Abstract
Human embryonic stem cells have the potential to differentiate into various cell types and, thus, may be useful as a source of cells for transplantation or tissue engineering. We describe here the differentiation steps of human embryonic stem cells into endothelial cells forming vascular-like structures. The human embryonic-derived endothelial cells were isolated by using platelet endothelial cell-adhesion molecule-1 (PECAM1) antibodies, their behavior was characterized in vitro and in vivo, and their potential in tissue engineering was examined. We show that the isolated embryonic PECAM1+ cells, grown in culture, display characteristics similar to vessel endothelium. The cells express endothelial cell markers in a pattern similar to human umbilical vein endothelial cells, their junctions are correctly organized, and they have high metabolism of acetylated low-density lipoprotein. In addition, the cells are able to differentiate and form tube-like structures when cultured on matrigel. In vivo, when transplanted into SCID mice, the cells appeared to form microvessels containing mouse blood cells. With further studies, these cells could provide a source of human endothelial cells that could be beneficial for potential applications such as engineering new blood vessels, endothelial cell transplantation into the heart for myocardial regeneration, and induction of angiogenesis for treatment of regional ischemia.
Human vascular endothelial cells are important for developing engineered vessels for treatment of vascular disease (1) and also may be useful for augmenting vessel growth to areas of ischemic tissue or after implantation (2). A potential source of cells for these applications are embryonic stem cells which, in murine systems, were shown to differentiate into endothelial cells that form vascular structures in a process called vasculogenesis (3). Vasculogenesis is defined as the in situ assembly of capillaries from undifferentiated endothelial cells, as opposed to angiogenesis, the sprouting of capillaries from preexisting blood vessels (4). The vasculogenic potential of the embryonic cells could be of use specifically in tissue engineering for the induction of tissue vascularization. Early endothelial progenitor cells isolated from differentiating mouse embryonic stem cells were shown to give rise to three blood vessel cell components, hematopoietic, endothelial, and smooth muscle cells (5). In addition, it was recently shown that endothelial progenitors and embryonic endothelial cells could differentiate into beating cardiomyocytes when cocultured with neonatal cardiomyocytes or when injected near a damaged heart area (6). It also has been shown that embryonic endothelial cells are critical for the earliest stages of liver and pancreas organogenesis (7, 8). Therefore, in addition to potential clinical applications, purified human embryonic endothelial cells could be important for studying early human development and the differentiation of embryonic stem cells into various tissues.
Differentiation of embryonic stem cells into endothelial cells and the formation of vessel structure have been studied extensively in murine embryogenesis, including maturation steps, molecular events, and growth factor involvement (9–11). However, lack of experimental cell systems has made it difficult to study these developmental processes in the human until now. Human embryonic stem cell lines (hES) recently established from the inner cell mass of human blastocytes (12) provide a unique system for studying these events in human embryonic development. hES cells have the potential to generate all embryonic cell lineages when they undergo differentiation (13). Differentiation of hES can be induced by removing the cells from their feeder layer and growing them in suspension. This differentiation in suspension results in aggregation of the cells and formation of embryoid bodies (EBs), in which successive differentiation steps occur (14).
In the present study, we show an increase in the expression of several endothelial cell-specific genes during EB differentiation, reaching a maximum between days 13–15, and development of extensive vasculature-resembling structures within the EB. We isolated human embryonic endothelial cells from day 13–15 EBs by using platelet endothelial cell-adhesion molecule-1 (PECAM1) antibodies and characterized their behavior in vitro and in vivo.
Materials and Methods
Cell Culture.
hES cells (H9 clone) were grown on mouse embryonic fibroblasts (Cell Essential, Boston, MA) in knockout medium, as described (14). Tissue culture plates were coated with 0.1% gelatin (Sigma). Cultures were grown in 5% CO2 and were routinely passaged every 5–6 days after disaggregating with 1 mg/ml collagenase type IV (GIBCO/BRL). To induce formation of EBs, hES colonies were digested by using either 1 mg/ml collagenase type IV or trypsin/EDTA (0.1%/1 mM) and transferred to Petri dishes to allow their aggregation and prevent adherence to the plate. Human EBs were grown in the same culture medium without lymphocyte inhibitory factor (LIF) and basic fibroblast growth factor (bFGF). Isolated PECAM1+ cells were grown on plates coated with 1% gelatin in endothelial growth medium, EGM-2 (Clonetics, San Diego) and passaged by using 0.025%/0.01% trypsin/EDTA (Clonetics). HUVEC cells (Clonetics) were grown on regular tissue culture plates in EGM-2 medium. For matrigel differentiation assay, cells removed from confluent culture by trypsin treatment were seeded in matrigel-coated 35-mm plates (BD Biosciences, Bedford, MA) at a concentration of 1 × 105 cells per 300 μl of culture medium. After 30 min of incubation at 37°C, 1 ml of medium was added. Cord formation was evaluated by contrast-phase microscopy 24 h or 3 days after seeding the cells.
Reverse Transcription (RT)-PCR Analysis.
Total RNAs from undifferentiating hES cells and from EBs were isolated by using RNEasy Mini Kit (Qiagen, Chatsworth, CA). RT-PCR was performed by using Qiagen OneStep RT-PCR kit with the addition of 10 units RNase inhibitor (GIBCO/BRL) and 40 ng RNA. To ensure semi-quantitative results of the RT-PCR assays, the number of PCR cycles for each set of primers was checked to be in the linear range of the amplification. In addition, all RNA samples were adjusted to yield equal amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Primer sequences, reaction conditions, and optimal cycle numbers are published as supporting information on the PNAS web site, www.pnas.org.
The amplified products were separated on 1.2% agarose gels with ethidium bromide (E-Gel, Invitrogen). For each time point, mean pixel intensities of each band were measured and normalized to mean pixel intensities of the GAPDH band. The values for three experiments then were averaged and graphed with SD.
Immunochemical Reagents and Procedures.
For staining, EBs were transferred to gelatin-coated cover slips with medium containing 10% (vol/vol) FBS. After attachment to the cover slips, EBs or cells grown on gelatin-coated cover slips were fixed with methanol for 5 min at −20°C or with 3% (wt/vol) paraformaldehyde at room temperature and stained for 30 min with the relevant primary antibodies: anti-human PECAM1, anti-human vinculin (Sigma), anti-human von Willebrand factor (vWF, Dako), purified monoclonal anti-N-cadherin (15), and anti-human VE-cad (7B4; ref. 16). The secondary antibodies were Cy3-labeled goat anti-mouse IgG (Jackson ImmunoResearch) and AlexaFluor goat anti-rabbit IgG (Molecular Probes). In some cases, cells or EBs also were stained with 4′,6-diamidino-2-phenylindole (DAPI) and FITC-phalloidin (Sigma). After the indirect immunolabeling, cells were mounted in Floromount-G (Southern Biotechnology) and were examined with either a conventional fluorescence microscope (Nikon) or Zeiss LSM 510 confocal microscope.
For uptake of Dill-labeled ac-LDL, PECAM1+ cells and control PECAM− cells were incubated with 10 μg/ml Dill-labeled ac-LDL (Biomedical Technologies, Stoughton, MA) for 4 h at 37°C. After incubation, cells were washed three times with PBS, fixed with 3% (wt/vol) paraformaldehyde for 30 min, and visualized with a fluorescent microscope (Nikon).
For immunohistology, tissues sections were deparaffinized, blocked with sniper (Biocare Medical, Walnut Creek, CA) for 5 min and stained by using Vector ABC or ARK (DAB) kits with 2-h incubation with the antibodies. The antibodies used include anti-human PECAM1, anti-human vWF (DAKO), and anti-human CD34 (Lab Vision, Fremont, CA).
Flow Cytometry.
For isolation of PECAM1+ cells, EBs at days 13–15 were dissociated with 0.025%/0.01% trypsin/EDTA, washed with PBS containing 5% (vol/vol) FBS, and incubated for 30 min with fluorescent-labeled PECAM1 antibodies (30884X, PharMingen) on ice. Fluorescent-labeled cells were isolated by using a FACStar flow cytometry cell sorter (Becton Dickinson) and plated on 1% gelatin-coated plates with endothelial cell growth medium (Clonetics). For analysis of endothelial cell markers, PECAM1+ cells (grown in culture for six passages) and HUVEC cells were dissociated by using cell dissociation buffer (GIBCO/BRL) and washed with PBS containing 5% (vol/vol) FBS. The cells were incubated with either isotype control (mouse IgG1κ, PharMingen) or antigen-specific antibodies: PECAM1-FITC (PharMingen), CD34-FITC (AC136, Miltenyi Biotec, Auburn, CA) and Flk-1/VEGFR-2-PE (ImClone Systems, New York). Cells were analyzed live (without fixation) by using propidium iodide to exclude dead cells on a FACScan with CELLQUEST software.
Electron Microscopy.
Cells seeded in Matrigel-coated 35 mm plates were fixed for 1 hr in 2.5% (wt/vol) glutaraldehyde, 3% (wt/vol) paraformaldehyde, and 7.5% (wt/vol) sucrose in 0.1 M sodium cacodylate buffer (pH 7.4) and then post-fixed in 1% (wt/vol) OsO4 in veronal-acetate buffer for 1 h. The cells were stained en bloc overnight with 0.5% uranyl acetate in veronal-acetate buffer (pH 6.0), dehydrated, and embedded in Spurrs resin. Sections were cut on a Reichert Ultracut E at a thickness of 70 nm with a diamond knife. Sections were examined with a Phillips EM410 electron microscope.
Biodegradable Polymer Matrix.
Porous sponges composed of poly-(l-lactic acid) (PLLA) and polylactic-glycolic acid (PLGA) were fabricated mainly as described (17). Briefly, PLLA (Polysciences) and PLGA (Boehringer Ingelheim) 1:1 were dissolved in chloroform to yield a solution of 5% (wt/vol) polymer; 0.24 ml of this solution was loaded into molds packed with 0.4 g of sodium chloride particles. The solvent was allowed to evaporate, and the sponges were subsequently immersed for 8 h in distilled water (changed every hour) to leach the salt and create an interconnected pore structure. The sponges, which had an average pore diameter of 250 μm, were cut to 0.5 × 4 × 5 mm3. Before transplantation, sponges were soaked in 70% (vol/vol) ethyl alcohol overnight and washed three times with PBS.
Transplantation into SCID Mice.
PECAM1+ cells (1 × 106) were resuspended in 50 μl of 1:1 mix of culture medium and matrigel (BD Biosciences) and allowed to absorb into the PLLA/PLGA polymer sponges. After a 30-min incubation at 37°C to allow for gelation of matrigel, the cells plus scaffolds were implanted s.c. in the dorsal region of 4-week-old SCID mice (CB.17.SCID, Taconic Farms). After transplantation (7 or 14 days), the implants were retrieved, fixed overnight in 10% (vol/vol) buffered formalin at 4°C, embedded in paraffin, and sectioned for histological examination.
Results
Endothelial Gene Expression During hEB Differentiation.
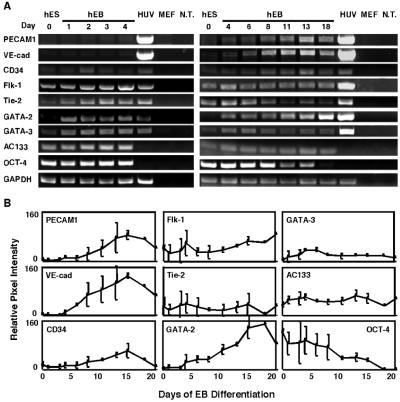
To isolate endothelial cells from hES cells, we first characterized their vasculogenic potential by analyzing the expression of endothelial-specific genes and proteins during hES differentiation. Spontaneous in vitro differentiation of H9 hES cells (12) into endothelial cells was investigated after removing undifferentiated cells from their mouse embryonic fibroblast (MEF) feeder layer and placing them into Petri dishes with culture medium lacking LIF and bFGF for induction of EB formation. At different time points during the differentiation process, the cultured hEBs were collected, and RNA was extracted for analysis of endothelial-related gene expression by using RT-PCR. The genes analyzed included endothelial cell adhesion molecules such as PECAM1/CD31 (18), vascular endothelial-cadherin (VE-cad; ref. 16) and CD34 (19); growth factor receptors such as vascular endothelial growth factor receptor-2 (VEGFR-2/Flk-1/KDR; ref. 20) and Tie-2 (21); transcription factors GATA-2 and GATA-3 (22); and AC133/CD133 a cell surface marker of vascular/hematopoietic stem and progenitor cells (23). As shown in Fig. 1, the levels of endothelial markers PECAM1, VE-cad, and CD34 increased during EB differentiation, reaching a maximum at days 13–15 and indicating a differentiation process toward endothelial cells. GATA-2 was expressed earlier and rose dramatically toward day 18. Unlike the mice system, the VEGF receptor Flk-1 is expressed in undifferentiated cells (also reported recently by Kaufman et al. in H1 line, ref. 24), and increased very slightly during differentiation. The tyrosine kinase receptor Tie-2 and the transcription factors GATA-3 also are expressed in hES cells, and their expression increased during the first 6 days of EB differentiation and then decreased (Fig. 1 A and B). AC133 is expressed in undifferentiated cells as well as in differentiated EB cells in a pattern similar to that of Flk-1. The levels of Oct-4, which is known to be expressed in undifferentiated cells (25), served as a control. Oct-4 expression shows the undifferentiated stage of the cells at day 0 as it is expressed in the cells in high levels. Oct-4 expression subsequently goes down, indicating that the differentiation process is proceeding in the EBs. HUVEC cells were used as a positive control for the expression of the various human endothelial genes. The MEF feeder layer cells were used as a negative control and did not express any of the human-specific genes examined. These data demonstrate an increase in expression of several endothelial cell genes during EB differentiation, reaching a maximum at days 13–15 (Fig. 1 A and B). Some genes were expressed in the undifferentiated cells in either high levels (Flk-1, AC133, Tie-2) or low levels (GATA-3, CD34), and others became notable after EB formation and differentiation (PECAM1, VE-cad, GATA-2; Fig. 1 A and B).
Figure 1.
Endothelial gene expression in hES-derived EBs by RT-PCR analysis. (A) RNA was isolated from undifferentiated hES cells and from hEBs at different time points (days) during differentiation and subjected to RT-PCR analysis. The negative controls, no template (N.T.) and MEF, and the HUVEC positive control (HUV) are shown to the right. (B) Quantitative analysis of gene expression. Relative pixel intensity corresponds to gene expression level; for each time point, mean pixel intensities of each band were measured and normalized to mean pixel intensities of GAPDH band. The results shown are mean values ±SD of three different experiments.
Formation of Vessel-Like Structure in Differentiating hEBs.
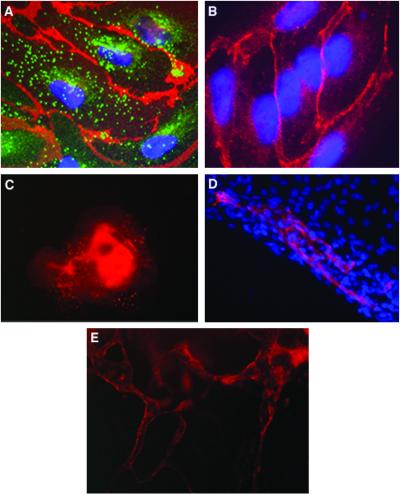
Analysis of endothelial-specific protein expression in day-13 EBs indicated that all EBs had defined cell areas expressing PECAM1 (Fig. 2C). Further analysis of PECAM1+ cells, with various endothelial specific proteins, indicated that these cells are endothelial-like-expressing PECAM1 and VE-cad adhesion molecules at cell–cell adhesion sites and vWF in large granules dispersed throughout the cytoplasm (Fig. 2 A and B). Within these EBs, the endothelial cells were not found as single cells but in groups organized in specific channel-like structures (Fig. 2 D and E), showing that hES cells cultivated as EBs spontaneously differentiate to endothelial cells and blood vessel-like structures.
Figure 2.
Expression of endothelial cell markers in vessel-like structure within hEBs. (A) EBs at day 13 stained with human PECAM1 antibodies (red), vWF antibodies (green), and DAPI for nuclear staining (blue). PECAM1 is organized at cell–cell junctions, whereas vWF is found in organelles in the cytoplasm. (B) EB cells stained with human VE-cadherin antibodies (red) and DAPI (blue) (magnification, ×1,000). (C) Low magnification (×100) of EB stained with PECAM1 antibodies. (D) Areas of PECAM1+ cells (red) within part of an EB, organized in elongated clusters. Cell nuclei stained with DAPI (blue) (magnification, ×400). (E) Channels forming PECAM1+ cells within a 13-day-old EB (magnification, ×200).
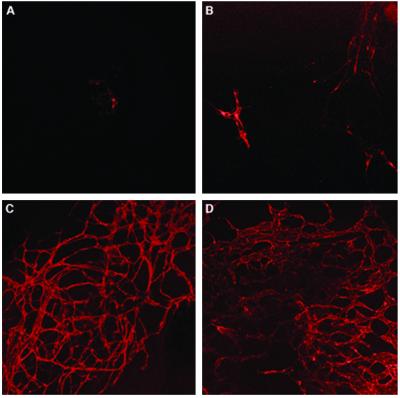
To study this vascularization-like process further, EBs at different time points were stained with PECAM1 antibodies and analyzed with confocal microscopy. Fig. 3 demonstrates that the capillary area increased during subsequent maturation steps up to day 13. On day 4, PECAM1+ cells were observed in a low percentage of the EBs and concentrated in small cell clusters (Fig. 3A). From day 6 onward, some sprouting of endothelial structures that resembled capillaries became evident (Fig. 3B). From day 10 onward, 100% of EBs contained extended areas of network-like capillary structures (Fig. 3C). The positive area was larger at day 13, and the network structure became more complex (Fig. 3D). The time course of cell differentiation and the development of extensive vasculature-resembling structures within the EB correlates with the RT-PCR analysis that demonstrates the subsequent increase in RNA levels of the endothelial genes PECAM1, VECAD, CD34, reaching a maximum between day 13–15 (Fig. 1).
Figure 3.
Confocal microscopy of EBs stained for PECAM1 showing three-dimensional network formations, vascular-like channels. (A) 4-day-old EB. (B) 6-day-old EB. (C) 10-day-old EB. (D) 13-day-old EB. Notice the intensive and complicated vascular network developed at day 10 of 13-day-old EB. (magnification, ×100).
Endothelial Cells Derived from hEBs.
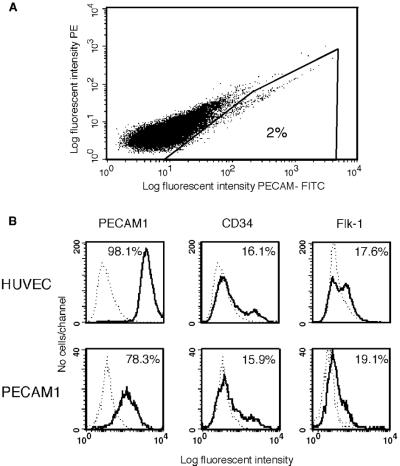
Based on the analysis of endothelial gene and protein expression, we determined the method and time point at which to isolate human embryonic endothelial cells. We decided to use antibodies against PECAM1 for the isolation, as PECAM1 has been shown as the definitive marker for mice embryonic endothelial cells (26), and, in human EBs, is expressed in vessel-like structures in correlation with VE-cad and vWF expression (Figs. 2 and 3), suggesting that it could serve as a marker for human embryonic-endothelial cells as well. EBs at day 13 were dissociated, stained with fluorescent-labeled anti-PECAM1 antibodies, and the PECAM1+ cells (2%) were sorted by using flow cytometry (Fig. 4A). To confirm an endothelial-like phenotype of PECAM1+ cells grown in culture, we assayed them for the expression of endothelial cell markers. Isolated PECAM1+ cells (after several passages in culture) and HUVEC cells were incubated with fluorescent-labeled antibodies and analyzed by fluorescence-activated cell sorter (FACS, Becton Dickinson). Fig. 4B shows that the expression profile of CD34 and Flk-1 in isolated PECAM1+ cells is similar to the HUVEC cells. Expression of PECAM1 also is comparable but with higher expression in the HUVEC cells (98%) compared with PECAM1+ isolated cells (78%). In addition to FACS analysis, we studied the distribution of adhesion molecules by immunofluorescence microscopy. PECAM1+ cells appear to present a correct organization of endothelial junctions (27); N-cadherin and the endothelium-specific VE-cadherin are distributed at adherent-type junctions (Fig. 5 C and D), a class of cell adhesions characterized by their interaction with the actin microfilament system. Actin stress fibers are found throughout the cells and end in both the cell–cell adherence junctions and focal contacts, as seen by double staining with vinculin (Fig. 5E). The tight-junction component, PECAM1, is distributed at the intercellular clefts, and the endothelial marker vWF is highly expressed in the cytoplasm (Fig. 5 A and B).
Figure 4.
Isolation of endothelial cells from human embryoid bodies by using fluorescent-labeled anti-PECAM1 antibodies and analysis of the sorted cells. (A) EBs at day 13 were dissociated and incubated with PECAM1 antibodies. Fluorescent-labeled cells were isolated by using a flow cytometry cell sorter. (B) Flow cytometric analysis of endothelial cell markers in PECAM1+ cells grown in culture for six passages and HUVEC cells. The cells were dissociated and incubated with either isotype control (dashed lines) or antigen-specific antibodies, as indicated (solid lines). Percent positive cells are shown.
Figure 5.
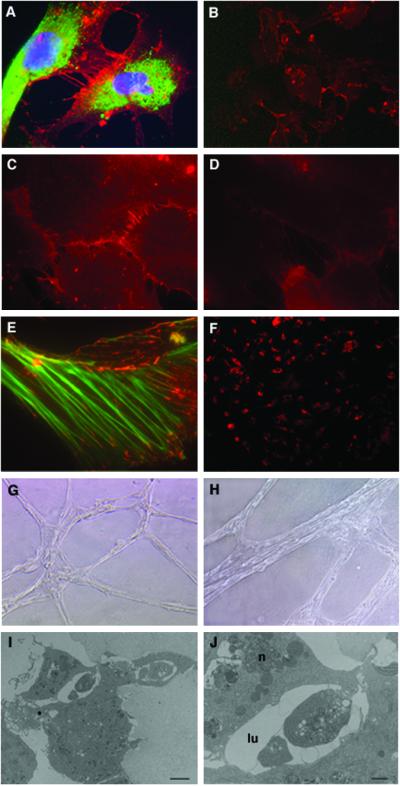
Characterization of hES-derived endothelial cells grown in culture. (A) Immunofluorescence staining of PECAM1 (red) at cell–cell junctions and vWF (green) in the cytoplasm. The nuclei are stained with DAPI (blue). (B) Cells stained for PECAM1. (C) N-cadherin and (D) VE-cadherin staining in cell–cell adherent junctions. (E) Double staining for vinculin (red) and actin (green). Vinculin is found in both focal contacts and cell–cell adherent junctions where it associates with actin stress fiber ends (magnification: A and C–E, ×1,000; B, ×200). (F) Uptake of Dill-labeled ac-LDL by PECAM1+ cells. (G and H) Cords formation by PECAM1+ cells 24 h (G) or 3 days (H) after seeding the cells in matrigel (magnification: G, ×100; H, ×200). (I) Electron microscopy of the cord cross-section showing lumen formation (bar = 2 μm) and (J) higher magnification of the lumen (lu) area showing cell–cell interactions closing the lumen and the nucleus (n) of one cell (bar = 8 μm).
Take-up of ac-LDL has been used to characterize endothelial cells (28). To evaluate whether embryonic-derived PECAM1+ cells are able to incorporate ac-LDL, cells were incubated with Dill-ac-LDL and subsequently examined by fluorescence microscopy. As shown in Fig. 5F, embryonic-derived PECAM1+ cells were brightly fluorescent, whereas the fluorescence intensity of PECAM1 cells was at background levels.
The characteristics of human embryonic PECAM1+ cells also were assessed by culture in matrigel, an extracellular matrix basement membrane that can be used to promote differentiation of endothelial cells (29). When PECAM1+ cells were cultured on matrigel, they were able to spontaneously reorganize in cord-like structures when maintained in culture for several days (Fig. 5 G and H). Electron microscopy analysis of the cord cross section indicated that the cords have a lumen (Fig. 5 I and J), suggesting that the cells have the capacity to differentiate and form tube-like structures under suitable conditions.
Transplantation of PECAM+ Cells into SCID Mice.
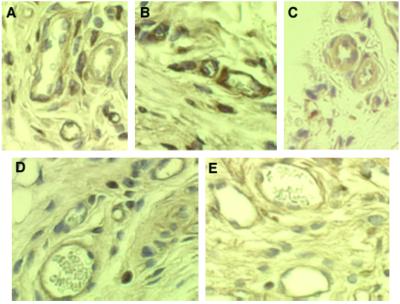
To analyze the therapeutic potential of hES-derived endothelial cells, we studied their behavior in vivo. The cells were seeded on highly porous PLLA/PLGA biodegradable polymer scaffolds, commonly used as scaffolds for tissue engineering (30). Sponges seeded with embryonic-derived PECAM+ cells were implanted in the s.c. tissue of SCID mice. At the time of implant retrieval (up to 14 days), no signs of infection were detected, and inflammation was minimal. Implants maintained in mice for at least 7 days became encapsulated by fibrous connective tissue that was permeated by mouse blood vessels. Histological examination with antibodies that are human specific and do not react with mice microvessels show microvessels that are immunoreactive with human PECAM1 and CD34 (Fig. 6 A–E). Some of these human-positive vessels had mouse blood cells in their lumen, suggesting that microvessels had formed and anastomosed with the mouse vasculature, becoming functional blood-carrying microvessels.
Figure 6.
Transplantation of embryonic endothelial cells (PECAM1+) in SCID mice. PECAM1+ cells were seeded onto PLLA/PLGA polymer scaffolds as described in Materials and Methods. The cells plus scaffolds were implanted s.c. in the dorsal region of 4-week-old SCID mice. (A–C) Immunoperoxidase (brown) staining of 7-day implants with anti-human PECAM1 antibodies and (D and E) of 14-day implants with anti-human CD34 antibodies, showing microvessels that are immunoreactive with these human-specific antibodies. Some of these human-positive microvessels have mouse blood cells in their lumen (magnification, ×400).
Discussion
This study indicates that human ES cells, when induced to form EBs, can spontaneously differentiate into the endothelial lineage, ultimately forming vascular structures. Our data demonstrate an increase in expression of several endothelial cell genes during EB differentiation, reaching a maximum at days 13–15. Some genes were expressed in undifferentiated cells in either high levels (Flk-1, AC133, Tie-2) or lower levels (GATA-3, CD34), and others (PECAM1, VE-cad, GATA-2) became notable after EB formation and differentiation. In the mouse, these genes are not expressed in ES (or expressed in very low levels that disappear by day 1 as EBs are formed, e.g., PECAM1 and Tie-2) and start to appear only around day 3 and later. (Flk-1 at day 2–3; PECAM and Tie–2 at day 4; VE-cad and Tie-1 at day 5; refs. 3 and 31). Mouse and human ES cells differ in morphology, population doubling time, and growth factor requirements. Undifferentiated mouse cells, for example, can be maintained as undifferentiated cells independently of feeder layer if growth factors such as LIF are added to the media (32). However, human cells will differentiate if grown without feeder layer or feeder layer-conditioned medium even in the presence of LIF (12, 33). Thus, different mechanisms of response to LIF and LIF removal between mouse and human ES cells may affect differences in gene expression patterns observed in the transition from the undifferentiated to the differentiated stage of the cells. It is possible that gene expression of endothelial markers in undifferentiated hES cells can be related to the “escape” of some cells from the undifferentiated stage of hES cells or to different basic definitions (regarding gene expression) of the undifferentiated state of hES cells kept in current culture conditions. However, because of significant differences between early human and mouse development and differences in the behavior of mouse and human ES cells, the pattern of human endothelial gene expression shown here might indicate differences in the mechanism of embryonic endothelial differentiation. Our preliminary results indicate that growth factor cocktails (including bFGF and VEGF) known to induce endothelial differentiation in mice EBs do not have the same effect on hEBs (data not shown), pointing again to potential differences between the two systems in the molecular mechanism underlying this process, and emphasizing the need to analyze developmental processes by using human systems.
The assembly of developing vascular-like structures could be observed during EBs outgrowth, as soon as the cells acquired the set of endothelial markers. The data also indicate that the capillary area in the EBs increased during subsequent maturation steps up to day 13, starting from cell clusters that later sprout into capillary-like structures and eventually become organized in a network-like arrangement. The increase in RNA expression of PECAM1, CD34, VE-cad, and GATA-2 genes during EB differentiation correlates with the observed increase in the number of endothelial cells expressing PECAM1 and VE-cad proteins, as demonstrated by antibody staining of differentiating EBs (Figs. 2 and 3). Antibody staining also indicates that at different stages of maturation, most markers seem to be coexpressed by the same cells. These data demonstrate that human ES cells, similar to mice ES cells, can spontaneously differentiate and organize in vitro in vessel-like structures in a pattern that resembles embryonic vascularization.
In the present study, we isolated and maintained in culture endothelial cells derived from hES cells differentiated in vitro. PECAM1 antibodies have been used in the mouse system for isolation of endothelial cells (34). The procedure used to obtain a pure culture of endothelial cells from hES is relatively simple and allowed us to culture large numbers of human embryonic endothelial cells that can be grown in culture without losing endothelial characteristics.
The isolation of human embryonic endothelial cells has potential therapeutic implications, including cell transplantation for repair of ischemic tissues and tissue engineering of vascular grafts. Recently, several studies demonstrated the use of adult endothelial progenitor cells for such applications (2, 35). It would be important to examine the efficiency of embryonic stem cells in such applications, because ES cells can be expanded without apparent limit (36), and ES cell-derived cells could be created in virtually unlimited amounts and available for potential clinical use. Another area in which embryonic endothelial cells can potentially be beneficial is the engineering of complex tissues where vascularization of the regenerating tissue is essential. Several approaches are now being developed to vascularize engineered tissue in vitro before transplantation (37, 38). Vascularization in vitro is important to enable cell viability during tissue growth, induce structural organization, and promote integration upon implantation. Because the formation of the first capillaries takes place mostly during early stages of embryogenesis when endothelial cells are generated from precursor cells (39), isolated human embryonic endothelial cells or progenitor cells can be critical for such applications. Future studies should include further analysis of the molecular mechanism underlying endothelial differentiation and vasculogenesis during hEB differentiation and investigation of growth factor involvement in this process. In addition, efforts to identify and isolate early embryonic progenitors are important to provide tools for elucidating regulatory elements in vasculogenesis and to potentially shed light on vasculogenic and angiogenic mechanisms involved in pathological situations affecting the vascular system.
Supplementary Material
Acknowledgments
We thank Nicki Watson for excellent assistance in preparation of the confocal and electron microscopy images, Robert Marini and Massachusetts Institute of Technology Division of Comparative Medicine for help with animal procedures and histology, and the Massachusetts Institute of Technology Flow Cytometry Unit. We thank Erin Lavik for her help in scaffold preparations. S.L. wishes to thank the European Molecular Biology Organization for a postdoctoral-fellowship.
Abbreviations
- hES
human embryonic stem cells
- EB
embryoid bodies
- PECAM1
platelet endothelial cell adhesion molecule-1
- HUVEC
human umbilical vain endothelial
- LIF
lymphocyte inhibitory factor
- RT-PCR
reverse transcription–PCR
- vWF
von Willebrand factor
- VE-cad
vascular endothelial-cadherin
References
- 1.Niklason L E, Gao J, Abbott W M, Hirschi K K, Houser S, Marini R, Langer R. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto A, Gwon H C, Iwaguro H, Yamaguchi J I, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner J M, Asahara T. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 3.Vittet D, Prandini M H, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 4.Yancopoulos G D, Klagsbrun M, Folkman J. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Nature (London) 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 6.Condorelli G, Borello U, De Angelis L, Latronico M, Sirabella D, Coletta M, Galli R, Balconi G, Follenzi A, Frati G, et al. Proc Natl Acad Sci USA. 2001;98:10733–10738. doi: 10.1073/pnas.191217898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto K, Yoshitomi H, Rossant J, Zaret K S. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 8.Lammert E, Cleaver O, Melton D. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 9.Keller G M. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 10.Darland D C, D'Amore P A. Curr Top Dev Biol. 2001;52:107–149. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- 11.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N. Blood. 1999;93:1253–1263. [PubMed] [Google Scholar]
- 12.Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 13.Odorico J S, Kaufman D S, Thomson J A. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 14.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 15.Volk T, Geiger B. J Cell Biol. 1986;103:1451–1464. doi: 10.1083/jcb.103.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampugnani M G, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco L P, Dejana E. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooney D J, Sano K, Kaufmann P M, Majahod K, Schloo B, Vacanti J P, Langer R. J Biomed Mater Res. 1997;37:413–420. doi: 10.1002/(sici)1097-4636(19971205)37:3<413::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.DeLisser H M, Newman P J, Albelda S M. Immunol Today. 1994;15:490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 19.Young P E, Baumhueter S, Lasky L A. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- 20.Yamaguchi T P, Dumont D J, Conlon R A, Breitman M L, Rossant J. Development (Cambridge) 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 21.Sato T N, Qin Y, Kozak C A, Audus K L. Proc Natl Acad Sci USA. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss M J, Orkin S H. Exp Hematol (Charlottesville, VA) 1995;23:99–107. [PubMed] [Google Scholar]
- 23.Peichev M, Naiyer A J, Pereira D, Zhu Z, Lane W J, Williams M, Oz M C, Hicklin D J, Witte L, Moore M A, Rafii S. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 24.Kaufman D S, Hanson E T, Lewis R L, Auerbach R, Thomson J A. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeom Y I, Fuhrmann G, Ovitt C E, Brehm A, Ohbo K, Gross M, Hubner K, Scholer H R. Development (Cambridge) 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 26.Vecchi A, Garlanda C, Lampugnani M G, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, et al. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- 27.Ayalon O, Sabanai H, Lampugnani M G, Dejana E, Geiger B. J Cell Biol. 1994;126:247–258. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voyta J C, Via D P, Butterfield C E, Zetter B R. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant D S, Lelkes P I, Fukuda K, Kleinman H K. In Vitro Cell Dev Biol Anim. 1991;27A:327–336. doi: 10.1007/BF02630910. [DOI] [PubMed] [Google Scholar]
- 30.Putnam A J, Mooney D J. Nat Med. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 31.Robertson S M, Kennedy M, Shannon J M, Keller G. Development (Cambridge) 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Inokuma M S, Denham J, Golds K, Kundu P, Gold J D, Carpenter M K. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 34.Balconi G, Spagnuolo R, Dejana E. Arterioscler Thromb Vasc Biol. 2000;20:1443–1451. doi: 10.1161/01.atv.20.6.1443. [DOI] [PubMed] [Google Scholar]
- 35.Kaushal S, Amiel G E, Guleserian K J, Shapira O M, Perry T, Sutherland F W, Rabkin E, Moran A M, Schoen F J, Atala A, et al. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amit M, Carpenter M K, Inokuma M S, Chiu C P, Harris C P, Waknitz M A, Itskovitz-Eldor J, Thomson J A. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 37.Black A F, Berthod F, L'Heureux N, Germain L, Auger F A. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 38.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa E R, Ravens M, Pien H, Cunningham B, Vacanti J P. Tissue Eng. 2000;6:105–117. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 39.Flamme I, Frolich T, Risau W. J Cell Physiol. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.