Abstract
Hepatic expression of the scavenger receptor class B type I (SR-BI) promotes selective uptake of HDL cholesterol by the liver and is believed to play a role in the process of reverse cholesterol transport (RCT). We hypothesized that hepatic SR-BI expression is a regulator of the rate of integrated macrophage-to-feces RCT and used an in vivo model to test this hypothesis. Cholesterol-loaded and [3H]cholesterol-labeled J774 macrophages were injected intraperitoneally into mice, after which the appearance of the [3H]cholesterol in the plasma, liver, and feces over 48 hours was quantitated. Mice overexpressing SR-BI in the liver had significantly reduced [3H]cholesterol in the plasma but markedly increased [3H] tracer excretion in the feces over 48 hours. Conversely, mice deficient in SR-BI had significantly increased [3H]cholesterol in the plasma but markedly reduced [3H] tracer excretion in the feces over 48 hours. These studies demonstrate that hepatic SR-BI expression, despite its inverse effects on steady-state plasma HDL cholesterol concentrations, is an important positive regulator of the rate of macrophage RCT.
Introduction
Plasma levels of HDL cholesterol (HDL-C) and its major protein apoA-I are inversely associated with the risk of atherosclerotic vascular disease. One mechanism by which HDL and apoA-I protect against atherosclerosis is probably by promoting reverse cholesterol transport (RCT) from macrophages to the liver, bile, and eventually feces (1, 2). RCT involves multiple steps, beginning with the efflux of unesterified cholesterol (UC) from macrophages to lipid-poor apolipoproteins as well as mature HDL (3). Some HDL UC is esterified by the HDL-associated enzyme lecithin-cholesterol acyltransferase to form cholesteryl ester (CE). Both HDL UC and CE can be selectively taken up by the liver via a process mediated by the hepatic scavenger receptor class B type I (SR-BI) (4, 5).
The relationship of hepatic SR-BI expression to HDL-C levels and atherosclerosis is paradoxical in light of human epidemiologic data. Hepatic overexpression of SR-BI in mice dramatically reduces plasma HDL-C levels (6, 7) but also reduces atherosclerosis (8–10). Conversely, gene deletion or attenuation of SR-BI in mice results in substantially increased HDL-C levels (11, 12) but markedly increased atherosclerosis (13–15). One possible explanation for these surprising results is that hepatic SR-BI expression is a positive regulator of the rate of RCT from macrophage to liver, bile, and feces, but this has never been proven. We developed an approach to trace reverse transport of labeled cholesterol specifically from macrophages to the liver and feces in vivo and showed that apoA-I overexpression promoted the macrophage-specific RCT in vivo (16). Here we utilize this approach to prove that modulation of hepatic SR-BI expression directly regulates the rate of macrophage RCT in vivo.
Results
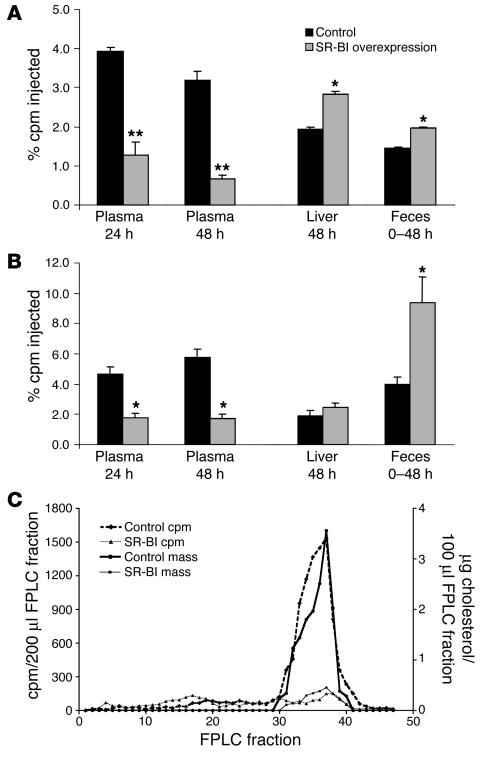
Hepatic overexpression of SR-BI in wild-type mice, compared with controls, reduced plasma levels of HDL-C (16 ± 14 vs. 55 ± 9 mg/dl). Macrophage-derived [3H]cholesterol in plasma at both 24 hours and 48 hours was also significantly lower in the mice overexpressing hepatic SR-BI compared with control mice (Figure 1A). However, mice overexpressing hepatic SR-BI had significantly higher [3H] tracer in the liver than control mice at 48 hours (Figure 1A). Furthermore, mice overexpressing SR-BI excreted significantly more [3H] tracer into the feces over 48 hours than did control mice (Figure 1A).
Figure 1.
Macrophage RCT in SR-BI–overexpressing mice. Mice were injected intravenously with SR-BI adenovirus or control adenovirus and then 3 days later injected intraperitoneally with [3H]-labeled cholesterol J774 foam cells. (A) [3H]cholesterol in plasma, liver, and feces of C57BL/6 mice (n = 6 per group). (B) [3H]cholesterol in plasma, liver, and feces of apoA-I–transgenic mice (n = 6 per group). (C) Cholesterol mass and [3H]cholesterol lipoprotein profile of pooled plasma samples drawn 24 hours after injection of J774 cells from apoA-I–transgenic mice subjected to FPLC analysis. *P < 0.05; **P < 0.01.
In a separate experiment, hepatic overexpression of SR-BI in human apoA-I–transgenic mice also resulted in markedly lower plasma HDL-C levels compared with controls (23 ± 2 vs. 122 ± 23 mg/dl). Plasma macrophage–derived [3H]cholesterol was again significantly lower in SR-BI–overexpressing versus control mice (Figure 1B). The [3H]cholesterol in plasma tracked closely with the cholesterol mass in plasma lipoproteins, present mostly in the HDL fraction (Figure 1C). apoA-I–transgenic mice overexpressing hepatic SR-BI had a substantial increase in [3H] tracer excretion in the feces over 48 hours (Figure 1B). Thus, hepatic SR-BI overexpression reduces steady-state plasma levels of HDL-C mass but significantly increases the fecal excretion of macrophage-derived [3H]cholesterol, indicating an acceleration of the rate of macrophage-to-feces RCT.
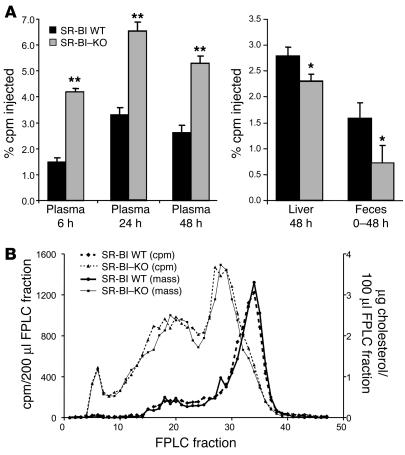
We also performed 2 independent studies in SR-BI–knockout mice compared with wild-type littermates, and because the results were very similar, the results were pooled for analysis. As expected, the SR-BI–knockout mice had higher HDL-C levels than wild-type control mice (158 ± 14 vs. 80 ± 15 mg/dl). The plasma macrophage–derived [3H]cholesterol levels at 6, 24, and 48 hours were significantly higher in SR-BI–knockout mice compared with controls (Figure 2A). Furthermore, at 48 hours, the percent of plasma [3H]cholesterol as UC was 78% in SR-BI–knockout mice, compared with only 38% in wild-type mice. The distribution of plasma [3H]cholesterol among lipoproteins was virtually identical to the distribution of cholesterol mass in both wild-type and SR-BI–knockout mice (Figure 2B). However, SR-BI–knockout mice had significantly less [3H] tracer in the liver at 48 hours and excreted significantly less [3H] tracer into the feces over 48 hours than did the wild-type control mice (Figure 2A). Thus, deficiency of SR-BI results in significantly reduced the fecal excretion of macrophage-derived cholesterol, indicating an overall slowing of the rate of macrophage-to-feces RCT.
Figure 2.
Macrophage RCT in SR-BI–knockout mice. SR-BI–/– mice and wild-type littermates were injected intraperitoneally with [3H]cholesterol-labeled J774 foam cells in 2 independent experiments and results pooled for analysis (n = 11 per group). (A) [3H]cholesterol in plasma, liver, and feces. (B) Cholesterol mass and [3H]cholesterol lipoprotein profile of pooled plasma samples drawn 24 hours after injection of J774 cells subjected to FPLC analysis. *P < 0.05; **P < 0.01.
Discussion
In this study, we demonstrate that hepatic SR-BI expression has a direct and substantial effect on the rate of macrophage-to-feces RCT in mice in vivo. Mice overexpressing SR-BI in the liver had markedly reduced plasma HDL-C levels and yet markedly increased macrophage-derived [3H]sterol excretion in the feces. Conversely, mice lacking SR-BI had markedly increased plasma levels of HDL-C and yet greatly reduced macrophage-derived [3H]sterol excretion in the feces. These data indicate that hepatic SR-BI expression regulates the rate of macrophage RCT inversely to its effects on plasma HDL-C levels. These experiments directly demonstrate that the rate of macrophage RCT is not simply a function of steady-state plasma concentrations of HDL-C and cannot be predicted based simply on measurement of plasma HDL-C.
Overexpression of SR-BI in the liver, while reducing levels of HDL-C in plasma, is known to reduce atherosclerosis in mice (8–10). This suggested that hepatic SR-BI overexpression may promote RCT, but this concept had never been proven. It is well established that hepatic overexpression increases the rate of uptake of HDL CE and HDL UC by the liver (7, 17–19). However, increased hepatic uptake of HDL-C alone would not necessarily be expected to increase the overall rate of RCT from macrophages to liver. The studies presented here strongly support the concept that hepatic overexpression of SR-BI does in fact promote the rate of macrophage RCT. One possible mechanism contributing to the effect of hepatic SR-BI overexpression on increasing macrophage RCT is that the action of hepatic SR-BI on HDL may generate remnant HDL particles and or lipid-poor apoA-I that serve as more efficient acceptors of cholesterol efflux from macrophages. Thus, hepatic SR-BI may generate HDL acceptor particles that promote peripheral macrophage efflux at one end of the RCT pathway, while at the other end promote the uptake of HDL-C from the plasma to liver and direct it to biliary excretion, thus promoting overall macrophage RCT.
Conversely, SR-BI–knockout mice have markedly increased atherosclerosis despite increased plasma levels of HDL-C (13–15), suggesting the possibility of impaired RCT. SR-BI–deficient or –attenuated mice have been shown to have substantially reduced uptake of HDL-C by liver (12, 18, 20, 21). However, if the increased HDL-C levels in SR-BI–deficient mice were highly efficient in promoting macrophage cholesterol efflux, the reduced rate of uptake into liver alone would not necessarily be expected to reduce the overall rate of macrophage RCT. Our studies indicate that SR-BI–deficient mice do have significantly reduced macrophage-to-feces RCT. The mechanisms by which hepatic SR-BI deficiency impairs net macrophage RCT have yet to be fully determined. In addition to impaired hepatic uptake of HDL-C, there may also be an impairment of mobilization of cholesterol from the macrophages as a result of hepatic SR-BI deficiency. Consistent with the argument posed above for SR-BI overexpression, the lack of SR-BI–mediated processing of HDL by the liver may result in HDL particles that have an impaired capacity to efflux macrophage cholesterol. How important impaired RCT is to the accelerated atherogenesis in SR-BI–knockout mice remains to be determined, and liver-specific SR-BI–knockout mice will be of great interest in this regard.
Notably, the levels of [3H]cholesterol tracer in plasma tracked with plasma cholesterol mass in both SR-BI–overexpressing (reduced) and SR-BI–deficient (increased) mice. The [3H]cholesterol tracer in plasma also tracked closely with the cholesterol mass with regard to distribution among lipoproteins. Fast protein liquid chromatography (FPLC) lipoprotein separations done in both SR-BI–overexpressing and SR-BI–knockout mice indicate that the macrophage-derived [3H]cholesterol tracer was distributed among plasma lipoproteins in proportion to cholesterol mass, and the specific activities were comparable. These data indicate that once effluxed from the macrophages, the [3H]cholesterol tracer is metabolized in a way comparable to the endogenous cholesterol mass in the animals and is influenced by the same input and output vectors that influence plasma levels of HDL-C mass. Thus, as for HDL-C mass measurements, plasma levels of macrophage-derived [3H]cholesterol in plasma are not themselves a marker of flux of tracer though the plasma compartment. While our conclusions in this report are based solely on measures of tracer and not mass, we suggest that the tracer in these studies is tracking the flux of macrophage-derived cholesterol mass.
While direct measures of flux of macrophage-derived cholesterol mass would clearly be desirable, it is impossible to detect the small pool of cholesterol mass derived specifically from macrophages. Furthermore, conclusions based on “whole body” peripheral cholesterol mass efflux and RCT may not faithfully reflect the specific effects of genetic manipulation or pharmacologic intervention on macrophage-specific cholesterol efflux and RCT. For example, several approaches designed to test the hypothesis that mice overexpressing apoA-I have increased whole body peripheral RCT were negative (22–24); in contrast, we later showed using our macrophage-specific tracer approach that apoA-I overexpression significantly increased macrophage RCT (16). Indeed, hepatic overexpression of SR-BI was not associated with reduced cholesterol content or increased cholesterol synthesis in peripheral tissues (19), which might have been expected if it promoted general peripheral efflux and RCT. Macrophages may differ from the majority of peripheral tissue with regard to the regulation of cholesterol efflux, and factors that do not influence general whole body RCT could still affect macrophage-specific RCT. Thus, it may not always be the case that the rate of macrophage RCT can be inferred from studies of mass that reflect the entire generalized RCT process.
A variety of studies have suggested that HDL UC is preferentially targeted toward the bile (25–27). Studies using HDL unesterified sitosterol as a tracer indicated that it was directly targeted to biliary excretion (28, 29). SR-BI–overexpressing mice have increased biliary cholesterol secretion (6), and SR-BI–deficient mice have reduced biliary cholesterol secretion (11, 30, 31), in each case without changes in biliary bile acid or phospholipid composition. Mice overexpressing hepatic SR-BI were noted to have increased hepatic uptake of HDL UC and with attenuated hepatic SR-BI expression to have reduced hepatic uptake of HDL UC (18). SR-BI–deficient mice have large HDL particles that are substantially enriched in UC (3.2-fold) to a greater extent than even CE (1.3-fold) compared with normal murine HDL (31). We noted in our studies that the percent of [3H]cholesterol as UC in plasma was substantially higher in SR-BI–knockout mice, compared with wild-type mice, consistent with the enrichment in HDL UC mass. UC enrichment of the HDL particle may be one factor that reduces its ability to serve as an effective acceptor of macrophage cholesterol efflux. Thus, hepatic SR-BI expression may have a particularly important effect on macrophage RCT through its effects on hepatic selective uptake of HDL UC.
One important limitation of these studies in extrapolating the results to human physiology is that mice, in contrast to humans, lack the CE transfer protein (CETP). CETP transfers CEs from HDL to apoB-containing lipoproteins in exchange for triglyceride and therefore has an important role in HDL-C metabolism (32). CETP-mediated transfer of HDL CE to apoB-containing lipoproteins with subsequent receptor-mediated uptake in the liver may be an important route of RCT in species that express CETP. Indeed, a kinetic study in humans suggested that the vast majority of an injected HDL CE tracer that eventually was excreted into the bile was taken up by the liver after transfer to apoB-containing lipoproteins (27). Importantly, however, the majority of an injected HDL UC tracer that was excreted in bile was transferred directly to the liver from HDL (27). In any case, the quantitative role of hepatic SR-BI in regulating macrophage RCT in humans and other species that express CETP may be different than in mice.
Our focus here is on the role of hepatic SR-BI in macrophage RCT because the overexpression studies were liver specific and because the absence of hepatic SR-BI in the knockout mice is the most likely cause of the observed results. However, SR-BI is also expressed in the intestine (33–36), and recent data suggest that the intestine may also play a role in directly excreting cholesterol as a liver-independent pathway in RCT (37, 38). In the small intestine, SR-BI is expressed both apically and basolaterally (33, 36). Although there is no direct evidence that SR-BI participates in selective uptake of plasma HDL-C into the enterocytes of the small intestine, some studies have suggested this could be the case (38). Thus, our data showing reduced macrophage-to-feces RCT in SR-BI–knockout mice could potentially be influenced by the lack of intestinal SR-BI in these mice.
SR-BI is also expressed in macrophages and can promote cholesterol efflux to HDL in vitro (39, 40). Mice lacking SR-BI specifically in bone marrow–derived cells develop accelerated atherosclerosis compared with wild-type mice (15, 41, 42) (though this effect may depend on the stage of atherogenesis; ref. 42). To our knowledge, the converse experiment of reconstituting macrophage SR-BI expression in SR-BI–deficient mice has not been reported. Importantly, mice deficient in bone marrow–derived cell SR-BI have considerably reduced atherosclerosis compared with total SR-BI–knockout mice, suggesting that deficiency of SR-BI in other tissues contributes substantially to the increased atherosclerosis. In the present studies, the labeled, injected J774 cells expressed SR-BI, and therefore our studies do not directly address the role of macrophage SR-BI in RCT. Future studies are required to determine the functional significance of macrophage SR-BI to RCT in vivo. Of note, SR-BI is also highly expressed in the adrenals, where is serves to provide cholesterol for steroidogenesis (40). In our studies in SR-BI–deficient mice, we harvested adrenals and found that the SR-BI–deficient mice had significantly reduced [3H]cholesterol at 48 hours compared with wild-type mice (0.15% ± 0.03% vs. 0.27% ± 0.04% of injected [3H]cholesterol; P < 0.05).
In summary, we demonstrate that hepatic SR-BI expression plays an important regulatory role in the rate of macrophage-to-feces RCT in a manner that is opposite to the effects of hepatic SR-BI expression on steady-state plasma levels of HDL-C. The influence of hepatic SR-BI expression on macrophage RCT may help to explain the effect of hepatic SR-BI expression on atherosclerosis and serves as the clearest demonstration to date of the principle that steady-state plasma concentrations of HDL-C are not necessarily predictive of the rate of macrophage RCT.
Methods
J774 cell culture, acetylated LDL cholesterol loading, and [3H]cholesterol labeling.
J774 cells were grown in suspension in RPMI 1640 supplemented with 10% FBS as previously described (17). Cells were radiolabeled with 5 μCi/ml [3H]cholesterol and cholesterol enriched with acetylated LDL for 48 hours, washed twice, equilibrated in RPMI plus 0.2% BSA for 6 hours, spun down, and resuspended in RPMI medium immediately before use. On average, the cell suspension contained 10 × 106 cells/ml at 11.8 × 106 cpm/ml and the percent CE in macrophages was 65%.
In vivo studies.
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Two independent experiments using hepatic SR-BI overexpression were performed in female mice, one with wild-type mice and one with human apoA-I–transgenic mice. All mice were fed a standard chow diet. Wild-type C57BL/6 or human apoA-I–transgenic mice on a C57BL/6 background (The Jackson Laboratory) were injected intravenously with second-generation recombinant adenoviral vector encoding the human SR-BI cDNA (n = 6) or control null adenovirus vector (n = 6) (1.0 × 1011 particles per mouse). Three days after vector injection, cholesterol-loaded and [3H]cholesterol-labeled J774 cells (0.5 ml) were injected intraperitoneally. and the mice were caged separately. Plasma was collected at 24 hours and 48 hours and was used for liquid scintillation counting and lipoprotein analysis. Plasma radioactivity is expressed as percent of total injected [3H]cholesterol per milliliter plasma. Feces were collected from 0 to 48 hours and stored at –20°C prior to lipid extraction. At 48 hours, mice were anesthetized and perfused with cold PBS, and the liver was collected and stored at –20°C until lipid extraction.
Two independent experiments were performed in SR-BI–knockout mice (The Jackson Laboratory). Male SR-BI–knockout mice (on a mixed B6, 126 background) were bred to female C57BL/6 mice, then SR-BI heterozygotes were cross-bred, and SR-BI homozygotes and wild-type littermates were used for experiments. All mice were fed a standard chow diet. In both experiments, female wild-type (n = 5–6) and SR-BI–knockout mice (n = 5–6) were injected intraperitoneally with [3H]cholesterol-labeled J774 cells. Results were similar in both experiments and were therefore pooled for analysis (n = 11 per group).
Lipid extractions.
Fecal cholesterol and bile acid were extracted as described by Batta et al. (43). Values are expressed as a percent of total injected [3H]cholesterol. Tissue lipids were extracted by the Bligh-Dyer method (44) and expressed as a percent of total [3H]cholesterol-injected/whole organ.
Plasma lipid and lipoprotein analysis.
Plasma total cholesterol, HDL-C, UC, CE, triglyceride, phospholipid, and human apoA-I levels were measured on a Cobas Fara (Roche Diagnostics Corp.) with the use of Sigma-Aldrich reagents, and the levels were expressed in milligrams/deciliters. Pooled plasma (150 μl) from wild-type and SR-BI mice was analyzed by FPLC gel filtration on 2 Superose 6 columns (Amersham Biosciences), as previously described (16). The cholesterol concentrations in the FPLC fractions were determined using an enzymatic assay (Wako Pure Chemical Industries Ltd.). FC and CE were separated by thin-layer chromatography.
Statistics.
Plasma lipid values are presented as mean ± SD, and all [3H]cholesterol data are presented as mean ± SEM. Results were analyzed by 2-tailed Student’s t test with the use of GraphPad Prism Software version 4. Statistical significance for all comparisons was assigned at P < 0.05.
Acknowledgments
D.J. Rader is supported by grants from the National Heart, Lung and Blood Institute (NHLBI) of the NIH (P01 HL22633, P50 HL70128, and R01 HL55323), a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, and an Alternative Drug Discovery Initiative award funded by GlaxoSmithKline. G.H. Rothblat is supported by grants P01 HL22633 and R01 HL63768 from the NHLBI. We are indebted to Aisha Faruqi, Archana Vemulapalli, Dawn Marchadier, Anna DiFlorio, and Linda Morrell for expert technical assistance.
Footnotes
See the related commentary beginning on page 2699.
Nonstandard abbreviations used: CE, cholesteryl ester; CETP, CE transfer protein; FPLC, fast protein liquid chromatography; HDL-C, HDL cholesterol; RCT, reverse cholesterol transport; SR-BI, scavenger receptor class B type I; UC, unesterified cholesterol.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 2.Groen AK, Oude Elferink RP, Verkade HJ, Kuipers F. The ins and outs of reverse cholesterol transport. Ann. Med. 2004;36:135–145. doi: 10.1080/07853890310020635. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR. Role of ABCA1 in cellular cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2003;23:710–711. doi: 10.1161/01.ATV.0000068683.51375.59. [DOI] [PubMed] [Google Scholar]
- 4.Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23:1732–1738. doi: 10.1161/01.ATV.0000091363.28501.84. [DOI] [PubMed] [Google Scholar]
- 5.Zanlungo S, Rigotti A, Nervi F. Hepatic cholesterol transport from plasma into bile: implications for gallstone disease. Curr. Opin. Lipidol. 2004;15:279–286. doi: 10.1097/00041433-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Kozarsky KF, et al. Overexpression of the HDL receptor SR-B1 alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Arai T, Ji Y, Rinninger F, Tall AR. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J. Biol. Chem. 1998;273:32920–32926. doi: 10.1074/jbc.273.49.32920. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 9.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, et al. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J. Biol. Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- 11.Rigotti A, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varban ML, et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trigatti B, et al. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun A, et al. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 15.Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 17.Ueda Y, et al. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 1999;274:7165–7171. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, et al. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J. Biol. Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 19.Alam K, Meidell RS, Spady DK. Effect of up-regulating individual steps in the reverse cholesterol transport pathway on reverse cholesterol transport in normolipidemic mice. J. Biol. Chem. 2001;276:15641–15649. doi: 10.1074/jbc.M010230200. [DOI] [PubMed] [Google Scholar]
- 20.Out R, et al. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J. Lipid Res. 2004;45:2088–2095. doi: 10.1194/jlr.M400191-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Brundert M, et al. Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:143–148. doi: 10.1161/01.ATV.0000149381.16166.c6. [DOI] [PubMed] [Google Scholar]
- 22.Stein O, et al. High levels of human apolipoprotein A-I and high density lipoproteins in transgenic mice do not enhance efflux of cholesterol from a depot of injected lipoproteins. Relevance to regression of atherosclerosis? Atherosclerosis. 1999;144:367–374. doi: 10.1016/s0021-9150(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 23.Osono Y, Woollett LA, Marotti KR, Melchior GW, Dietschy JM. Centripetal cholesterol flux from extrahepatic organs to the liver is independent of the concentration of high density lipoprotein-cholesterol in plasma. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4114–4119. doi: 10.1073/pnas.93.9.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolley CD, Woollett LA, Turley SD, Dietschy JM. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J. Lipid Res. 1998;39:2143–2149. [PubMed] [Google Scholar]
- 25.Schwartz CC, Halloran LG, Vlahcevic ZR, Gregory DH, Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978;200:62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz CC, et al. Multicompartmental analysis of cholesterol metabolism in man. Characterization of the hepatic bile acid and biliary cholesterol precursor sites. J. Clin. Invest. 1978;61:408–423. doi: 10.1172/JCI108952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J. Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Robins SJ, Fasulo JM. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to bile. J. Clin. Invest. 1997;99:380–384. doi: 10.1172/JCI119170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins SJ, Fasulo JM. Delineation of a novel hepatic route for the selective transfer of unesterified sterols from high-density lipoproteins to bile: studies using the perfused rat liver. Hepatology. 1999;29:1541–1548. doi: 10.1002/hep.510290518. [DOI] [PubMed] [Google Scholar]
- 30.Mardones P, et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- 31.Van Eck M, et al. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J. Biol. Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 32.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 33.Voshol PJ, et al. Down-regulation of intestinal scavenger receptor class B, type I (SR-BI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem. J. 2001;356:317–325. doi: 10.1042/0264-6021:3560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werder M, et al. Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry. 2001;40:11643–11650. doi: 10.1021/bi0109820. [DOI] [PubMed] [Google Scholar]
- 35.Altmann SW, et al. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. . Biochim. Biophys. Acta. 2002; 1580:77–93. doi: 10.1016/s1388-1981(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 36.Cai SF, Kirby RJ, Howles PN, Hui DY. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J. Lipid Res. 2001;42:902–909. [PubMed] [Google Scholar]
- 37.Plosch T, et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 38.Kruit JK, et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Ji Y, et al. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholersterol efflux. J. Biol. Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 40.Williams DL, et al. Scavenger receptor BI and cholesterol trafficking. Curr. Opin. Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, et al. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- 42.Van Eck M, Bos IS, Hildebrand RB, Van Rij BT, Van Berkel TJ. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batta AK, et al. Simultaneous quantitation of fatty acids, sterols and bile acids in human stool by capillary gas-liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;775:153–161. doi: 10.1016/s1570-0232(02)00289-1. [DOI] [PubMed] [Google Scholar]
- 44.Bligh E, Dyer N. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]