Abstract
One of the most common genetic risk factors for Parkinson’s disease (PD) is variants in GBA1, which encodes the lysosomal enzyme glucocerebrosidase (GCase). GCase deficiency has been associated with an increased PD risk, but not all individuals with low GCase activity are carriers of GBA1 mutations, suggesting other factors may be acting as modifiers. We aimed to discover common variants associated with GCase activity, as well as replicate previously reported associations, by performing a genome-wide association study using two independent cohorts: a Columbia University cohort consisting of 697 PD cases and 347 controls and the Parkinson’s Progression Markers Initiative (PPMI) cohort consisting of 357 PD cases and 163 controls. As expected, GBA1 variants have the strongest association with decreased activity, led by N370S (beta = − 4.36, se = 0.32, p = 5.05e − 43). We also identify a novel association in the GAA locus (encoding for acid alpha-glucosidase, beta = − 0.96, se = 0.17, p = 5.23e − 09) that may be the result of an interaction between GCase and acid alpha-glucosidase based on various interaction analyses. Lastly, we show that several PD-risk loci are potentially associated with GCase activity. Further research will be needed to replicate and validate our findings and to uncover the functional connection between acid alpha-glucosidase and GCase.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-025-04996-1.
Keywords: Genome-wide association study, Glucocerebrosidase, Parkinson’s disease, GBA1, Lysosomal metabolism
Introduction
Parkinson’s disease (PD) is a complex neurodegenerative disorder, characterized by the accumulation of alpha synuclein in Lewy bodies and a progressive loss of dopaminergic neurons in the substantia nigra [1]. Although the exact mechanisms remain unknown, there is a clear role for genetic factors in PD. One gene of particular interest is GBA1. In addition to being one of the most common genetic risk factors associated with PD, it is also associated with a faster rate of motor and non-motor progression [2].
GBA1 encodes for glucocerebrosidase (GCase), a lysosomal hydrolase whose biallelic deficiency causes the lysosomal storage disorder (LSD) known as Gaucher disease [3]. Heterozygous GBA1 variants associated with reduced GCase activity are important risk factors for both PD and dementia with Lewy bodies (DLB), and a substantial portion of patients with these disorders have reduced GCase activity, albeit not carrying GBA1 variants [4, 5]. This observation suggests that other factors, genetic or environmental, may modify GCase activity. One such factor may be TMEM175 variants, which have been associated with reduced GCase activity in patient data [6] and in cell models [7]. Additionally, variants in LRRK2 have also been linked to modified GCase activity, although the directionality of the effect is still unclear [4, 8–10].
Understanding the genetic variation influencing GCase activity could be informative in several ways. First, it can provide a better understanding of the mechanisms underlying the role GCase plays in PD. Furthermore, it would allow for proper adjustments or stratification in future research and clinical trials. In the present study, we aimed to identify genetic factors associated with GCase activity by performing a genome-wide association study (GWAS) including a total of 1054 PD cases and 510 healthy controls. A secondary analysis was conducted to identify PD-associated variants that may act as modifiers of GCase activity.
Methods
Study Population
The study population consisted of two separate cohorts with available genotype and GCase activity data: (1) a cohort of 697 PD cases and 347 controls collected from Columbia University in New York and (2) a cohort of 357 PD cases and 163 controls from the Parkinson’s Progression Markers Initiative (PPMI). Cohort demographics can be found in Table 1. Diagnosis inclusion and exclusion criteria for Columbia [9, 11] and PPMI [4] have been previously described (https://www.ppmi-info.org/study-design/study-cohorts#overview/). All subjects were of European descent, confirmed with principal component analysis. Informed consent forms were signed by all participants prior to entering their respective studies, and the study protocol was approved by the institutional review boards.
Table 1.
Cohort demographics for individuals with available genotype and GCase data
| Cohort | N cases | N controls | Status | Males (%) | Females (%) | Mean age (± SD) |
|---|---|---|---|---|---|---|
| Columbia | 697 | 347 | cases | 65.1 | 34.9 | 65.74 (11.05) |
| controls | 38.3 | 61.7 | 64.25 (10.05) | |||
| PPMI | 357 | 163 | cases | 66.7 | 33.3 | 61.85 (9.49) |
| controls | 67.5 | 32.5 | 60.79 (11.34) |
N number, SD standard deviation, PPMI Parkinson’s progression markers initiative
Enzyme Activity
Enzymatic activity for GCase, acid alpha-glucosidase (GAA), acid sphingomyelinase (ASM), alpha-galactosidase A (GLA), and galactosylceramidase (GALC) was measured from dried blood spots in the Columbia cohort, the protocol for which has been previously described [12, 13]. To summarize, enzyme activity was measured by Sanofi laboratories using liquid chromatography-tandem mass spectrometry (LC–MS/MS) from dried blood spots, using a multiplex assay. The dried blood spots were incubated in a reaction cocktail with substrates for the enzymes and buffer. Enzyme activity measurements were determined by calculating the amount of product obtained after the incubation, under the assumption that this is a direct indication of the activity of the enzymes. Lysosomal enzyme activity for the PPMI cohort was measured from frozen whole blood, which was slowly thawed and processed similarly to the Columbia cohort. GCase outliers were identified as those with activity measurements lying outside ± 1.5 times the interquartile range (IQR) and were subsequently removed in both cohorts.
Genome-Wide Association Study
Genotyping was performed with the OmniExpress GWAS array for the Columbia cohort and the NeuroX array for the PPMI cohort, according to the manufacturer’s protocols (Illumina Inc.). Quality control for individual and variant data was completed as previously described (https://github.com/neurogenetics/GWAS-pipeline). In brief, samples that were heterozygosity outliers (inclusion criteria of − 0.15 ≤ F ≤ 0.15), call rate outliers (missingness > 95%), had mismatched genetic and reported sex, or were identified as European ancestry outliers based on HapMap3 principal component analysis (PCA) in plink v1.9 were removed [14]. Additionally, we removed samples with relatedness closer than third degree relatives (pihat > 0.125). Individual SNPs were excluded on the basis of variant missingness (> 95%), differences in missingness between cases and controls (p < 1e − 04), haplotype missingness (p < 1e − 04), and deviation from the Hardy–Weinberg equilibrium in controls (p < 1e − 04). Imputation was performed on filtered data with the Michigan Imputation Server using the Haplotype Reference Consortium reference panel r1.1 2016 and default settings [15]. Linear regressions of GCase activity were performed in plink v1.9 using hard-call variants (R2 > 0.8) and a minor allele frequency (MAF) threshold of > 0.01, using a standard p-value significance threshold of p < 5E − 08. We manually added the PD-relevant variant GBA1 N370S to the Columbia analyses, as well as GBA1 T369M and LRRK2 G2019S after filtering, due to all being under the minor allele threshold. We believed these variants were important to include, considering all have been demonstrated as relevant for both GCase activity and Parkinson’s disease in previous studies [2, 4, 9, 10, 16]. We have used the traditional nomenclature for GBA1 variants, which omits the first 39 amino acids in the sequence. The Columbia cohort included adjustments for age, sex, disease status, Ashkenazi Jewish ancestry, LRRK2 G2019S status, and lysosomal enzyme activities (GAA, GLA, GALC, and ASM) to examine the isolated effect on GCase activity. The G2019S mutation was included as a covariate due to it being a known GCase modifier [4, 8, 9]. Lysosomal enzymes were adjusted for because they have been shown to correlate with one another in a previous study [17]. The PPMI analysis included the same adjustments, with the addition of white blood cell count (WBC) as previously suggested [9]. Samples were excluded from analyses if they were missing data included in the respective regression model. Missing data for variables outside of those used in the given model were not grounds for exclusion. Fixed-effect meta-analyses of PPMI and Columbia cohorts were performed with METAL in plink v1.9. Principal components (PCs) were calculated using PCA with plink v1.9, and the top 10 PCs were included as covariates for each cohort. Conditional and joint analyses were performed with GCTA-COJO to identify independent variants after adjusting for lead SNPs [18]. ANOVA, linear regressions with interaction terms, mediation analysis, and interaction plot generation were performed in R v4.3.1 [19]. Linkage disequilibrium Manhattan plots were created using LocusZoom [20].
Data Statement
All code for analyses used in this project can be found at https://github.com/gan-orlab/GCase_GWAS. PPMI data used for this study can be obtained by qualified researchers upon completion of a data access application (https://www.ppmi-info.org/access-data-specimens/download-data). Columbia cohort data can be obtained by request. The GCase GWAS summary statistics can be found on the GWAS catalog (https://www.ebi.ac.uk/gwas/).
Results
GAA and GBA1 Loci Are Associated with GCase Activity
After quality control and imputation, a total of 556 cases, 284 controls, and 13,327,381 variants in the Columbia cohort and 328 cases, 144 controls, and 941,882 variants in the PPMI cohort were available for analysis. The mean GCase activity was similar between Columbia (mean = 11.1 µmol/l/h, sd = ± 3.14) and PPMI (mean = 11.63 µmol/l/h, sd = ± 2.59) and was also similar between cases and controls within each cohort (Supplementary Table 2).
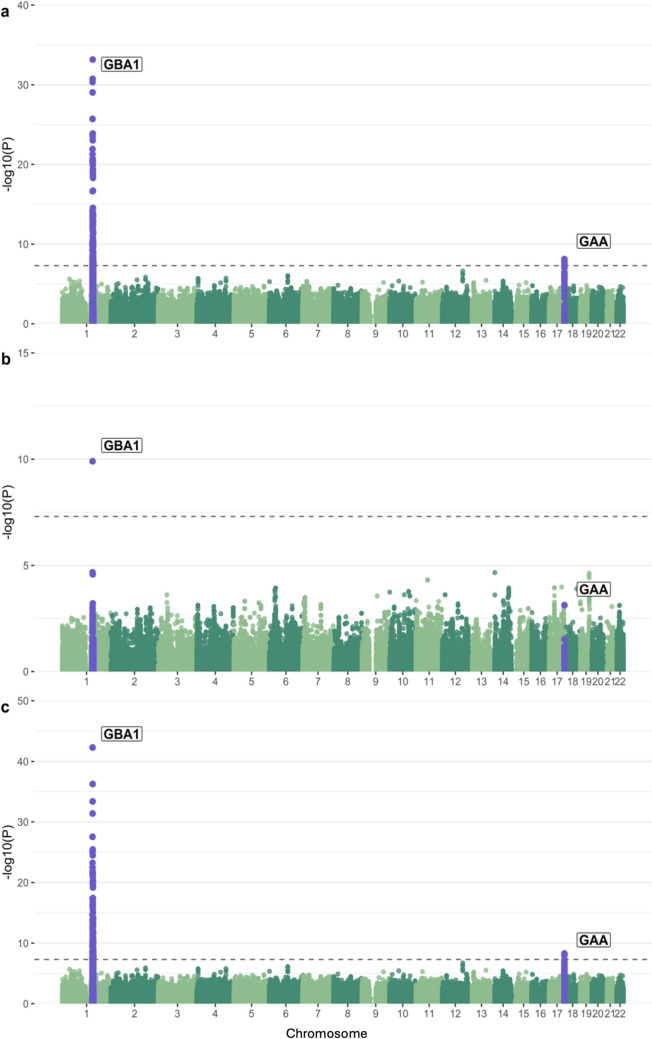
We performed a GWAS to identify potential associations between common variants and GCase activity in both cohorts separately, followed by a meta-analysis. We looked at the genomic inflation factors and QQ plots of each analysis to test for systematic bias and found them to be acceptable (Columbia λ = 0.99, PPMI λ = 1.04, Supplementary Fig. 1). The strongest associated locus in Columbia was in the GBA1 locus, as expected (Fig. 1A). This signal is driven by N370S (rs76763715, beta = − 4.21, se = 0.35, p = 4.55e − 31), which is in linkage disequilibrium (LD) with the lead SNP in the locus (rs745550122, beta = − 4.46, se = 0.35, p = 6.61e − 34, R2 = 0.78, D′ = 0.91). A second signal can be seen in chromosome 17 in the GAA locus, with the strongest associated variants comprising a high-LD region (Supplementary Fig. 2) and the lead SNP having a negative direction of effect (beta = − 0.96, se = 0.17, p = 7.55e − 9). Applying conditional and joint analyses revealed no secondary independent associations in either signal. We performed the same analysis in cases and controls separately to assess if there were any notable differences in associations between the two disease groups (Supplementary Fig. 3). The same GBA1 N370S peak is observed in both analyses with varied strengths of association (cases, b = − 4.18, se = 0.46, p = 9.16e − 19; controls, b = − 4.07, se = 0.56, p = 4.92e − 12), and no other genome-wide significant loci were observed. Notably, we do not see the GAA locus association in either analysis, which is due to a lack of power from the decrease in cohort size considering that the top variants in both analyses are in linkage disequilibrium with the same direction of effect (cases, rs9899138, b = − 0.86, se = 0.2, p = 3.19e − 05; controls, rs9905685, b = − 1.22, se = 0.3, p = 6.96e − 05; R2 = 0.72, D′ = 0.91). The GBA1 signal was replicated in the PPMI cohort, with N370S once again driving the association (Fig. 1B, beta = − 5.05, se = 0.76, p = 1.25e − 10). The GAA locus does surpass nominal significance in PPMI, although the lead SNP is different and has an opposite direction of effect compared to the lead SNP from Columbia (Table 2). The meta-analysis likewise showed associations in the GBA1 and GAA loci, but did not result in any additional associations (Fig. 1C). The GBA1 variants T369M (rs75548401) and E326 K (rs2230288) were associated with GCase activity, although the only association passing GWAS significance was T369M in the meta-analysis (Table 3). Both variants had similar effect sizes, directions, and p-values across both analyses, and patients who carried these mutations had similar GCase activity means to control carriers except for E326 K where patients show a decrease in activity in both cohorts (Supplementary Table 2). We also repeated these analyses with the inclusion of GCase outliers and found the results to be highly similar, with the GBA1 signals increasing in strength (N370S, beta = − 4.62, se = 0.31, p = 6.95e − 50; T369M, beta = − 3.66, se = 0.53, p = 5.04e − 12; E326 K, beta = − 1.4, se = 0.41, p = 0.00071) and the GAA signal remaining mostly the same (rs9899138, beta = − 0.99, se = 0.16, p = 1.37e − 08, Supplementary Fig. 4).
Fig. 1.
Manhattan plot of log adjusted p-values at each genomic position in (a) the Columbia cohort with adjustments for age, sex, disease status, Ashkenazi Jewish status, LRRK2 G2019S, ASM activity, GAA activity, GLA activity, GALC activity, and the top 10 PCs; (b) the PPMI cohort with adjustments for age, sex, disease status, LRRK2 G2019S genotype, ASM activity, GAA activity, GLA activity, GALC activity, white blood cell count, and the top 10 PCs; and (c) the meta-analysis of these two analyses
Table 2.
Comparison of lead GAA locus variants in the Columbia and PPMI cohorts
| SNP | Cohort | Beta | se | p |
|---|---|---|---|---|
| 17:78,061,141:T:G | Columbia | − 1.009 | 0.18 | 1.15e − 08 |
| PPMI | − 0.29 | 0.19 | 0.14 | |
| 17:78,096,483:A:C | Columbia | 0.39 | 0.162 | 0.017 |
| PPMI | 0.60 | 0.18 | 7.44e − 4 |
SNP single nucleotide polymorphism, se standard error, PPMI Parkinson’s progression markers initiative
Table 3.
GBA1 T369M and E326K association statistics for Columbia and PPMI cohorts
| Variant | Cohort | Beta | se | p |
|---|---|---|---|---|
| T369M | Columbia | − 3.28 | 0.78 | 2.79e − 05 |
| PPMI | − 2.91 | 0.68 | 2.11e − 05 | |
| Meta-analysis | − 3.067 | 0.51 | 1.787e − 09 | |
| E326K | Columbia | − 1.62 | 0.59 | 0.0063 |
| PPMI | − 1.43 | 0.5 | 0.0047 | |
| Meta-analysis | − 1.51 | 0.38 | 8.027e − 05 |
se standard error, PPMI Parkinson’s progression markers initiative
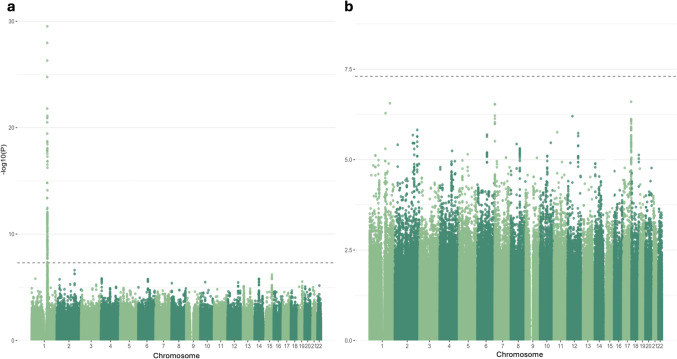
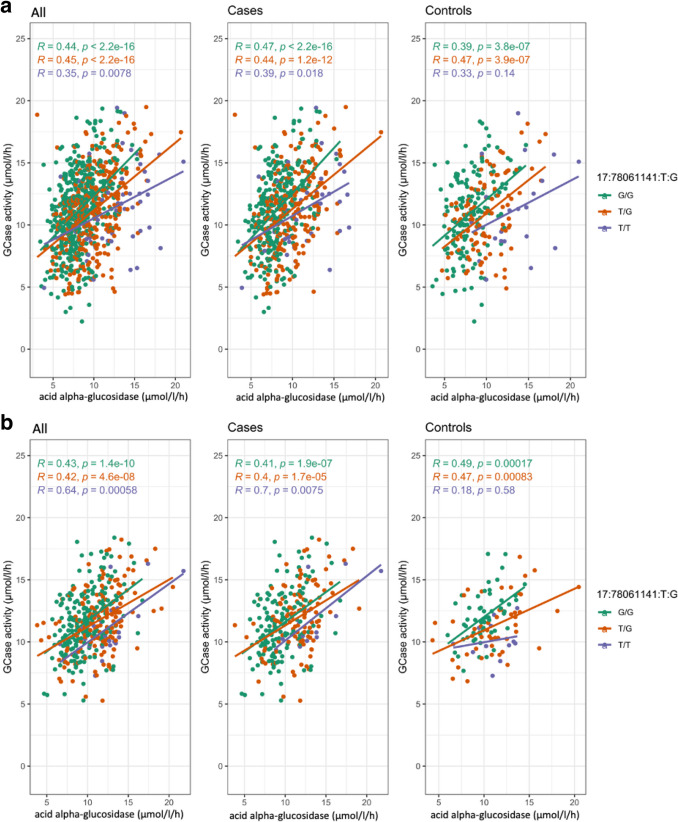
Interestingly, analysis in the Columbia cohort without adjusting for acid alpha-glucosidase activity eliminates any association from the GAA locus (Fig. 2A). Adding adjustments for common PD-relevant GBA1 variants N370S, E326K, and T369M results in both the GBA1 and GAA peaks dropping below GWAS-level significance (Fig. 2B). To better understand if these outcomes could be due to an interaction between the acid alpha-glucosidase and GCase enzymes, we constructed an interaction plot of enzyme activities by genotype of the top GAA SNP that was common in both cohorts (Fig. 2B). The genotype of the lead SNP in the GAA locus (17:78,061,141:T:G) appears to impact the interaction of GCase and acid alpha-glucosidase enzyme activities in Columbia, with correlations becoming progressively weaker in an additive manner with the addition of the T allele (Fig. 3). This trend is not as clear in controls, which could be due to the lower number of samples in this group. The effect is also not apparent in PPMI, reflective of the lack of association in this cohort. To further investigate the presence of an interaction, both an ANOVA and a linear regression using an interaction term were performed. The ANOVA used the absolute difference between GCase and acid alpha-glucosidase activity, which did significantly vary by the genotype status of the lead GAA variant (df = 2, sum Sq = 130, mean Sq = 64.75, F = 13.62, p = 1.52e − 06). The linear regression was performed using the same covariates as the previous analysis, with the addition of a GAA*GCase interaction term. The results indicated that the T/T genotype status was significant (beta = − 0.25, se = 0.13, p = 0.048), supporting the presence of an interaction effect between GAA genotype status, acid alpha-glucosidase activity, and GCase activity. Notably, all enzymes in this model had a significant effect on GCase activity (GAA, beta = 0.63, se = 0.068, p = 2e − 16; ASM, beta = − 0.1, se = 0.025, p = 2.78e − 05; GALC, beta = 0.12, se = 0.057, p = 0.037; GLA, beta = 0.32, se = 0.067, p = 2.56e − 06). An analysis of mediation provided evidence that acid alpha-glucosidase activity levels significantly mediate the relationship between GAA genotype and GCase activity (beta = 1.05, se = 0.12, p < 0.0001).
Fig. 2.
Manhattan plot of log adjusted p-values at each genomic position in the Columbia cohort with adjustments for (a) age, sex, disease status, Ashkenazi Jewish status, LRRK2 G2019S, ASM activity, GLA activity, GALC activity, and the top 10 PCs; and (b) age, sex, disease status, Ashkenazi Jewish status, LRRK2 G2019S, GBA1 N370S, GBA1 T369M, GBA1 E326 K, ASM activity, GAA activity, GLA activity, GALC activity, and the top 10 PCs
Fig. 3.
Interaction plots of GCase and acid alpha-glucosidase activity colored by 17:78,061,141:T:G genotype in (a) Columbia and (b) PPMI
To examine if the GAA locus has any role in PD, we investigated the association of this region with PD risk and various measurements of progression using GWAS summary statistics from previous studies. We identified 26 variants nominally associated in the GWAS of PD risk, four with age at onset, five with UPDRS III scores, 79 with MoCA scores, and six with MMSE scores (Supplementary Table 1), although no variants passed Bonferroni correction for multiple comparisons [21–23]. There were no GAA locus variants even nominally associated in the GWASs of motor, cognitive, and composite progression [24]. Of the associated variants, eight variants from the GWAS of PD risk, two from the GWAS of age at onset, 33 from the GWAS of MoCA scores, and one from the GWAS of MMSE scores were also nominally associated with GCase activity in the present meta-analysis.
PD-Related Variants Are Associated with GCase Activity
We investigated PD-related variants nominated from the largest PD GWAS to date to determine if any were associated with GCase activity in our cohorts (Nalls et al., 2019). There were 17 loci below nominal significance in the main GWAS for Columbia, seven in the GWAS for PPMI, and 14 in the meta-analysis (Table 4). Of these, only N370S (rs76763715) and rs35749011 (in strong LD with E326K) in the GBA1 locus, as well as rs13117519 in the ANK2 locus passed multiple testing correction in the meta-analysis. For our case and control stratified analyses, in addition to the GBA1 N370S signal, two PD-risk loci in SNCA became significantly associated with GCase activity in PD cases after applying multiple testing correction (rs356203, beta = 0.53, se = 0.17, p = 0.0015; rs356182, beta = 0.55, se = 0.17, p = 0.0017). When repeating the analysis with the inclusion of GCase outliers, we find the GBA1 E326K (rs35749011, beta = − 2.29, se = − 0.66, p = 0.001) and ANK2 (rs13117519, beta = 0.43, se = 0.14, p = 0.0011) loci to no longer be significant after Bonferroni correction. We then attempted to replicate previously reported associations in TMEM175 and LRRK2 with GCase activity by investigating these loci in a simplified meta-analysis using only age, sex, disease status, 10 PCs, and PD-relevant GBA1 SNPs including N370S, E326K, and T369M as covariates (Supplementary Fig. 4). There were three intronic variants in LRRK2 that passed nominal significance (rs116911375, beta = − 1.7, se = 0.79, p = 0.03; rs191242488, beta = − 1.041, se = 0.52, p = 0.044; 12:40,668,909:T:G, beta = − 1.041, se = 0.52, p = 0.044), and no variants in TMEM175. No variants passed multiple testing corrections. The nominally significant variants do not appear to be in LD with G2019S or M1646T. Although these variants are not associated with GCase activity in our analysis, G2019S and M1646T do show a consistent positive direction of effect with what has been demonstrated in previous research (Table 5) [4, 9, 10]. The difference seen in our results compared to the nominal association of M1646T found in a very similar analysis in these cohorts performed by Sosero et al. [10] is due to the removal of GCase outliers in our study. With the inclusion of outliers, we also see an association of this variant in our meta-analysis (b = 1.16, se = 0.38, p = 0.002, rs35303786).
Table 4.
Parkinson’s disease risk SNPs associated with changes in GCase activity
| Cohort | SNP | rsID | Beta | SE | MAF | p | Locus |
|---|---|---|---|---|---|---|---|
| Columbia | 1:155,205,634:T:C | rs76763715 | − 4.21 | 0.35 | 0.041 | 4.55E − 31 | GBA1 |
| 20:6,006,041:C:T | rs77351827 | 0.63 | 0.19 | 0.16 | 0.00083 | CRLS1 | |
| 6:112,243,291:A:G | rs997368 | − 0.49 | 0.16 | 0.24 | 0.0017 | FYN | |
| 4:114,369,065:C:T | rs13117519 | 0.53 | 0.19 | 0.16 | 0.006 | ANK2 | |
| 4:90,666,041:C:T | rs356203 | 0.37 | 0.14 | 0.41 | 0.008 | SNCA | |
| 4:90,626,111:G:A | rs356182 | 0.36 | 0.14 | 0.37 | 0.011 | SNCA | |
| 1:155,135,036:G:A | rs114138760 | − 1.61 | 0.64 | 0.012 | 0.012 | GBA1 | |
| 21:38,852,361:G:A | rs2248244 | 0.39 | 0.16 | 0.26 | 0.012 | DYRK1A | |
| 4:90,636,630:G:A | rs5019538 | 0.33 | 0.14 | 0.33 | 0.022 | SNCA | |
| 17:43,933,307:T:C | rs7221167 | − 0.3 | 0.14 | 0.4 | 0.029 | MAPT | |
| 14:37,989,270:T:C | rs12147950 | − 0.3 | 0.14 | 0.43 | 0.032 | MIPOL1 | |
| 4:90,607,126:C:G | rs356228 | 0.28 | 0.13 | 0.46 | 0.034 | SNCA | |
| 18:31,304,318:G:T | rs1941685 | − 0.29 | 0.14 | 0.47 | 0.037 | ASXL3 | |
| 3:28,705,690:T:C | rs6808178 | 0.3 | 0.14 | 0.36 | 0.039 | LINC00693 | |
| 13:97,865,021:T:C | rs4771268 | − 0.32 | 0.16 | 0.28 | 0.044 | MBNL2 | |
| 14:95,194,760:G:C | rs4905237 | 0.38 | 0.19 | 0.15 | 0.047 | SERPINA13P | |
| PPMI | 1:155,205,634:T:C | rs76763715 | − 5.048 | 0.76 | 0.0087 | 1.25E − 10 | GBA1 |
| 1:155,135,036:G:A | rs35749011 | − 1.43 | 0.5 | 0.02 | 0.0047 | GBA1 | |
| 1:154,898,185:G:C | rs114138760 | − 1.61 | 0.62 | 0.014 | 0.0097 | GBA1 | |
| 4:114,369,065:C:T | rs13117519 | 0.48 | 0.19 | 0.19 | 0.01 | ANK2 | |
| 1:171,719,769:C:T | rs11578699 | 0.47 | 0.19 | 0.19 | 0.012 | VAMP4 | |
| 14:88,464,264:G:T | rs979812 | − 0.48 | 0.2 | 0.45 | 0.018 | GALC | |
| 6:32,578,772:C:A | rs504594 | − 0.46 | 0.22 | 0.14 | 0.035 | HLA-DRB1 | |
| Meta | 1:155,205,634:T:C | rs76763715 | − 4.36 | 0.32 | 0.036 | 5.05E − 43 | GBA1 |
| 1:155,135,036:G:A | rs35749011 | − 1.50 | 0.39 | 0.017 | 0.00014 | GBA1 | |
| 4:114,369,065:C:T | rs13117519 | 0.50 | 0.13 | 0.17 | 0.00016 | ANK2 | |
| 20:6,006,041:C:T | rs77351827 | 0.45 | 0.14 | 0.15 | 0.0013 | CRLS1 | |
| 4:90,666,041:C:T | rs356203 | 0.31 | 0.1 | 0.41 | 0.0028 | SNCA | |
| 4:90,636,630:G:A | rs5019538 | 0.3 | 0.11 | 0.33 | 0.0041 | SNCA | |
| 6:112,243,291:A:G | rs997368 | − 0.34 | 0.12 | 0.22 | 0.0053 | FYN | |
| 14:88,464,264:G:T | rs979812 | − 0.34 | 0.13 | 0.44 | 0.0068 | GALC | |
| 4:90,626,111:G:A | rs356182 | 0.2784 | 0.1037 | 0.3811 | 0.007286 | SNCA | |
| 1:154,898,185:G:C | rs114138760 | − 1.606 | 0.618 | 0.0138 | 0.009358 | GBA1 | |
| 21:38,852,361:G:A | rs2248244 | 0.3915 | 0.1556 | 0.2634 | 0.01187 | DYRK1A | |
| 18:31,304,318:G:T | rs1941685 | − 0.2872 | 0.1376 | 0.4749 | 0.03687 | ASXL3 | |
| 4:90,607,126:C:G | rs356228 | 0.2054 | 0.1005 | 0.4607 | 0.04097 | SNCA | |
| 2:135,438,789:G:A | rs4954162 | 0.2489 | 0.1246 | 0.2193 | 0.04585 | TMEM163 | |
| 14:95,194,760:G:C | rs4905237 | 0.3794 | 0.1904 | 0.1481 | 0.0463 | SERPINA13P |
SNP single-nucleotide polymorphism, se standard error, MAF minor allele frequency, PPMI Parkinson’s progression markers initiative
Table 5.
LRRK2 G2019S and M1646T association statistics for Columbia and PPMI cohorts
| Variant | Cohort | Beta | se | p |
|---|---|---|---|---|
| G2019S | Columbia | 0.64 | 0.4 | 0.11 |
| PPMI | − 0.053 | 1.12 | 0.96 | |
| Meta-analysis | 0.56 | 0.56 | 0.14 | |
| M1646T | Columbia | 0.41 | 0.45 | 0.36 |
| PPMI | 0.82 | 0.54 | 0.13 | |
| Meta-analysis | 0.58 | 0.58 | 0.094 |
se standard error, PPMI Parkinson’s progression markers initiative
Discussion
In the present GWAS, we identified variants in the GAA locus as potential modifiers of GCase activity, in addition to the known associations with GBA1 variants. GAA encodes acid alpha-glucosidase, and deficiency in this enzyme leads to the LSD Pompe disease [25]. There is no known association between Pompe disease and PD, and the variants in the GAA locus are not associated with PD risk, age at onset, or various measures of progression. This is an important observation, since it may suggest that changes in GCase activity alone are insufficient to cause PD, or that the effects of GAA variants on GCase activity are too small to have a meaningful clinical effect. It was hypothesized that the mechanism underlying GBA1-associated PD is an imbalance or disturbance in the lysosomal glycosphingolipid metabolism pathway overall, rather than reduced GCase activity on its own [26]. This is supported by the identification of other genes in this pathway that are associated with PD, such as SMPD1, GALC, ARSA, and ASAH1 [13, 27–34]. Moreover, burden analyses of LSD genes have implicated this pathway in both European and Chinese populations [35, 36]. Previous work has also demonstrated that lysosomal enzymes are highly correlated, especially for GCase, acid alpha-glucosidase, and alpha galactosidase A, which is encoded by GLA [17]. Taken into consideration with the interaction effect observed in the present study, there seems to be mounting evidence in favor of this hypothesis. The mechanism behind the interaction of GCase and acid alpha-glucosidase is still unknown. The products of GCase are lipids and glucose, while acid alpha-glucosidase hydrolyzes glycogen into glucose [37]. Our observation of genotype-dependent correlation between GCase and acid alpha-glucosidase activities may therefore indicate that the GAA locus variants associated with GCase activity could maintain equilibrium in this pathway, as they affect both GCase and alpha-glucosidase activities. Overall, our results support that the association between GAA and GCase activity is complex, with additional genetic, enzymatic, and functional studies being required to determine the presently unknown mechanism behind it.
We further examined the association between known PD-associated loci and GCase activity. As to be expected, we found three PD variants in the GBA1 locus to be associated with GCase activity. We also found an additional association with the ANK2 locus. Phosphorylation in the ANK2 region has been proposed to play a role in PD neurodegeneration through the inhibition of organelle autophagy, but its connection to GCase activity is not clear from the current literature and will need further research [38]. There are also multiple notable associations with ties to GCase activity that may have only failed multiple testing corrections due to sample size. One such locus is GALC, encoding the enzyme galactosylceramidase which, similar to GCase, breaks down large sphingolipids into lipids and ceramide. The association of GALC variants with GCase activity may suggest that a disturbance in lysosomal metabolism could be a causal mechanism in PD, which is also supported by a previous research that found galactosylceramide and GCase to be correlated [17]. We also found nominal associations with multiple SNCA variants, complementing previous associations found between levels of alpha synuclein aggregates and Gcase activity [39–41]. A third interesting locus that fell just shy of the multiple testing correction thresholds was FYN. This gene encodes a kinase that has been shown to phosphorylate alpha-synuclein, which is a key step towards its aggregation into Lewy bodies [42]. We did not replicate previously reported associations with LRRK2 or TMEM175 variants, which are likely due to additional adjustments in our study and the removal of GCase outliers compared to previous studies. Studies in larger cohorts will be required to confirm the associations of these PD loci.
This study has several limitations. The first limitation is a lack of statistical power. Our discovery and replication analyses utilized the largest cohorts with enzyme activity measurements that were available at the time of performing the study and allowed us to complete the first GWAS of GCase activity. This enabled us to discover common variants with moderate effect sizes, such as the lead SNP of the GAA locus (rs9899138, beta = − 0.99, MAF = 0.28) or rarer variants with large effect sizes, such as GBA1 N370S (rs76763715, beta = − 4.21, MAF = 0.038). However, we are lacking in power to detect variants of smaller effect sizes and rarer allele frequencies, and thus are likely missing more modulators of GCase activity yet to be uncovered. Considering this is an exploratory analysis, we believe the methodology should be replicated in a larger, well-powered cohort when data is available. A second limitation is our inability to assess rare pathogenic GBA1 mutations, such as L444P, in our analysis of GCase activity. These variants were not present in our imputed genotype dataset and thus could not be analyzed, but could still exert significant effects on GCase activity. Studying these in future research will be important for understanding the relationship between GBA1 mutations and GCase. Another limitation is the use of only individuals of European and Ashkenazi Jewish ancestry, as there was insufficient data to carry out the study in other populations. Next, our study had minor differences in age and notable differences in sex, particularly in the Columbia cohort, between cases and controls. Both age and sex were adjusted for in all analyses to account for these differences. An additional limitation is the method of enzyme measurement used. Lysosomal activity was measured using dried blood spots, the methodology for which has been thoroughly tested and optimized [12]. However, this method is not guaranteed to capture the true enzyme activity within a functioning lysosomal environment. These results will be strengthened if replicated in future studies that use lysosomal-specific methods of enzyme activity, in cells more representative of disease pathogenesis. Next, we were not able to adequately adjust for disease subtypes or severity in our analyses due to the data available for each cohort. This is a limitation of this study and similar studies that do not take these factors into account, as disease severity could have an effect on the results. Lastly, again due to the limitation of data available, we are undoubtedly missing adjustments for the influence of potentially influential confounding variables. We are also limited in how many confounding variables we can include in our analyses as our sample size increases the risk of overfitting.
In conclusion, we found a novel potential association in the GAA locus associated with GCase activity, which may represent an interaction effect between GCase and acid alpha-glucosidase and could be indicative of a homeostatic relationship between lysosomal enzymes. We also support previously suggested connections of multiple PD-related genes with GCase activity, such as GALC and SNCA, as well as identifying a novel potential association with ANK2. These findings could be significant for improving our understanding of how GCase deficiency is related to overall lysosomal dysfunction and how GCase functions in relation to other lysosomal enzymes. Additionally, associated loci can be taken into account in future research and clinical trials to help control for genetic influences on GCase activity. Due to the novelty of our results and lack of confident association in our replication cohort, further genetic and functional research will be required to validate our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the research participants for contributing to this study. We thank Meron Teferra for her assistance.
Author Contributions
E.N.S. conducted all analyses, interpreted data, developed the methodology, and wrote the manuscript. L.K. developed the methodology and contributed to data analysis and result interpretation. K.S. and E.Y. contributed to data analysis and result interpretation. J.A., F.A., J.A.R., D.S., S.F., C.W., and S.P.S. performed data collection and curation. R.N.A. developed the methodology and performed data collection and curation. Z.G.O. developed the research idea, developed the methodology, contributed to result interpretation, and supervised the study. All authors read, approved, and contributed to editing the final manuscript.
Funding
This study was financially supported through grants from the Michael J. Fox Foundation (MJFF, No. 020700), the Canadian Consortium on Neurodegeneration in Aging (CCNA, No. 049–14), and the Canada First Research Excellence Fund (CFREF, No. 247908), awarded to McGill University for the Healthy Brains for Healthy Lives initiative (HBHL). Additionally, the G-Can (GBA1-Canada) Initiative, an open-science collaborative initiative aimed at addressing GBA1 mutation-based Parkinson’s disease, has made contributions to this research. G-Can is supported by The Hilary & Galen Weston Foundation, Silverstein Foundation, and J. Sebastian van Berkom and Ghislaine Saucier. The Columbia cohort was funded by the National Institutes of Health (no. K02 NS080915 and no. UL1 TR000040, formerly the National Center for Research Resources, Grant No. UL1 RR024156). PPMI—a public–private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s, AskBio, Avid Radiopharmaceuticals, BIAL, BioArctic, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Capsida Biotherapeutics, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Jazz Pharmaceuticals, Johnson & Johnson Innovative Medicine, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Neuron23, Neuropore, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager. Z.G.O. is supported by the Fonds de recherche du Québec—Santé (FRQS) Chercheurs-Boursiers award and is a William Dawson Scholar. K.S. is supported by a post-doctoral fellowship from the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives initiative (HBHL). E.N.S. gratefully acknowledges funding support for this research from Parkinson Canada.
Data Availability
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/access-data-specimens/download-data). For up-to-date information on the study, visit www.ppmi-info.org. Columbia cohort data can be obtained by request. The GCase GWAS summary statistics can be found on the GWAS catalog (https://www.ebi.ac.uk/gwas/). All code for analyses used in this project can be found at https://github.com/gan-orlab/GCase_GWAS. PPMI data used for this study can be obtained by qualified researchers upon completion of a data access application (https://www.ppmi-info.org/access-data-specimens/download-data).
Declarations
Consent to Publish
All authors have read and consented to publish this manuscript.
Competing Interests
Z.G.O received consultancy fees from Lysosomal Therapeutics Inc. (LTI), Idorsia, Prevail Therapeutics, Ono Therapeutics, Denali, Handl Therapeutics, Neuron23, Bial Biotech, Bial, UCB, Capsida, Vanqua bio, Congruence Therapeutics, Takeda, Jazz Guidepoint, Lighthouse and Deerfield.
Ethics Approval and Consent to Participate
Informed consent forms were signed by all participants prior to entering their respective studies, and the study protocol was approved by the institutional review boards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong MJ, Okun MS (2020) Diagnosis and treatment of parkinson disease: a review. JAMA 323(6):548–560. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- 2.Gan-Or Z, Liong C, Alcalay RN (2018) GBA-associated Parkinson’s disease and other synucleinopathies. Curr Neurol Neurosci Rep 18(8):44. 10.1007/s11910-018-0860-4 [DOI] [PubMed] [Google Scholar]
- 3.Hruska KS, LaMarca ME, Scott CR, Sidransky E (2008) Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 29(5):567–583. 10.1002/humu.20676 [DOI] [PubMed] [Google Scholar]
- 4.Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo SH, Mazzoni P, Pauciulo MW et al (2015) Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain 138(Pt 9):2648–2658. 10.1093/brain/awv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moors TE, Paciotti S, Ingrassia A, Quadri M, Breedveld G, Tasegian A, Chiasserini D, Eusebi P et al (2019) Characterization of brain lysosomal activities in gba-related and sporadic Parkinson’s disease and dementia with lewy bodies. Mol Neurobiol 56(2):1344–1355. 10.1007/s12035-018-1090-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krohn L, Ozturk TN, Vanderperre B, Ouled Amar Bencheikh B, Ruskey JA, Laurent SB, Spiegelman D, Postuma RB et al (2020) Genetic, structural, and functional evidence link TMEM175 to synucleinopathies. Ann Neurol 87(1):139–153. 10.1002/ana.25629 [DOI] [PubMed] [Google Scholar]
- 7.Jinn S, Drolet RE, Cramer PE, Wong AH, Toolan DM, Gretzula CA, Voleti B, Vassileva G et al (2017) TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc Natl Acad Sci U S A 114(9):2389–2394. 10.1073/pnas.1616332114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ysselstein D, Nguyen M, Young TJ, Severino A, Schwake M, Merchant K, Krainc D (2019) LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat Commun 10(1):5570. 10.1038/s41467-019-13413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcalay RN, Wolf P, Chiang MSR, Helesicova K, Zhang XK, Merchant K, Hutten SJ, Scherzer C et al (2020) Longitudinal measurements of glucocerebrosidase activity in Parkinson’s patients. Ann Clin Transl Neurol 7(10):1816–1830. 10.1002/acn3.51164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosero YL, Yu E, Krohn L, Rudakou U, Mufti K, Ruskey JA, Asayesh F, Laurent SB et al (2021) LRRK2 p. M1646T is associated with glucocerebrosidase activity and with Parkinson’s disease. Neurobiol Aging 103:142141–142145. 10.1016/j.neurobiolaging.2021.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakanaka K, Waters CH, Levy OA, Louis ED, Chung WK, Marder KS, Alcalay RN (2014) Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns 23(1):114–120. 10.1007/s10897-013-9618-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuser AJ, Verheijen FW, Bali D, van Diggelen OP, Germain DP, Hwu WL, Lukacs Z, Muhl A et al (2011) The use of dried blood spot samples in the diagnosis of lysosomal storage disorders–current status and perspectives. Mol Genet Metab 104(1–2):144–148. 10.1016/j.ymgme.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 13.Senkevich K, Zorca CE, Dworkind A, Rudakou U, Somerville E, Yu E, Ermolaev A, Nikanorova D et al (2023) GALC variants affect galactosylceramidase enzymatic activity and risk of Parkinson’s disease. Brain 146(5):1859–1872. 10.1093/brain/awac413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY et al (2016) Next-generation genotype imputation service and methods. Nat Genet 48(10):1284–1287. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedariti M, Frattini E, Baden P, Cogo S, Civiero L, Ziviani E, Zilio G, Bertoli F et al (2022) LRRK2 kinase activity regulates GCase level and enzymatic activity differently depending on cell type in Parkinson’s disease. NPJ Parkinsons Dis 8(1):92. 10.1038/s41531-022-00354-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcalay RN, Wolf P, Levy OA, Kang UJ, Waters C, Fahn S, Ford B, Kuo SH et al (2018) Alpha galactosidase A activity in Parkinson’s disease. Neurobiol Dis 112:85–90. 10.1016/j.nbd.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Ferreira T, Morris AP, Medland SE, Genetic Investigation of ATC, Replication DIG, Meta-analysis C, Madden PA, Heath AC, Martin NG, Montgomery GW, Weedon MN, Loos RJ, Frayling TM, McCarthy MI, Hirschhorn JN, Goddard ME, Visscher PM (2012) Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet 44 (4):369–375, S361–363. 10.1038/ng.2213 [DOI] [PMC free article] [PubMed]
- 19.R Development Core Team (2023) R: A language and environment for statistcal computing. 4.3.1 edn. R Foundation for Statistical Computing, Vienna, Austria
- 20.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR et al (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26(18):2336–2337. 10.1093/bioinformatics/btq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blauwendraat C, Heilbron K, Vallerga CL, Bandres-Ciga S, von Coelln R, Pihlstrom L, Simon-Sanchez J, Schulte C et al (2019) Parkinson’s disease age at onset genome-wide association study: Defining heritability, genetic loci, and alpha-synuclein mechanisms. Mov Disord 34(6):866–875. 10.1002/mds.27659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwaki H, Blauwendraat C, Leonard HL, Kim JJ, Liu G, Maple-Grodem J, Corvol JC, Pihlstrom L et al (2019) Genomewide association study of Parkinson’s disease clinical biomarkers in 12 longitudinal patients’ cohorts. Mov Disord 34(12):1839–1850. 10.1002/mds.27845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA et al (2019) Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol 18(12):1091–1102. 10.1016/S1474-4422(19)30320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan MMX, Lawton MA, Jabbari E, Reynolds RH, Iwaki H, Blauwendraat C, Kanavou S, Pollard MI et al (2021) Genome-wide association studies of cognitive and motor progression in Parkinson’s disease. Mov Disord 36(2):424–433. 10.1002/mds.28342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens D, Milani-Nejad S, Mozaffar T (2022) Pompe disease: a clinical, diagnostic, and therapeutic overview. Curr Treat Options Neurol 24(11):573–588. 10.1007/s11940-022-00736-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machtel R, Boros FA, Dobert JP, Arnold P, Zunke F (2023) From lysosomal storage disorders to Parkinson’s disease - challenges and opportunities. J Mol Biol 435(12):167932. 10.1016/j.jmb.2022.167932 [DOI] [PubMed] [Google Scholar]
- 27.Alcalay RN, Mallett V, Vanderperre B, Tavassoly O, Dauvilliers Y, Wu RYJ, Ruskey JA, Leblond CS et al (2019) SMPD1 mutations, activity, and alpha-synuclein accumulation in Parkinson’s disease. Mov Disord 34(4):526–535. 10.1002/mds.27642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, Gana-Weisz M, Raymond D et al (2013) The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology 80(17):1606–1610. 10.1212/WNL.0b013e31828f180e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senkevich K, Rudakou U, Gan-Or Z (2022) New therapeutic approaches to Parkinson’s disease targeting GBA, LRRK2 and Parkin. Neuropharmacology 202:108822. 10.1016/j.neuropharm.2021.108822 [DOI] [PubMed] [Google Scholar]
- 30.Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, International Parkinson’s Disease Genomics C, Heutink P, Shulman JM (2017) Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140 (12):3191-3203. 10.1093/brain/awx285 [DOI] [PMC free article] [PubMed]
- 31.Makarious MB, Lake J, Pitz V, Fu AY, Guidubaldi JL, Solsberg CW, Bandres-Ciga S, Leonard HL et al (2023) Large-scale rare variant burden testing in Parkinson’s disease. Brain 146(11):4622–4632. 10.1093/brain/awad214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan HX, Wang YG, Zhao YW, Zeng Q, Wang Z, Fang ZH, Zhang Y, Zhou X et al (2022) Evaluating the role of ARSA in Chinese patients with Parkinson’s disease. Neurobiol Aging 109:269–272. 10.1016/j.neurobiolaging.2021.08.008 [DOI] [PubMed] [Google Scholar]
- 33.Deng S, Deng X, Song Z, Xiu X, Guo Y, Xiao J, Deng H (2016) Systematic genetic analysis of the SMPD1 gene in Chinese patients with Parkinson’s disease. Mol Neurobiol 53(7):5025–5029. 10.1007/s12035-015-9426-5 [DOI] [PubMed] [Google Scholar]
- 34.Mao CY, Yang J, Wang H, Zhang SY, Yang ZH, Luo HY, Li F, Shi M et al (2017) SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Parkinsonism Relat Disord 34:59–61. 10.1016/j.parkreldis.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 35.Straniero L, Rimoldi V, Monfrini E, Bonvegna S, Melistaccio G, Lake J, Soldà G, Aureli M et al (2022) Role of lysosomal gene variants in modulating associated Parkinson’s disease risk. Movement Disord 37(6):1202–1210. 10.1002/mds.28987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao YW, Pan HX, Liu ZH, Wang YG, Zeng Q, Fang ZH, Luo TF, Xu K et al (2021) The association between lysosomal storage disorder genes and Parkinson’s disease: a large cohort study in Chinese mainland population. Front Aging Neurosci 13:749109. 10.3389/fnagi.2021.749109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roig-Zamboni V, Cobucci-Ponzano B, Iacono R, Ferrara MC, Germany S, Bourne Y, Parenti G, Moracci M et al (2017) Structure of human lysosomal acid alpha-glucosidase-a guide for the treatment of Pompe disease. Nat Commun 8(1):1111. 10.1038/s41467-017-01263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arango D, Bittar A, Esmeral NP, Ocasion C, Munoz-Camargo C, Cruz JC, Reyes LH, Bloch NI (2021) Understanding the potential of genome editing in Parkinson’s disease. Int J Mol Sci 22(17):9241. 10.3390/ijms22179241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tayebi N, Parisiadou L, Berhe B, Gonzalez AN, Serra-Vinardell J, Tamargo RJ, Maniwang E, Sorrentino Z et al (2017) Glucocerebrosidase haploinsufficiency in A53T alpha-synuclein mice impacts disease onset and course. Mol Genet Metab 122(4):198–208. 10.1016/j.ymgme.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz SS, Petersen D, Marlet FR, Kucukkose E, Galvagnion C (2021) The interplay between glucocerebrosidase, alpha-synuclein and lipids in human models of Parkinson’s disease. Biophys Chem 273:106534. 10.1016/j.bpc.2020.106534 [DOI] [PubMed] [Google Scholar]
- 41.Fishbein I, Kuo YM, Giasson BI, Nussbaum RL (2014) Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain 137(Pt 12):3235–3247. 10.1093/brain/awu291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura T, Yamashita H, Takahashi T, Nakamura S (2001) Activated Fyn phosphorylates alpha-synuclein at tyrosine residue 125. Biochem Biophys Res Commun 280(4):1085–1092. 10.1006/bbrc.2000.4253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/access-data-specimens/download-data). For up-to-date information on the study, visit www.ppmi-info.org. Columbia cohort data can be obtained by request. The GCase GWAS summary statistics can be found on the GWAS catalog (https://www.ebi.ac.uk/gwas/). All code for analyses used in this project can be found at https://github.com/gan-orlab/GCase_GWAS. PPMI data used for this study can be obtained by qualified researchers upon completion of a data access application (https://www.ppmi-info.org/access-data-specimens/download-data).