Abstract
Protein kinases are important mediators of much of the signal transduction that occurs in eukaryotic cells. Unfortunately, the identification of protein kinase substrates has proven to be a difficult task, and we generally know few, if any, of the physiologically relevant targets of any particular kinase. Here, we describe a sequence-based approach that simplified this substrate identification process for the cAMP-dependent protein kinase (PKA) in Saccharomyces cerevisiae. In this method, the evolutionary conservation of all PKA consensus sites in the S. cerevisiae proteome was systematically assessed within a group of related yeasts. The basic premise was that a higher degree of conservation would identify those sites that are functional in vivo. This method identified 44 candidate PKA substrates, 5 of which had been described. A phosphorylation analysis showed that all of the identified candidates were phosphorylated by PKA and that the likelihood of phosphorylation was strongly correlated with the degree of target site conservation. Finally, as proof of principle, the activity of one particular target, Atg1, a key regulator of autophagy, was shown to be controlled by PKA phosphorylation in vivo. These data therefore suggest that this evolutionary proteomics approach identified a number of PKA substrates that had not been uncovered by other methods. Moreover, these data show how this approach could be generally used to identify the physiologically relevant occurrences of any protein motif identified in a eukaryotic proteome.
Keywords: Ras proteins, sequence conservation, stationary phase
Protein kinases are key components of signal transduction pathways that regulate many aspects of eukaryotic biology (1, 2). The protein kinase gene family is one of the largest in eukaryotic organisms and typically constitutes almost 2% of all protein-encoding genes (3, 4). In general, these enzymes catalyze the transfer of the terminal phosphate from ATP to the hydroxyl group of particular serine, threonine, or tyrosine residues in a defined set of protein targets. This phosphorylation ultimately alters cell physiology by modifying the activities associated with these substrate proteins. A complete understanding of the biology of any protein kinase therefore requires the identification of the particular substrates of this enzyme. Unfortunately, this identification process is often a difficult and labor-intensive task, and, as a result, we generally know few of the physiologically relevant substrates of any protein kinase (5).
The cAMP-dependent protein kinase (PKA) has been extensively studied and is one of the best understood members of the protein kinase family (6, 7). In Saccharomyces cerevisiae, PKA is a key regulator of cell growth and is regulated largely by the small GTP-binding Ras proteins (8–10). The two Ras proteins, Ras1 and Ras2, bind to adenylyl cyclase and stimulate the production of cAMP (11, 12). This stimulation results in elevated PKA activity and the increased phosphorylation of substrates that are presumably important for cell growth and proliferation (13). Although several PKA substrates have been described, the biological activities of these proteins are not sufficient to explain the global effect that PKA activity has on S. cerevisiae growth.
In the past decade, there has been a tremendous accumulation of DNA sequence information for a wide variety of organisms. One of the major challenges for modern biology is the development of methods to mine the information inherent in these data so as to further our understanding of basic biology and to provide insights into human disease. Comparative analyses between related species is one approach that seems to hold great promise in this pursuit. By comparing the DNA sequence of organisms separated by a range of evolutionary distances, experimenters have been able to identify important features of both entire genomes and individual genes and their protein products (14–18). In this report, we describe a comparative approach that uses sequence information to identify the biologically relevant occurrences of a protein motif of interest. In this approach, the evolutionary conservation of all occurrences of a particular sequence element in the proteome is systematically assessed within a group of related organisms. The underlying premise is that a higher degree of sequence conservation would identify those elements that are functional in vivo (15, 16).
The general utility of this approach was assessed here by examining whether the evolutionary conservation of a consensus phosphorylation site would identify physiologically relevant substrates of a particular protein kinase, PKA, in S. cerevisiae. By comparing the sequences of orthologous proteins present in a series of related budding yeast species, we identified 44 candidate substrates for this protein kinase. A phosphorylation analysis indicated that all of these candidates can be phosphorylated by PKA in vitro and suggested that these proteins might be in vivo targets of this enzyme. A more detailed analysis of one particular target, the autophagy-related protein kinase, Atg1, showed that this protein was phosphorylated and regulated by PKA in vivo. In all, these data demonstrate the general potential this type of a comparative approach has for determining the physiological relevance of any sequence element found in any type of protein.
Methods
Protein Sequence Comparisons. The pattern match program, patmatch, at the Saccharomyces Genome Database (SGD) web site (www.yeastgenome.org) was used to identify the consensus PKA sites present in the S. cerevisiae proteome. The proteins containing these PKA sites were then aligned with their likely orthologs from the other budding yeast species used in this analysis with the blastp and dialign alignment programs. The final sequence alignments were also examined by eye to ensure that no conserved PKA site had been missed. The protein sequences for the five Saccharomyces species used in this analysis were obtained from the web site for the Genome Sequencing Center at Washington University (genome.wustl.edu). The Candida albicans sequences were obtained from the CandidaDB website (www.pasteur.fr/Galar_Fungail/CandidaDB) developed by the Galar Fungail European Consortium. The PKA consensus site used here, R–3-R–2-x–1-S/T-B+1, was deduced from a variety of studies, including work with combinatorial peptide libraries and an analysis of known PKA target sites (19–22). In this site, “x” refers to any amino acid, “B” to a residue with a hydrophobic side chain and the “S/T” to the serine or threonine residue that is the site of phosphate addition. A second consensus site of R–6-x–5-x–4-R–3-x–2-x–1-S/T-B+1 has been identified for mammalian PKA enzymes (23). However, because it is not yet known whether this site is also recognized by the S. cerevisiae enzyme, we have focused on the former consensus site in this study. Also, it should be pointed out that previous studies have indicated that PKA phosphorylation can occur at sequences that differ from both of these potential consensus sites. Such potential targets would also be missed by this analysis.
Alkaline Phosphatase-Based Autophagy Assays. Autophagy levels were measured with an alkaline phosphatase-based assay that has been described (24, 25). Autophagy was induced by transferring cells to a medium that lacks a nitrogen source, SD-N, and alkaline phosphatase levels were assessed after 0 and 15 h at 30°C. SD-N consists of 0.17% yeast nitrogen base lacking amino acids and ammonium sulfate (Difco) and 2% glucose.
Analysis of Protein Phosphorylation. In general, the in vitro phosphorylation assays were performed with GST fusion proteins that were under the control of the GAL1 promoter in the yeast strain, Y258 (MATa his4-580 ura3-52 leu2-3,112 pep4-3) (26). The strains were grown to mid log phase in raffinose medium and transferred to galactose for 4 h at 30°C. The GST fusion proteins were then isolated on glutathione-agarose beads (Pierce) and incubated with 1 μCi (1 Ci = 37 GBq) [γ-32P]ATP (PerkinElmer) and either 0 or 5 units of bovine PKA catalytic subunit (Sigma) as described (27). A Western immunoblot was performed with an α-GST antibody (Cell Signaling Technology, Beverly, MA) to quantify the relative amount of GST fusion protein present.
The Protein A (PrA)-Atg1 fusion proteins were constructed by subcloning PCR fragments encoding Atg1 residues 345–559 into a plasmid, pPHY1044, that contains two repeats of the Ig-binding region of PrA from Staphylococcus aureus (28). The hemagglutinin (HA)-tagged full-length Atg1 and PrA-Atg1 fusion proteins were expressed in the protease-deficient strain, TVY614 (MATα his3-Δ200 leu2-3,112 lys2-801 suc2-Δ9 trp1-101 ura3-52 prc1Δ::HIS3 pep4Δ::LEU2 prb1Δ::hisG) (29). The site-directed mutageneses were performed as described (28, 30, 31). For the in vivo phosphorylation experiments, yeast cells were labeled with [32P]inorganic orthophosphate (32), and the labeled PrA-Atg1 was precipitated as described (27). The PKA-minus strain used for this analysis, NB13-14D (MATa ade8 his3 leu2 trp1 ura3 tpk1::URA3 tpk2::HIS3 tpk3::TRP1 rim15::kanMX2), has been described (33).
Fluorescence Microscopy. The CFP-Atg11, RFP-Atg11, and YFP-Atg1 fusions were under the control of the inducible promoter from the yeast CUP1 gene (34). Expression of these fusion proteins was induced by the addition of 100 μM CuSO4 for 1 h at 30°C. For the starvation experiments, the cells were transferred to SD-N medium containing 100 μM CuSO4 for 1 h at 30°C. The GFP-Atg23 fusion protein has been described (35). The samples were imaged with an Axioplan 2 Imaging E Mot microscope (Zeiss) equipped with a ×100 Plan Neofluar objective (1031-172) and filter sets 31044v2 (CFP), 41017 (Endow GFP), and 41028 (YFP) (Chroma Technology, Rockingham, VT) and model C4742-95-12ERG charge-coupled device (CCD) (Hamamatsu Photonics, Hamamatsu City, Japan). Image processing and contrast enhancement were performed with openlab 3 (Improvision, Lexington, MA) and photoshop (Adobe Systems, San Jose, CA) software.
Results
Assessing the Evolutionary Conservation of the Consensus PKA Sites in the S. cerevisiae Proteome. The comparative analysis used here consisted of two major steps. In the first, a pattern match program was used to identify the S. cerevisiae proteins that contain a consensus PKA phosphorylation site (see Methods). For this study, we used the consensus site, R–3-R–2-x–1-S/T-B+1, that had been defined by previous work with PKA enzymes from a variety of sources, including yeast and humans (19–22). A search of the S. cerevisiae proteome found 553 occurrences of this consensus sequence in 491 proteins (Table 2, which is published as supporting information on the PNAS web site). Fifty-one proteins were found to have multiple sites, with 5 sites being the most present in any one protein.
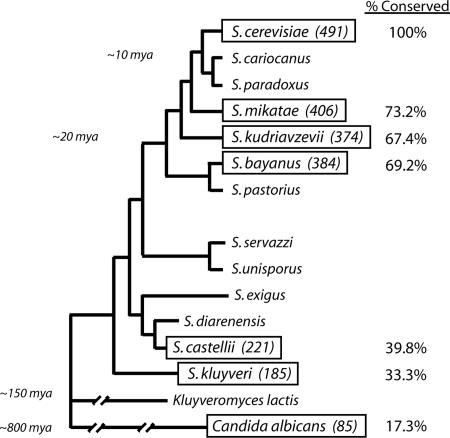
The second stage of this analysis assessed whether these consensus PKA sites were conserved in the likely orthologous proteins present in six different budding yeasts, including five Saccharomyces species that represent the three major subgroups of this genus and the pathogenic yeast, C. albicans (Fig. 1A) (17, 36). The Saccharomyces species used in this analysis were the sensu stricto species S. mikatae, S. kudriavzevii, and S. bayanus, the sensu lato species S. castellii, and the petite-negative species S. kluyveri. It is important to point out that recent work has indicated that PKA activity is regulated, at least in part, by the Ras proteins in C. albicans, the yeast that is most distantly related to S. cerevisiae in this analysis (37, 38). These observations therefore suggest that a Ras/PKA signaling pathway is functional in all of the yeasts being used for this study.
Fig. 1.
An evolutionary proteomics approach identified 85 potential substrates of PKA in S. cerevisiae. The relative number of S. cerevisiae candidates that have PKA sites conserved in the likely orthologous protein present in each of the indicated budding yeast species is shown. The approximate evolutionary distance to S. cerevisiae is shown for several positions on this phylogenetic tree (36, 49).
As expected, this analysis found that the number of conserved PKA sites dropped as the evolutionary distance from S. cerevisiae increased (Fig. 1 A). Only 92 of the original 553 sites (≈17%) present in S. cerevisiae were conserved in the other Saccharomyces species and C. albicans. The 85 proteins that contain these conserved sites are involved in a wide variety of processes important for cell growth (Table 3, which is published as supporting information on the PNAS web site). This observation is consistent with both the highly pleiotropic phenotypes associated with mutations affecting the Ras/PKA pathway and with a model proposing that Ras/PKA signaling activity might be functioning as part of a general growth checkpoint mechanism in S. cerevisiae (9, 10). It is important to point out that many of these 85 proteins are highly conserved among these budding yeasts, and thus it was unclear whether the observed conservation of their PKA sites was significant. Therefore, we limited the subsequent analysis to those candidates that possessed a conserved PKA site in a region exhibiting <50% identity between the S. cerevisiae and C. albicans proteins. This constraint reduced the number of potential PKA substrates to 44 proteins, or <1% of the total proteome (Table 1). Significantly, these candidates included 5 of the best characterized PKA substrates in the S. cerevisiae literature (Table 1).
Table 1. The 44 candidate PKA substrates identified by the evolutionary proteomics approach described here.
| Biological process | Gene name |
|---|---|
| Transport (8) | ATG1, ATG13, ATG18, AVT1, MNR2, MRS6, TOM71, YOL075c |
| Signal transduction (6) | BCY1*, IRA2, IRA1, RIM15*, SYG1, YAK1* |
| RNA pol II transcription (5) | ACA1, CST6, MSN2*, STP1, STP2 |
| Cell size regulation (3) | SFP1, WHI3, WHI4 |
| Chromatin assembly (3) | STB2, STB3, STB6 |
| Cell wall synthesis (2) | SSD1, ECM3 |
| Spindle assembly (2) | DAM1, SPC105 |
| Bud site selection (1) | BUD4 |
| Lipid biosynthesis (1) | CKI1* |
| Protein synthesis (1) | IFH1 |
| RNA pol III transcription (1) | MAF1 |
| Sporulation (1) | MDS3 |
| Unknown (10) | IKS1, PSP1, YBL029w, YBR028c, YHR032w, YJL046w, YLL029w, YLR177w, YLR419w, YPL260w |
The genes encoding the 5 proteins previously shown to be substrates of PKA are indicated with an asterisk. The genes encoding the 23 candidates that were phosphorylated by PKA in this study are underlined.
The Candidates Identified by the Evolutionary Proteomics Approach Were Phosphorylated by PKA in Vitro. The underlying premise in this analysis was that the more highly conserved PKA consensus sites would be correspondingly more likely to represent bona fide PKA phosphorylation sites. To test this hypothesis, we asked whether proteins with highly conserved sites were more likely than other S. cerevisiae proteins to be phosphorylated by PKA in an in vitro assay. Representative proteins from five different groups were examined: proteins lacking a PKA consensus site; proteins with sites only in S. cerevisiae; proteins with sites conserved among the sensu stricto Saccharomyces species; proteins with sites conserved to the sensu lato and petite-negative Saccharomyces species; and proteins with sites conserved to C. albicans.
In general, we found that the likelihood of phosphorylation correlated well with the degree of conservation of the PKA consensus sites. In particular, all of the candidates tested with sites conserved to C. albicans (23/23) were phosphorylated by PKA (Fig. 2 A and E). In contrast, only 20–33% of the proteins containing less conserved sites were labeled in this assay (Fig. 2 B and C). Finally, none of the proteins that lacked a consensus site were efficiently phosphorylated by PKA (Fig. 2D). Thus, the presence of a highly conserved PKA consensus site was a very strong predictor of PKA phosphorylation (Fig. 2E). The striking correlation between the conservation of the PKA site and its tendency to be phosphorylated suggested that the candidates with the most highly conserved sites might all be in vivo targets of PKA. Consistent with this prediction, five of these candidate proteins, Bcy1, Cki1, Msn2, Rim15, and Yak1, have been previously shown to be substrates of this kinase (Table 1) (33, 39–42). Finally, it is important to point out that several proteins with less conserved sites were phosphorylated by PKA in this study and could also be relevant targets of this enzyme in vivo (Fig. 2 B–D). The key point here is that the degree of evolutionary conservation can be used as a predictive tool to assess the likely physiological relevance of a given sequence motif in the proteome.
Fig. 2.
Proteins with highly conserved consensus PKA sites were more likely to be phosphorylated by PKA than proteins with less conserved sites. Shown is a phosphorylation analysis of representative proteins from the following five groups identified by the comparative analysis performed here: (i) proteins with consensus PKA sites conserved to C. albicans (A); (ii) proteins with sites conserved to the sensu lato and petite-negative Saccharomyces species; (iii) proteins with sites conserved amongst the sensu stricto Saccharomyces species (B); (iv) proteins with PKA sites in S. cerevisiae only (C); (v) proteins with no consensus PKA sites (D). For this analysis, the amount of label incorporated into a full-length GST fusion protein from [γ-32P]ATP was assessed with an in vitro assay performed in the presence or absence of PKA as described in Methods (26, 27). Note that the labeled protein bands have been appropriately lined up to facilitate comparisons between samples. Western immunoblots were performed with an α-GST antibody to quantify the relative amount of fusion protein present. (E) A summary graph indicating the percentage of candidates in each of the above groups that was phosphorylated by PKA.
The Autophagy-Related Protein Kinase, Atg1, Is a Substrate of PKA. As a proof of principle, we tested whether the function of one particular candidate was indeed regulated by PKA phosphorylation in vivo. For this analysis, we chose to examine the autophagy-related protein kinase, Atg1. Autophagy is a highly conserved, membrane-trafficking pathway responsible for much of the protein and membrane turnover in eukaryotic cells (43, 44). Atg1 is a serine/threonine-specific protein kinase that is a key regulator of the initial induction stage of this degradative pathway (43, 45). Interestingly, three proteins important for autophagy, Atg1, Atg13 and Atg18, were all identified in this study as potential substrates for PKA. These observations suggested that the Ras/PKA pathway was regulating autophagic activity in yeast cells, and recent work from our lab has confirmed this possibility (Fig. 3A) (25).
Fig. 3.
The autophagy-related protein kinase, Atg1, is a substrate of PKA. (A) Elevated levels of Ras/PKA signaling activity inhibited autophagy. Autophagy levels were assessed with an alkaline phosphatase-based assay that has been described (24). The values shown represent the difference between the alkaline phosphatase levels found in starved and nonstarved cultures of the indicated yeast strains. HC-TPK1, high-copy plasmid encoding a catalytic subunit of PKA. (B) The full-length Atg1 was phosphorylated by PKA in vitro at the serine residues within the two consensus PKA sites. The residues at positions 508 and 515 within the two PKA sites are indicated: S, serine; A, alanine. Note that this experiment was performed with a kinase-inactive variant of Atg1, Atg1-K54A, to avoid the background autophosphorylation signal. (Lower) A Western immunoblot control indicating the relative levels of Atg1 present in each kinase reaction. (C and D) The in vitro (C) and in vivo (D) phosphorylation of an Atg1 fusion protein depended upon both PKA activity and the two PKA sites in Atg1. This fusion protein contained two repeats of the Ig-binding region of protein A fused in frame to residues 345–559 of Atg1 (28). In both cases, the Upper panel is the phosphorylation assay and the Lower is the Western immunoblot control. For the in vivo experiments, yeast cultures were incubated with [32P]orthophosphate, and the amount of label incorporated into the PrA-Atg1 fusion proteins was assessed by autoradiography. The pkaΔ strain lacks all three catalytic subunits of the S. cerevisiae PKA enzyme (33).
Atg1 has two conserved sequences, R-R-P-S508-L and R-R-L-S515-I, that conform to the PKA consensus site discussed above. Interestingly, we found that the full-length Atg1 was phosphorylated in vitro by PKA and that this phosphorylation required the presence of the serine residues in these two consensus sites (Figs. 3B and 5, which is published as supporting information on the PNAS web site). The ability of PKA to phosphorylate an Atg1 fusion protein in vitro was also dependent upon these same serine residues (Fig. 3C). Finally, the in vivo phosphorylation of this Atg1 fusion protein also required the presence of the two PKA consensus sites and PKA activity (Fig. 3D). In mutants lacking all three of the PKA catalytic subunits, Atg1 was not phosphorylated appreciably at Ser-508 or Ser-515. In all, these data indicated that Atg1 was likely a bona fide substrate for PKA in S. cerevisiae.
PKA Phosphorylation Regulates the Association of Atg1 with the Preautophagosomal Structure (PAS). Two observations indicated that Atg1 protein kinase activity was not regulated by PKA phosphorylation. First, the loss of the consensus PKA sites had no significant effect on Atg1 protein kinase activity in vitro in assays measuring either autophosphorylation or the phosphorylation of an exogenous substrate (Fig. 6A, which is published as supporting information on the PNAS web site). Second, the in vivo level of Atg1 autophosphorylation was not affected by alterations of the two PKA sites in this protein (Fig. 6B). Thus, PKA phosphorylation must be affecting some other aspect of Atg1 function in vivo. Because previous work had indicated that the subcellular localization of Atg1 is influenced by nutrient availability, we tested whether PKA might be regulating this facet of Atg1 behavior.
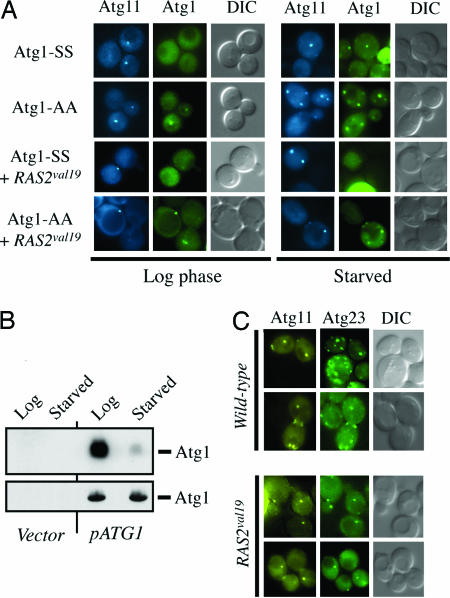
Atg1 is predominantly cytoplasmic in dividing cells but is recruited to a specialized site, known as the PAS, upon the induction of autophagy (34, 46) (Fig. 4A). The PAS is thought to be the site of autophagosome formation; the autophagosome is the double-membrane intermediate responsible for transporting bulk cytoplasm to the vacuole/lysosome during autophagy (34, 43, 46). We therefore tested whether PKA phosphorylation might control the association of Atg1 with the PAS. In support of this possibility, we found that an Atg1 variant lacking both PKA sites, Atg1-AA, was constitutively localized to the PAS (Fig. 4A). In addition, the introduction of a constitutively active allele of RAS2, known as RAS2val19, blocked Atg1 localization to the PAS in a manner that depended upon the two PKA sites in Atg1 (Fig. 4A). In the RAS2val19 mutant, wild-type Atg1 was not found at the PAS in either growing or starved cells. PKA phosphorylation therefore seems to control the recruitment of Atg1 to the PAS during nutrient limitation, conditions that normally induce the autophagy pathway.
Fig. 4.
PKA phosphorylation regulates the association of Atg1 with the preautophagosomal structure, or PAS. (A) A fluorescence microscopy analysis of the subcellular localization of a YFP-Atg1 fusion protein in either log-phase or starved cultures of the indicated strains. The location of the PAS is shown by a CFP-Atg11 reporter construct (34). (B) The level of Atg1 phosphorylation decreased upon nitrogen starvation. Wild-type cells containing either a control vector (Vector) or a plasmid encoding a Protein A-Atg1 fusion protein (pATG1) were incubated with [32P]orthophosphate either before (Log) or after (Starved) a 2-h incubation in a medium lacking nitrogen. The amount of radiolabel incorporated into Atg1 was assessed by autoradiography. (Lower) The Western immunoblot control. (C) The presence of the RAS2val19 allele resulted in the redistribution of a GFP-Atg23 fusion protein from a number of punctate structures within the cell to the PAS. The identity of these punctate structures is not yet known. The location of the PAS is indicated by an RFP-Atg11 fusion protein.
The data here suggest that, when nutrients are plentiful, Ras/PKA signaling levels are high and Atg1 is phosphorylated and largely cytoplasmic. Upon nutrient deprivation, Atg1 would become dephosphorylated and associate with the PAS. This model is consistent with previous work indicating that PKA activity decreases upon nutrient limitation (47). We tested the basic tenet of this model by examining the in vivo phosphorylation of an Atg1 fusion protein in dividing and starved cells. The phosphorylation of this fusion is completely dependent upon the presence of the PKA consensus sites and PKA activity (see above). Consistent with the model, we found that the relative level of Atg1 phosphorylation decreased by >10-fold after a period of nitrogen starvation (Fig. 4B).
We also tested whether the presence of the RAS2val19 allele had any effect on the subcellular localization of Atg23. Atg23 is a peripheral membrane protein that has been shown to cycle between the PAS and unknown structures in the cytoplasm in an Atg1-dependent manner (35). In wild-type cells, an Atg23-GFP fusion is found associated with a number of punctate foci in cells, some that correspond to the PAS and others that do not (48) (Fig. 4C). In contrast, in atg1 mutants, Atg23 is found at the PAS only and not with the other punctate structures seen in wild-type cells (35). These results have been interpreted as evidence that Atg1 is required for the recycling of Atg23 from the PAS. Consistent with a role for the Ras/PKA pathway in the regulation of this Atg1 activity, we found that Atg23 was also restricted to the PAS in RAS2val19 mutants (Fig. 4C). Because Atg1 (and its regulatory partner, Atg13) is the only Atg protein known to be required for the proper recycling of Atg23, these data reinforced the above phosphorylation analysis and suggested that the Ras/PKA pathway was regulating Atg1 activity in S. cerevisiae cells.
Discussion
This report makes use of a sequence-based, comparative method for finding functionally relevant sequences within a eukaryotic proteome. In this approach, the evolutionary conservation of all occurrences of a given sequence motif is systematically assessed within a group of related organisms. The underlying premise is that the physiologically relevant motifs would be distinguished by a higher degree of sequence conservation. The general utility of this approach was assessed here with an attempt to identify candidate substrates for the protein kinase, PKA, in S. cerevisiae. In this study, the conservation of consensus PKA target sites was assessed within a group of budding yeast species that are separated by up to 800 million years of evolutionary distance (49). This analysis identified 44 proteins as potential substrates of PKA, and, remarkably, we found that all of the candidates tested were phosphorylated by this enzyme in an in vitro assay. Moreover, our data indicated that the degree of sequence conservation was a very strong predictor of the likelihood of PKA phosphorylation. The more highly conserved the PKA site, the more likely it was to be phosphorylated by PKA. In all, the data suggested that this evolutionary proteomics approach had successfully identified a number of novel substrates of the S. cerevisiae PKA.
Similar types of comparative approaches have been used extensively to identify functional motifs in individual proteins. However, this study emphasizes how this strategy can be systematically applied over an entire proteome. In this case, we were examining a short sequence motif that was relatively low in information content. Nonetheless, we were able to successfully identify proteins that had been previously shown to be targets of PKA and many additional candidate substrates. In theory, this approach should be applicable to any type of sequence element in any protein. In fact, structure-function studies of proteins often identify similarly short, but biologically interesting, sequence domains. This study demonstrates how the biological relevance of these elements can be assessed. The only prerequisite is that there must be sequence information available for an appropriate group of evolutionarily related organisms.
The power of these comparative approaches is illustrated by the fact that more potential PKA substrates were identified here than had been found in the past two decades by other means. Although further work is obviously needed to show that these proteins are indeed regulated by PKA phosphorylation, this candidate pool already contains five previously identified substrates of PKA. The work presented here adds to this list by showing that the autophagy-related kinase, Atg1, is both phosphorylated and regulated by PKA in vivo. Interestingly, the activities of several of the other candidates have already been linked to the Ras/PKA signaling pathway. For example, a recent study has suggested that Ras/PKA pathway regulates the activity of Ifh1 and Sfp1, two key regulators of ribosome synthesis and cell size in S. cerevisiae (50, 51). Finally, it is important to point out that the proteins identified here are equally likely to be substrates for PKA in the pathogenic yeast, C. albicans. This possibility is especially interesting in light of recent work indicating that PKA activity is important for virulence in this yeast (37, 38).
This study identified three proteins important for autophagy, Atg1, Atg13 and Atg18, as potential substrates for PKA. These observations led us to investigate the role of the Ras/PKA signaling pathway in the control of this degradative process. Our work indicates that Ras/PKA activity inhibits an early step in autophagy, a step that precedes the formation of the autophagosome (25). Interestingly, all three of these Atg proteins seem to act at this stage of the autophagy process (43, 52, 53). In this study, we found that the PKA phosphorylation of Atg1 regulates the localization of this protein in accordance with nutrient availability and ensures that Atg1 is associated with the PAS only under conditions that result in the induction of autophagy. Because most Atg proteins are present constitutively at the PAS, we feel that this PKA phosphorylation is likely indirectly influencing Atg1 protein kinase activity by regulating its ability to associate with its most likely substrates in the cell. Finally, it is important to point out that Atg1 does not seem to be the only PKA target important for the control of autophagy. This assertion follows from our observation that the hyperactive RAS2val19 allele still inhibits autophagy in mutants carrying the Atg1-AA variant (our unpublished observations). It will be important to test whether Atg13 and/or Atg18 might be these other relevant targets of PKA phosphorylation in vivo.
In summary, this report describes a systematic, sequence-based approach that uses a basic tenet of the theory of natural selection to identify the functionally relevant occurrences of a given sequence element in a eukaryotic proteome. The success we had identifying potential substrates of the S. cerevisiae PKA illustrates the potential inherent in this general strategy. In addition to identifying autophagy as an important target of the Ras/PKA pathway, the other substrates identified here are likely to provide fundamental insights into the manner in which this signaling pathway controls the growth of these budding yeasts. Finally, further comparisons between the consensus sites that are phosphorylated by PKA and those that are not could identify additional sequence elements that are important for PKA substrate recognition.
Supplementary Material
Acknowledgments
We thank Russell Hill for the use of his fluorescence microscope and his assistance with the imaging software; Daniel Klionsky (University of Michigan, Ann Arbor), Yoshinori Ohsumi (National Institute for Basic Biology, Okazaki, Japan), Steven Osmani (Ohio State University), and Jonathan Warner (Albert Einstein College of Medicine, Bronx, NY) for plasmids and strains; Michael Snyder (Yale University, New Haven, CT) for the yeast GST-ORF fusion library; and Susie Howard for comments on the manuscript. This work was supported by National Institutes of Health Grant GM65227 (to P.K.H.).
Author contributions: Y.V.B., J.S.S., S.J.D., and P.K.H. designed research; Y.V.B., J.S.S., and S.J.D. performed research; Y.V.B., J.S.S., S.J.D., and P.K.H. contributed new reagents/analytic tools; Y.V.B., J.S.S., S.J.D., and P.K.H. analyzed data; and Y.V.B., J.S.S., and P.K.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PKA, cAMP-dependent protein kinase; PAS, pre-autophagosomal structure; PrA, protein A.
References
- 1.Cohen, P. (2002) Nat. Rev. Drug Discov. 1, 309–315. [DOI] [PubMed] [Google Scholar]
- 2.Hunter, T. (2000) Cell 100, 113–127. [DOI] [PubMed] [Google Scholar]
- 3.Hunter, T. (1987) Cell 50, 823–829. [DOI] [PubMed] [Google Scholar]
- 4.Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. (2002) Science 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- 5.Manning, B. D. & Cantley, L. C. (2002) Sci. STKE 162, 1–4. [DOI] [PubMed] [Google Scholar]
- 6.Taylor, S. S., Yang, J., Wu, J., Haste, N. M., Radzio-Andzelm, E. & Anand, G. (2004) Biochim. Biophys. Acta 1697, 259–269. [DOI] [PubMed] [Google Scholar]
- 7.Taylor, S. S., Buechler, J. A. & Yonemoto, W. (1990) Annu. Rev. Biochem. 59, 971–1005. [DOI] [PubMed] [Google Scholar]
- 8.Broach, J. R. (1991) Trends Genet. 7, 28–33. [DOI] [PubMed] [Google Scholar]
- 9.Herman, P. K. (2002) Curr. Opin. Microbiol. 5, 602–607. [DOI] [PubMed] [Google Scholar]
- 10.Thevelein, J. M. & de Winde, J. H. (1999) Mol. Microbiol. 33, 904–918. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki, N., Choe, H. R., Nishida, Y., Yamawaki-Kataoka, Y., Ohnishi, S., Tamaoki, T. & Kataoka, T. (1990) Proc. Natl. Acad. Sci. USA 87, 8711–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field, J., Xu, H. P., Michaeli, T., Ballester, R., Sass, P., Wigler, M. & Colicelli, J. (1990) Science 247, 464–467. [DOI] [PubMed] [Google Scholar]
- 13.Toda, T., Cameron, S., Sass, P., Zoller, M. & Wigler, M. (1987) Cell 50, 277–287. [DOI] [PubMed] [Google Scholar]
- 14.Cliften, P. F., Hillier, L. W., Fulton, L., Graves, T., Miner, T., Gish, W. R., Waterston, R. H. & Johnston, M. (2001) Genome Res. 11, 1175–1186. [DOI] [PubMed] [Google Scholar]
- 15.Tagle, D. A., Koop, B. F., Goodman, M., Slightom, J. L., Hess, D. L. & Jones, R. T. (1988) J. Mol. Biol. 203, 439–455. [DOI] [PubMed] [Google Scholar]
- 16.Hardison, R. C., Oeltjen, J. & Miller, W. (1997) Genome Res. 7, 959–966. [DOI] [PubMed] [Google Scholar]
- 17.Cliften, P., Sudarsanam, P., Desikan, A., Fulton, L., Fulton, B., Majors, J., Waterston, R., Cohen, B. A. & Johnston, M. (2003) Science 301, 71–76. [DOI] [PubMed] [Google Scholar]
- 18.Rubin, G. M., Yandell, M. D., Wortman, J. R., Gabor Miklos, G. L., Nelson, C. R., Hariharan, I. K., Fortini, M. E., Li, P. W., Apweiler, R., Fleischmann, W., et al. (2000) Science 287, 2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis, C. L., Kemp, B. E. & Zoller, M. J. (1991) J. Biol. Chem. 266, 17932–17935. [PubMed] [Google Scholar]
- 20.Songyang, Z., Blechner, S., Hoagland, N., Hoekstra, M. F., Piwnica-Worms, H. & Cantley, L. C. (1994) Curr. Biol. 4, 973–982. [DOI] [PubMed] [Google Scholar]
- 21.Tegge, W., Frank, R., Hofmann, F. & Dostmann, W. R. (1995) Biochemistry 34, 10569–10577. [DOI] [PubMed] [Google Scholar]
- 22.Shabb, J. B. (2001) Chem. Rev. 101, 2381–2411. [DOI] [PubMed] [Google Scholar]
- 23.Smith, C. M., Radzio-Andzelm, E., Madhusudan, Akamine, P. & Taylor, S. S. (1999) Prog. Biophys. Mol. Biol. 71, 313–341. [DOI] [PubMed] [Google Scholar]
- 24.Noda, T., Matsuura, A., Wada, Y. & Ohsumi, Y. (1995) Biochem. Biophys. Res. Commun. 210, 126–132. [DOI] [PubMed] [Google Scholar]
- 25.Budovskaya, Y. V., Stephan, J. S., Reggiori, F., Klionsky, D. J. & Herman, P. K. (2004) J. Biol. Chem. 279, 20663–20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, H., Bilgin, M., Bangham, R., Hall, D., Casamayor, A., Bertone, P., Lan, N., Jansen, R., Bidlingmaier, S., Houfek, T., et al. (2001) Science 293, 2101–2105. [DOI] [PubMed] [Google Scholar]
- 27.Chang, Y. W., Howard, S. C. & Herman, P. K. (2004) Mol. Cell 15, 107–116. [DOI] [PubMed] [Google Scholar]
- 28.Budovskaya, Y. V., Hama, H., DeWald, D. B. & Herman, P. K. (2002) J. Biol. Chem. 277, 287–294. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt, B., Kordas, T. J., Thompson, C. M., Patel, P. & Vida, T. (1998) J. Biol. Chem. 273, 15818–15829. [DOI] [PubMed] [Google Scholar]
- 30.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K. (1995) Current Protocols in Molecular Biology (Wiley, New York).
- 31.Kunkel, T. A. (1985) Proc. Natl. Acad. Sci. USA 82, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman, P. K., Stack, J. H. & Emr, S. D. (1991) EMBO J. 10, 4049–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinders, A., Burckert, N., Boller, T., Wiemken, A. & De Virgilio, C. (1998) Genes Dev. 12, 2943–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, J., Huang, W. P., Stromhaug, P. E. & Klionsky, D. J. (2002) J. Biol. Chem. 277, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reggiori, F., Tucker, K. A., Stromhaug, P. E. & Klionsky, D. J. (2004) Dev. Cell 6, 79–90. [DOI] [PubMed] [Google Scholar]
- 36.Barnett, J. A. (1992) Yeast 8, 1–23.1496857 [Google Scholar]
- 37.Rocha, C. R., Schroppel, K., Harcus, D., Marcil, A., Dignard, D., Taylor, B. N., Thomas, D. Y., Whiteway, M. & Leberer, E. (2001) Mol. Biol. Cell 12, 3631–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leberer, E., Harcus, D., Dignard, D., Johnson, L., Ushinsky, S., Thomas, D. Y. & Schroppel, K. (2001) Mol. Microbiol. 42, 673–687. [DOI] [PubMed] [Google Scholar]
- 39.Kuret, J., Johnson, K. E., Nicolette, C. & Zoller, M. J. (1988) J. Biol. Chem. 263, 9149–9154. [PubMed] [Google Scholar]
- 40.Kim, K. H. & Carman, G. M. (1999) J. Biol. Chem. 274, 9531–9538. [DOI] [PubMed] [Google Scholar]
- 41.Gorner, W., Durchschlag, E., Wolf, J., Brown, E. L., Ammerer, G., Ruis, H. & Schuller, C. (2002) EMBO J. 21, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrett, S., Menold, M. M. & Broach, J. R. (1991) Mol. Cell. Biol. 11, 4045–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noda, T., Suzuki, K. & Ohsumi, Y. (2002) Trends Cell Biol. 12, 231–235. [DOI] [PubMed] [Google Scholar]
- 44.Reggiori, F. & Klionsky, D. J. (2002) Eukaryot. Cell 1, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuura, A., Tsukada, M., Wada, Y. & Ohsumi, Y. (1997) Gene 192, 245–250. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki, K., Kirisako, T., Kamada, Y., Mizushima, N., Noda, T. & Ohsumi, Y. (2001) EMBO J. 20, 5971–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell, M., Bradshaw-Rouse, J., Markwardt, D. & Heideman, W. (1993) Mol. Biol. Cell 4, 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucker, K. A., Reggiori, F., Dunn, W. A., Jr. & Klionsky, D. J. (2003) J. Biol. Chem. 278, 48445–48452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heckman, D. S., Geiser, D. M., Eidell, B. R., Stauffer, R. L., Kardos, N. L. & Hedges, S. B. (2001) Science 293, 1129–1133. [DOI] [PubMed] [Google Scholar]
- 50.Jorgensen, P., Rupes, I., Sharom, J. R., Schneper, L., Broach, J. R. & Tyers, M. (2004) Genes Dev. 18, 2491–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin, D. E., Soulard, A. & Hall, M. N. (2004) Cell 119, 969–979. [DOI] [PubMed] [Google Scholar]
- 52.Tsukada, M. & Ohsumi, Y. (1993) FEBS Lett. 333, 169–174. [DOI] [PubMed] [Google Scholar]
- 53.Guan, J., Stromhaug, P. E., George, M. D., Habibzadegah-Tari, P., Bevan, A., Dunn, W. A., Jr. & Klionsky, D. J. (2001) Mol. Biol. Cell 12, 3821–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.