [DOI] [PubMed] [Google Scholar]
- von Wachenfeldt C., de Vries S., van der Oost J. The CuAsite of the caa3-type oxidase of Bacillus subtilis is a mixed-valence binuclear copper centre. FEBS Lett. 1994 Feb 28;340(1-2):109–113. doi: 10.1016/0014-5793(94)80182-7. [DOI] [PubMed] [Google Scholar]


