Abstract
Healthy older men manifest concomitant hypoandrogenemia and attenuation of LH pulse size. Because exogenous GnRH remains effective, a plausible intuition is that aging reduces hypothalamic GnRH secretion, thus mediating relative hypogonadotropic hypogonadism. To assess the impact of age on central GnRH outflow indirectly, we quantitated graded suppression of pulsatile LH secretion by saline and escalating doses of a potent and selective GnRH-receptor antagonist, ganirelix, in 18 healthy men ages 23–72 yr. The rationale is that ganirelix should reduce the amplitude of LH pulses in proportion to both drug concentration and endogenous GnRH feedforward. To this end, blood was sampled every 10 min for 2 h before and 16 h after sc administration of saline or ganirelix and for 3 additional hours after iv injection of a fixed dose of GnRH (100 ng/kg); concentrations of LH and ganirelix were measured by immunochemiluminometry and RIA, respectively; and pulsatile LH secretion was quantitated by a deconvolution procedure. Log-linear regression analysis was used to estimate the sensitivity of pulsatile LH secretion to inhibition by a unit increase in serum ganirelix concentrations in each subject. Statistical analyses revealed that increasing age markedly attenuated the capability of ganirelix to decrease LH pulse size (viz., r = −0.648; P = 0.004). In contrast, age did not modify the competitive interaction between injected GnRH and ganirelix. These joint outcomes support the clinical hypothesis that age diminishes hypothalamic GnRH outflow without impairing GnRH action in healthy men.
Abbreviations: CV, Coefficient(s) of variation; Te, testosterone
Aging is marked by a 30–50% decline in systemic testosterone (Te) availability in healthy community-dwelling men (1–5). Epidemiological studies have correlated hypoandrogenemia with sarcopenia, osteopenia, diminished physical stamina, sexual dysfunction, visceral adiposity, reduced quality of life, depressive mood, and cognitive deficits (6–13). Te supplementation ameliorates some of these signs and symptoms (14–17). Despite the high prevalence of aging-associated hypoandrogenemia, the precise cause remains unknown (18).
Mechanistic studies indicate that both the calculated mass of LH secreted in bursts (and therefore the incremental amplitude of LH pulses) and systemic Te availability are reduced significantly in older men, thereby mimicking hypogonadotropic hypogonadism (19–25). Smaller LH secretory bursts are not attributable to impaired gonadotrope responsiveness to GnRH, inasmuch as injected GnRH stimulates LH release normally acutely and over 14 d in healthy elderly individuals (26–28). An alternative clinical hypothesis is that aging decreases hypothalamic GnRH secretion, thereby limiting feedforward drive to LH pulses. This mechanistic consideration is significant, inasmuch as the amplitude of LH pulses correlates with Te secretion in vitro and in vivo (28–33). In addition, iv infusion of discrete pulses of biosynthetic LH can restore normal Te secretion in young men when pituitary LH output is reduced experimentally by a potent GnRH-receptor blocker (34). Accordingly, understanding the basis for attenuation of high-amplitude LH secretory bursts in the aging male is relevant to elucidating possible central mechanisms that contribute to hypoandrogenemia.
Neuronal GnRH secretion cannot be measured directly in the human (18). To probe hypothalamic GnRH outflow indirectly, the present study compares the capability of graded doses (and thereby increasing serum concentrations) of a potent and specific GnRH-receptor antagonist to suppress pulsatile LH secretion in healthy men ages 23–72 yr. The mechanistic assumption is that selectively blocking central GnRH action will decrease pulsatile LH secretion in proportion to opposing GnRH feedforward (35). This reasoning follows from the physiological observations that: 1) GnRH is the primary agonist of burst-like LH release (36, 37); and 2) GnRH-receptor blockers acutely repress the pulsatile rather than basal component of LH secretion (38, 39).
Subjects and Methods
Clinical screening
Eighteen men, ages 23 to 72 yr (average age, 45 yr; two or three men in each decade), were enrolled after providing voluntary written informed consent, as approved by the Mayo Clinic Institutional Review Board. Participants were healthy community-dwelling men within 20% of ideal body weight, who had not undertaken transmeridian travel within 10 d or consumed alcohol, caffeine, or prescribed medications for 48 h or 5 half-lives. Detailed medical inventory excluded a history of infertility, systemic disease, recent weight change (more than 2 kg change in the preceding 6 wk), hormonal therapy, or psychoactive drug use. Medical history (including libido and erectile function), physical examination (including testis size), and fasting morning (0800 h) biochemical tests of renal, hepatic, hematological and metabolic function (fasting plasma glucose, electrolytes, and thyroid function) were within normal limits for age. Subjects were compensated for the time spent in the study according to an IRB-defined schedule.
Sampling protocol
Eligible volunteers were admitted to the General Clinical Research Center (GCRC) for four separate randomly ordered, overnight inpatient studies scheduled at least 1 wk apart. Blood samples (1.0 ml) were withdrawn every 10 min beginning at 1800 h for a total of 21 h through forearm iv catheters. Samples were allowed to clot at room temperature, and sera were frozen at −20C for later assay of LH, ganirelix, and Te concentrations.
Ganirelix is a potent, selective antagonist of GnRH action that binds competitively to the cognate receptor (40). The plasma half-life of ganirelix is 15 ± 2 h (41), which coincides with its 20- to 28-h inhibitory effect in men after sc injection (34). Ganirelix doses of 0 (saline), 0.1, 0.3, or 1.0 mg/m2 were administered sc in double-blind fashion on separate days in randomly assigned order at 2000 h (2 h after the beginning of blood sampling). A single submaximally effective GnRH stimulus (100 ng/kg iv bolus) was given 16 h after ganirelix administration (viz., 3 h before the end of blood sampling) to verify competitive inhibition in each subject.
Assays
Serum LH concentrations were measured in each 10-min sample in duplicate by automated chemiluminescence assay (ACS 180, Bayer, Norwood, MA), using the First International Reference Preparation as the standard. Intraassay coefficients of variation (CV) were 4.7, 3.5, and 3.8%, and interassay CV were 8, 3.7, and 4.7% at LH concentrations of 4.4, 18, and 39 IU/liter, respectively. Procedural sensitivity was 0.2 IU/liter. All samples were measurable (3 or more sd values above LH-deficient serum). Total Te concentrations were assayed in serum collected at the beginning of blood sampling (1800 h). Intra- and inter-assay CV were 6.8 and 8.3%, and assay sensitivity was 8 ng/dl (multiply by 0.0347 to convert to nanomoles per liter) (21, 42). Thus, 9,144 separate samples were assayed for LH and Te (total, 18,288).
Serum ganirelix concentrations were measured in duplicate by RIA using polyclonal rabbit antisera (Anaspec Inc., San Jose, CA), as initially described by others (43). The antiserum does not cross-react detectably with native GnRH at concentrations ranging from 30–1000 ng/ml. Ganirelix was radioiodinated via the chloramine-T reaction. Incubations were conducted with 50 μl serum, 5000 dpm radiolabeled ganirelix, and an antibody dilution of 1:3000 in RIA buffer [0.1 m phosphate buffer (pH 7.4), 0.8% NaCl, 0.5% BSA, 0.01% thimerosal, 0.01% Triton X-100, 0.1 mm EDTA]. Bound and free ligand were separated by precipitation with goat antirabbit antiserum. Mean intraassay CV were 8.9, 5.7, and 18.7%, and interassay CV were 8.2, 4.0, and 9.2% at 0.5, 1.0, and 10 ng/ml, respectively. Assay sensitivity was 0.05 ng/ml. Ganirelix concentrations were determined in a 2-h serum pool collected just before GnRH injection. A 2-h pool is valid, given the prolonged serum half-life of ganirelix in the human (41).
Analytical procedures
According to classical concepts of a competitive single ligand-receptor interaction, the magnitude of an observed biological response is determined 3-fold jointly by concentration(s) of the agonist and any competing antagonist and properties of the receptor-response pathway (35). These minimal assumptions are satisfied because: 1) the GnRH receptor-effector pathway responds normally or in an enhanced fashion to single and repeated (14-d) pulses of GnRH in older men (27, 28); 2) GnRH is the exclusive or predominant physiological agonist of the cognate human pituitary receptor (37); and 3) ganirelix acts strictly competitively in vitro and in vivo (44). In the last regard, we have shown that iv injection of 100 ng/kg GnRH is submaximally stimulatory (37) and inhibited competitively by ganirelix in men and women (34, 45, 46).
The primary outcome of interest is pulsatile LH secretion (the summed mass of LH secreted in bursts) over the last 8 h just before, and the 3 h immediately after, injection of GnRH in each of the four interventions (saline and three doses of ganirelix). The foregoing time intervals comprise inclusively 9–16 and 17–19 h after ganirelix administration, respectively, thereby encompassing periods of sustained LH suppression and exogenous GnRH-induced LH release (34, 45, 46).
Pulsatile LH secretion was estimated from each 21-h LH concentration time series by deconvolution analysis (47). The methodology assumed Gaussian-approximated secretory bursts and previously determined biexponential LH half-lives of 18 and 90 min, with 0.63 as the proportion of slow/total decay amplitude (48). The empirical estimates do not vary with age, as corroborated analytically and by infusion of biosynthetic LH in young and older men (23, 24, 46). The entire LH profile was analyzed, and then pulsatile secretion was segmented into respective 8-h and 3-h intervals for further statistical analysis.
Statistical analysis
Inhibitory sensitivity was estimated in each volunteer by regressing the natural logarithm of pulsatile LH secretion during the last 8 h before (or 3 h after) GnRH injection linearly on measured serum ganirelix concentrations. The slope of the relationship in any given subject provides an index of the sensitivity of pulsatile LH secretion to inhibition by increasing concentrations of ganirelix independently of absolute baseline LH concentrations. To test the hypothesis that age attenuates fractional inhibition by ganirelix, the 18 individual slope values were regressed linearly on age. The a priori postulate was that age decreases ganirelix’s inhibition of LH secretory-burst mass; i.e. the cohort slope on age is significantly negative (49).
Two-way repeated-measures ANOVA was applied to assess the relationship between serum ganirelix concentrations (dependent variable) and both ganirelix dose (four fixed factors) and age (one random factor) (independent variables) (49). The variance-covariance matrix in the generalized linear-model structure allowed for within-subject but not between-subject response correlations by dose (50). Estimation of model parameters was by maximum likelihood (51). Homogeneity of slopes was tested by an F ratio at P < 0.05. Post hoc comparisons among means were made by Tukey’s test. Analyses were performed using SAS Proc Mixed (version 8.02; SAS Institute, Inc., Cary, NC). Power was defined as 1 minus the estimated type II error expressed as a percentage (52).
Data are presented as the mean ± sem (or ± sd for individual slopes).
Results
Baseline concentrations of LH and total Te averaged over the 18-h interval before GnRH injection on the saline (zero-dose ganirelix) day were 3.4 ± 0.41 (range, 1.5–7.4) IU/liter and 460 ± 46 (range, 183–780) ng/dl (multiply by 0.0347 to convert to nanomoles per liter), respectively. Mean values did not differ by age, as reported previously in other healthy cohorts (28, 53, 54). Calculated free and bioavailable Te concentrations declined linearly with age (both P < 0.01) (55). Serum ganirelix concentrations after doses of 0, 0.1, 0.3, and 1.0 mg/m2 increased logarithmically (P < 0.001) (Fig. 1). Based on repeated-measures two-way ANOVA, age did not significantly alter dose-dependent increases in ganirelix concentrations.
Fig. 1.
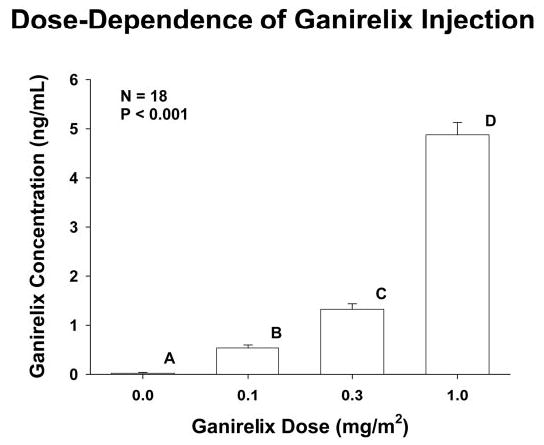
Relationship between serum ganirelix concentrations and logarithmically increasing doses of ganirelix in 18 normal men. The overall P value was estimated by repeated-measures ANOVA. Post hoc comparisons were made by the Tukey procedure. Means with different (unshared) alphabetic superscripts differ by P < 0.05. Data are the mean ± sem (n = 18).
Figure 2 depicts LH concentrations sampled every 10 min for 21 h stratified in relation to the four randomly ordered interventions in one 34-yr-old subject. By visual inspection, ganirelix suppressed peak LH concentrations dose-dependently. Repeated-measures one-way ANOVA revealed that absolute maximal (5-point moving average) LH concentrations over the 21 h decreased with increasing ganirelix dose(0, 0.1, 0.3, and 1.0 mg/m2), as follows: 10.3 ± 1.3, 6.7 ± 0.96, 5.7 ± 0.86, and 3.9 ± 0.62 IU/liter (P < 0.001). Post hoc comparisons revealed that responses differed significantly among any pair of saline and the lowest and highest inhibitor doses. Absolute nadir (5-point moving average) LH concentrations were 2.2 ± 0.31, 0.81 ± 1.12, 0.81 ± 0.21, and 0.55 ± 0.09 IU/liter and occurred 583 ± 85, 737 ± 50, 712 ± 60, and 820 ± 42 min after ganirelix administration. ANOVA disclosed that compared with saline the three doses of ganirelix enforced equivalent decreases in, and time delays to, nadir LH concentrations (each P < 0.01). Thus, nonzero ganirelix doses determine peak LH concentrations, but not nadir values or the time latency to reach the nadir.
Fig. 2.
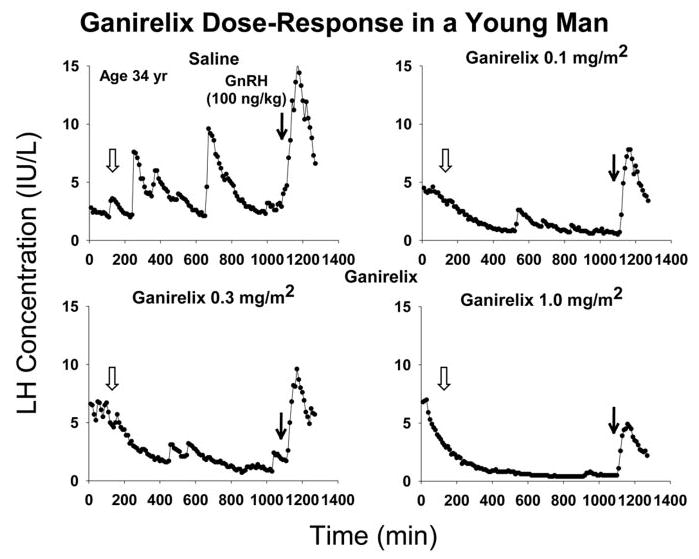
Ganirelix dose-response study illustrated in a 34-yr-old man. Each set of LH concentrations includes a 2-h baseline (0–120 min), 16 h of monitoring after saline or ganirelix injection (open arrow, at 130 min), and 3 additional hours of sampling after fixed iv GnRH bolus (bold arrow, at 1080 min). Data reflect 10-min sampling beginning at 1800 h.
Deconvolution analysis was used to quantitate pulsatile (putatively GnRH-dependent) LH secretion before (8 h) and after (3 h) GnRH injection (Fig. 3). Ganirelix reduced pulsatile LH secretion dose-dependently during endogenous GnRH drive (Fig. 3A) and after exogenous GnRH stimulation (Fig. 3B) (both P < 0.001). The highest ganirelix dose (1.0 mg/m2) was significantly more inhibitory than the lower two ganirelix doses, and all three doses were significantly inhibitory compared with saline (each P ≤ 0.007).
Fig. 3.
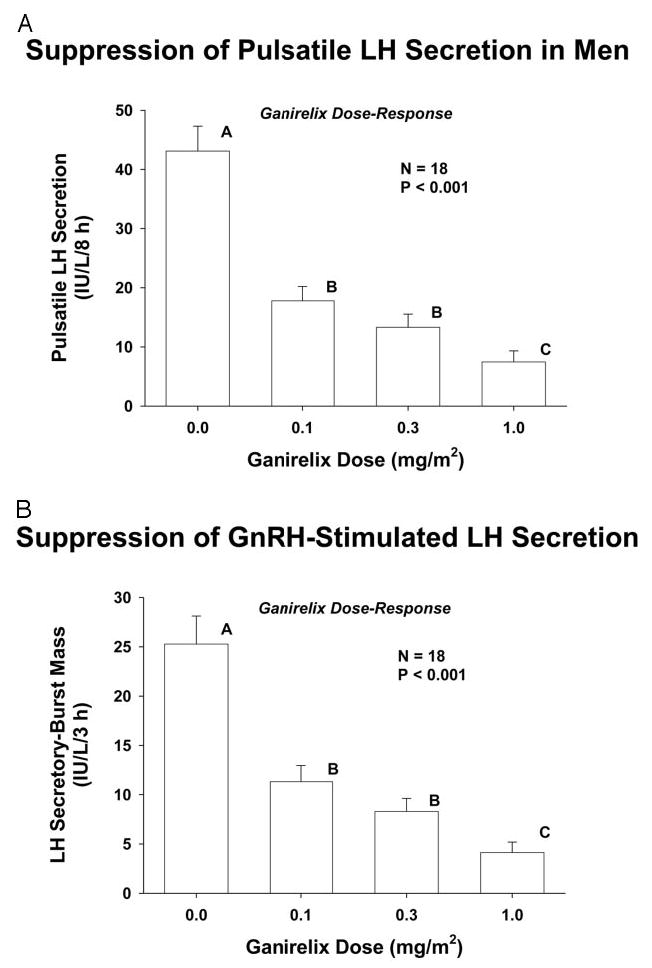
Ganirelix suppresses pulsatile LH secretion (A) and exogenous GnRH-stimulated LH release (B) dose-dependently. Unshared (unique) alphabetic superscripts identify significantly different means by the post hoc Tukey test. Data are the mean ± sem in 18 healthy men ages 23–72 yr.
Figure 4 illustrates log-linear regression of pulsatile LH secretion (dependent variable) on ganirelix concentrations (independent variable) in nine individual subjects whose ages (shown) spanned the six decades studied. The absolute value of the slope of each regression is a measure of inhibitory sensitivity in that particular subject; viz., the fractional degree to which pulsatile LH secretion is reduced by a unit increase in the serum ganirelix concentration.
Fig. 4.
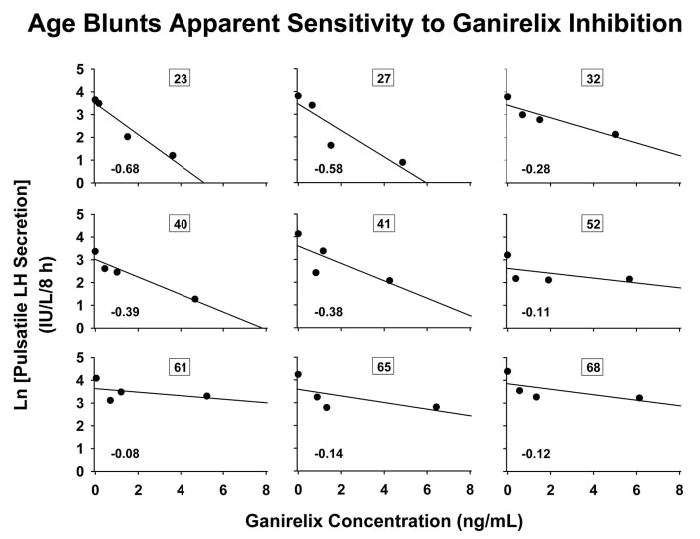
Illustration of log-linear relationship between pulsatile LH secretion (IU per liter per 8 h) and serum ganirelix concentrations (nanograms per milliliter) in nine men of the indicated ages (numbers in boxes) among the 18 subjects studied. The slope of an individual regression line (bold face number) provides an estimate of inhibitory sensitivity to ganirelix in that subject (viz., fractional suppression of pulsatile LH secretion per unit increase in ganirelix concentration; see Subjects and Methods). All 18 slope values are given in Fig. 5.
Figure 5 relates inhibitory sensitivity of pulsatile LH secretion (dependent variable) to age (independent variable) in the cohort of 18 men. Statistical analysis rejected the null hypothesis of a zero slope at P = 0.004 (r = −0.648). The estimated absolute slope (± sd) of the regression was 0.010 ± 0.003.
Fig. 5.
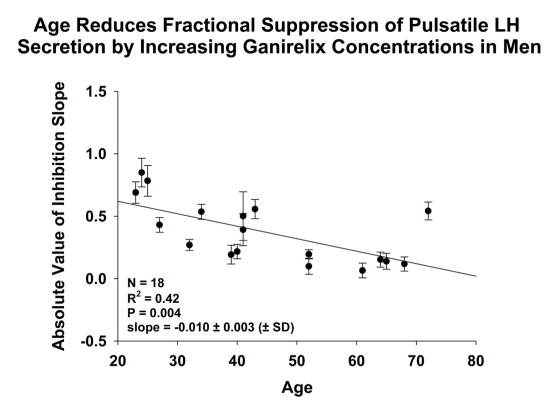
Linear regression of inhibitory sensitivity of pulsatile LH secretion on age in 18 men. Each point represents the slope (± sd) of the relationship between the logarithm of pulsatile LH secretion and the ganirelix concentration (slopes are illustrated in Fig. 4).
Figure 6 relates comparably estimated inhibitory sensitivity of GnRH-stimulated pulsatile LH secretion to age. Statistical analysis revealed that age does not alter ganirelix’s concentration-dependent inhibition of a constant GnRH stimulus. Statistical estimates predicted a power of at least 95% for detecting ≥ 30% contribution by age to the variance in slope at P < 0.05 when n = 18. Thus, the effect of age is not on GnRH action but on GnRH availability.
Fig. 6.
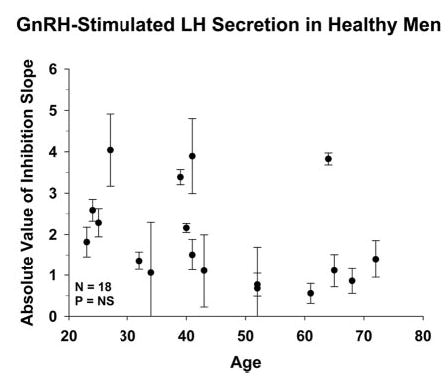
Lack of relationship between inhibitory sensitivity of exogenous GnRH-stimulated LH release (dependent variable) and age (independent variable) in 18 healthy men. The format of data presentation is that of Fig. 5.
Discussion
The present investigation in 18 normal men supports the postulate that age reduces hypothalamic outflow of GnRH to the pituitary gland, thereby resulting in low-amplitude LH pulses in the face of reduced Te availability. In particular, assuming that a burst of GnRH drives a pulse of LH (36, 37), impaired suppression of pulsatile LH secretion by escalating concentrations of a competitive GnRH-receptor antagonist would predict diminished GnRH availability and/or enhanced gonadotrope sensitivity to GnRH (35). The present data argue against the latter conjecture, inasmuch as age did not influence competitive inhibition of a fixed exogenous GnRH stimulus. In principle, attenuation of GnRH feedforward in aging men could reflect a smaller amount and/or an altered waveform of neuronal GnRH delivery to gonadotrope cells (24, 25, 56).
The accompanying investigative paradigm comprised graded suppression of endogenous and exogenous GnRH-driven LH secretory bursts by a logarithmic range of doses of a potent GnRH-receptor antagonist. Another GnRH blocker was used at a single dose earlier in a clinical study of the relative GnRH dependence of the preovulatory LH surge (57). The current paradigm adopts an analogous strategy in aging men but differs experimentally by way of: 1) evaluating GnRH-receptor blockade over a 10-fold range of antagonist doses in each subject; 2) measuring serum ganirelix concentrations, and thereby relating LH secretion to simultaneous concentrations of the inhibitor in each subject; 3) quantitating pulsatile LH secretion as the endpoint of GnRH-receptor antagonism, given that pulsatile but not basal LH secretion reflects GnRH drive acutely (36–38); and 4) employing a fixed exogenous GnRH stimulus to assess whether the GnRH-ganirelix-receptor interaction changes with age (35). Under this extended set of study conditions, older age predicts diminished feedforward drive of pulsatile LH secretion by endogenous, but not by exogenous, GnRH.
Basic laboratory studies point to decreased GnRH outflow in the aged male rat (18). For example, GnRH release by mediobasal hypothalamic fragments and castration-induced LH secretion are impaired; direct pituitary effects of GnRH are preserved; and LH pulse amplitude falls in the older rodent (58–64). Incremental LH pulse amplitude (or LH secretory-burst mass) also declines in older men despite retention of gonadotrope responsiveness to exogenous GnRH acutely and over a 2-wk interval of pulsatile iv infusions (20, 27, 28). In fact, the size of LH secretory bursts is approximately 50% lower in elderly than young men studied in each of the gonadally intact, low-Te, and low-estrogen milieus (24, 25, 65). The generality of diminished burst-like LH secretion in these contexts is consistent with the accompanying evidence for attenuated GnRH outflow.
Whether heightened sex-steroid negative feedback contributes to reduced hypothalamic GnRH drive in the older male is not known. In this regard, exogenous androgens reportedly are either more or less inhibitory and endogenous androgens are less inhibitory in older than young volunteers (24, 25, 32, 66). Other investigations establish that injections of human chorionic gonadotropin or recombinant human LH fail to elevate total or bioavailable Te concentrations maximally in aging subjects (46, 67, 68). According to biomathematical models of the hypothalamo-pituitary-gonadal axis, tripartite diminution of GnRH feedforward, androgenic negative feedback, and Leydig-cell steroidogenesis are necessary and sufficient to explicate the ensemble features of low-amplitude LH pulses, more irregular patterns of LH release, and hypoandrogenemia in the elderly male (24, 25, 53, 54). Thus, diminished GnRH drive in the older male may be part of a larger array of regulatory deficits.
The clinical implications of reduced hypothalamic GnRH drive to pulsatile LH secretion in older individuals are 2-fold. First, a proximate inference is that aging modifies one or more central neuronal pathways that direct pulsatile GnRH secretion. Candidate neurochemical signals would include glutamine, γ-aminobutyric acid, norepinephrine, neuropeptide Y, and the cocaine amphetamine-regulated transcript (69–71). And, secondly, a physiological issue is whether decreased GnRH secretion represents an adaptive process in aging. The query arises because experimental withdrawal of LH during young-adult life in the male rat significantly enhances testis responsiveness to the same gonadotropin in older age (72).
The validity of the accompanying inferences is supported by several considerations: 1) serum ganirelix concentrations varied in proportion to inhibitor dose but not age; 2) the GnRH antagonist suppressed both (deconvolved) LH secretory-burst mass and (model-free) LH peak height dose-dependently; 3) the within-subject dose-response design permitted regression of inhibitory responses on a 10-fold range of ganirelix concentrations; and 4) injection of a fixed GnRH stimulus corroborated competitive and age-invariant inhibition of exogenous GnRH action. By way of qualification, although age alone accounted for more than 60% of the variance in the sensitivity of pulsatile LH secretion to inhibition by ganirelix, longitudinal investigations in healthy aging volunteers would be required to define the precise age-dependence of decreased GnRH outflow.
In summary, increasing age markedly blunts the capability of graded blockade of the GnRH receptor to reduce endogenously stimulated LH pulses in healthy men. In contrast, age does not affect the relationship between ganirelix dose and serum ganirelix concentrations or exogenous GnRH action. These collective outcomes support the hypothesis that aging attenuates GnRH outflow to gonadotrope cells, thereby contributing to relative hypogonadotropic hypogonadism in older men.
Acknowledgments
We thank Kris Nunez for manuscript support and Jason Kerkvliet for performing the ganirelix assays.
Footnotes
This work was supported in part by the National Center for Research Resources (Rockville, MD), Grant M01 RR00585 to the General Clinical Research Center of the Mayo Clinic and Foundation, and National Institutes of Health (Bethesda, MD) Grants RO1 AG023133 and DK060717. P.Y.L. was supported by a Neil Hamilton Fairley Research Fellowship from the National Health and Medical Research Council of Australia (Grant ID 262025). This focused report necessarily omits many primary references because of editorial constraints.
References
- 1.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Kaiser FE, Perry 3rd HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 4.Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Kaiser F, Raum WJ, Perry 3rd HM, Flood JF, Jensen J, Silver AJ, Roberts E. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor I to growth hormone. Proc Natl Acad Sci USA. 1997;94:7537–7542. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nankin HR, Calkins JH. Decreased bioavailable testosterone in aging normal and impotent men. J Clin Endocrinol Metab. 1986;63:1418–1420. doi: 10.1210/jcem-63-6-1418. [DOI] [PubMed] [Google Scholar]
- 7.Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- 8.Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 9.Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ. Interrelationships of serum testosterone and free testosterone index with fat free mass and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–E294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 10.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 11.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab. 2003;88:5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 12.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 13.Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab. 2002;87:3125–3135. doi: 10.1210/jcem.87.7.8630. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 15.Ly LP, Jimenez M, Zhuang TN, Celermajer DS, Conway AJ, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab. 2001;86:4078–4088. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 16.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E280–E286. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 17.Liu PY, Swerdloff RS, Veldhuis JD. The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab. 2004;89:4789–4796. doi: 10.1210/jc.2004-0807. [DOI] [PubMed] [Google Scholar]
- 18.Veldhuis JD, Iranmanesh A, Keenan DM 2004 An ensemble perspective of aging-related hypoandrogenemia in men. In: Winters SJ, ed. Male hypogonadism: basic, clinical, and theoretical principles. Totowa, NJ: Humana Press; 261–284
- 19.Winters SJ, Troen P. Episodic luteinizing hormone (LH) secretion and the response of LH and follicle-stimulating hormone to LH-releasing hormone in aged men: evidence for coexistent primary testicular insufficiency and an impairment in gonadotropin secretion. J Clin Endocrinol Metab. 1982;55:560–565. doi: 10.1210/jcem-55-3-560. [DOI] [PubMed] [Google Scholar]
- 20.Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab. 1992;75:52–58. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling–a clinical research center study. J Clin Endocrinol Metab. 1995;80:3025–3031. doi: 10.1210/jcem.80.10.7559891. [DOI] [PubMed] [Google Scholar]
- 22.Vermeulen A, Deslypere JP, De Meirleir K. A new look at the andro-pause: altered function of the gonadotrophs. J Steroid Biochem. 1989;32:163–165. doi: 10.1016/0022-4731(89)90158-1. [DOI] [PubMed] [Google Scholar]
- 23.Keenan DM, Veldhuis JD, Yang R. Joint recovery of pulsatile and basal hormone secretion by stochastic nonlinear random-effects analysis. Am J Physiol. 1998;275:R1939–R1949. doi: 10.1152/ajpregu.1998.275.6.R1939. [DOI] [PubMed] [Google Scholar]
- 24.Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol. 2001;281:R1917–R1924. doi: 10.1152/ajpregu.2001.281.6.R1917. [DOI] [PubMed] [Google Scholar]
- 25.Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol. 2001;280:R1755–R1771. doi: 10.1152/ajpregu.2001.280.6.R1755. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman JM, Giri M, Deslypere JM, Thomas G, Vermeulen A. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab. 1991;72:1255–1260. doi: 10.1210/jcem-72-6-1255. [DOI] [PubMed] [Google Scholar]
- 27.Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and α-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]
- 28.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- 29.Rowe PH, Racey PA, Lincoln GA, Ellwood M, Lehane J, Shenton JC. The temporal relationship between the secretion of luteinizing hormone and testosterone in man. J Endocrinol. 1975;64:17–25. [PubMed] [Google Scholar]
- 30.Davies TF, Platzer M. The perifused Leydig cell: system characterization and rapid gonadotropin-induced desensitization. Endocrinology. 1981;108:1757–1762. doi: 10.1210/endo-108-5-1757. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, King JC, Urban RJ, Rogol AD, Evans WS, Kolp LA, Johnson ML. Operating characteristics of the male hypothalamo-pituitary-gonadal axis: pulsatile release of testosterone and follicle-stimulating hormone and their temporal coupling with luteinizing hormone. J Clin Endocrinol Metab. 1987;65:929–941. doi: 10.1210/jcem-65-5-929. [DOI] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Iranmanesh A, Keenan DM. Erosion of endogenous testosterone-driven negative feedback on pulsatile LH secretion in healthy aging men. J Clin Endocrinol Metab. 2004;89:5753–5761. doi: 10.1210/jc.2004-0399. [DOI] [PubMed] [Google Scholar]
- 33.Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements in interglandular signaling. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Iranmanesh A. Pulsatile intravenous infusion of recombinant human luteinizing hormone under acute gonadotropin-releasing hormone receptor blockade reconstitutes testosterone secretion in young men. J Clin Endocrinol Metab. 2004;89:4474–4479. doi: 10.1210/jc.2004-0203. [DOI] [PubMed] [Google Scholar]
- 35.Keenan DM, Veldhuis JD 2003 Mathematical modeling of receptor-mediated interlinked systems. Encyclopedia of Hormones. San Diego: Academic Press; 286–294
- 36.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuis JD, O’Dea LS, Johnson ML. The nature of the gonadotropin-releasing hormone stimulus-luteinizing hormone secretory response of human gonadotrophs in vivo. J Clin Endocrinol Metab. 1989;68:661–670. doi: 10.1210/jcem-68-3-661. [DOI] [PubMed] [Google Scholar]
- 38.Davis MR, Veldhuis JD, Rogol AD, Dufau ML, Catt KJ. Sustained inhibitory actions of a potent antagonist of gonadotropin-releasing hormone in postmenopausal women. J Clin Endocrinol Metab. 1987;64:1268–1274. doi: 10.1210/jcem-64-6-1268. [DOI] [PubMed] [Google Scholar]
- 39.Pavlou SN, Veldhuis JD, Lindner J, Souza KH, Urban RJ, Rivier JE, Vale WW, Stallard DJ. Persistence of concordant LH, testosterone and α-subunit pulses following LHRH antagonist administration in normal men. J Clin Endocrinol Metab. 1990;70:1472–1478. doi: 10.1210/jcem-70-5-1472. [DOI] [PubMed] [Google Scholar]
- 40.A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod. 1998;13:3023–3031. [PubMed] [Google Scholar]
- 41.Oberye JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 42.Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH and FSH secretion in young men. J Clin Endocrinol Metab. 2001;86:2600–2606. doi: 10.1210/jcem.86.6.7520. [DOI] [PubMed] [Google Scholar]
- 43.Nerenberg C, LaFargue J, Gee C, Chu F, Wolfe L, Chan R, Tarnowski T. Radioimmunoassay of ganirelix in plasma or serum. J Immunoassay. 1993;14:191–207. doi: 10.1080/15321819308019849. [DOI] [PubMed] [Google Scholar]
- 44.Beckers T, Bernd M, Kutscher B, Kuhne R, Hoffmann S, Reissmann T. Structure-function studies of linear and cyclized peptide antagonists of the GnRH receptor. Biochem Biophys Res Commun. 2001;289:653–663. doi: 10.1006/bbrc.2001.5939. [DOI] [PubMed] [Google Scholar]
- 45.McCartney CR, Bellows AB, Gingrich MB, Hu Y, Evans WS, Marshall JC, Veldhuis JD. Exaggerated 17-hydroxyprogesterone response to intravenous infusions of recombinant human LH in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2004;286:E902–E908. doi: 10.1152/ajpendo.00415.2003. [DOI] [PubMed] [Google Scholar]
- 46.Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol Endo Metab. 2005;288:E775–E781. doi: 10.1152/ajpendo.00410.2004. [DOI] [PubMed] [Google Scholar]
- 47.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veldhuis JD, Fraioli F, Rogol AD, Dufau ML. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest. 1986;77:1122–1128. doi: 10.1172/JCI112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuehl RO 1994 Split-plot designs. Statistical principles of research design and analysis. Belmont, CA: Duxbury Press; 473–498
- 50.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. [PubMed] [Google Scholar]
- 51.Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien R, Power of analysis for linear models. SAS Institute. Proc 11th Annual SAS Users Group International Conference, Cary, NC, 1987, pp 915–922
- 53.Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keenan DM, Veldhuis JD. Divergent gonadotropin-gonadal dose-responsive coupling in healthy young and aging men. Am J Physiol. 2004;286:R381–R389. doi: 10.1152/ajpregu.00376.2003. [DOI] [PubMed] [Google Scholar]
- 55.Veldhuis JD, Johnson ML. Analysis of nonequilibrium facets of pulsatile sex-steroid secretion in the presence of plasma binding proteins. Methods Enzymol. 2000;321:239–263. doi: 10.1016/s0076-6879(00)21197-x. [DOI] [PubMed] [Google Scholar]
- 56.Handelsman DJ, Cummins JT, Clarke IJ. Pharmacodynamics of gonadotropin-releasing hormone. I. Effects of gonadotropin-releasing hormone pulse contour on pituitary luteinizing hormone secretion in vivo in sheep. Neuroendocrinol. 1988;48:432. doi: 10.1159/000125045. [DOI] [PubMed] [Google Scholar]
- 57.Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfield DA, Crowley Jr WF. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA. 1994;91:6894–6898. doi: 10.1073/pnas.91.15.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaar CJ, Euker JS, Riegle GD, Meites J. Effects of castration and gonadal steroids on serum luteinizing hormone and prolactin in old and young rats. J Endocrinol. 1974;66:45–51. doi: 10.1677/joe.0.0660045. [DOI] [PubMed] [Google Scholar]
- 59.Bonavera JJ, Swerdloff RS, Sinha Hakim AP, Lue YH, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N-methyl-d-aspartate. J Neuroendocrinol. 1998;10:93–99. doi: 10.1046/j.1365-2826.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 60.Jarjour LT, Handelsman DJ, Swerdloff RS. Effects of aging on the in vitro release of gonadotropin-releasing hormone. Endocrinology. 1986;119:1113–1117. doi: 10.1210/endo-119-3-1113. [DOI] [PubMed] [Google Scholar]
- 61.Goldman JM, Cooper RL, Rehnberg GL, Gabel S, McElroy WK, Hein J, Conn PM. Age-related alterations in the stimulated release in vitro of catecholamines and luteinizing hormone-releasing hormone from the male rat hypothalamus. Neurochem Res. 1987;12:651–657. doi: 10.1007/BF00971015. [DOI] [PubMed] [Google Scholar]
- 62.Karpas AE, Bremner WJ, Clifton DK, Steiner RA, Dorsa DM. Diminished luteinizing hormone pulse frequency and amplitude with aging in the male rat. Endocrinology. 1983;112:788–791. doi: 10.1210/endo-112-3-788. [DOI] [PubMed] [Google Scholar]
- 63.Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superlano L, Sinha-hikim AP, Wang C. In the male Brown-Norway (BN) male rat reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18:359–365. [PubMed] [Google Scholar]
- 64.Kaler LW, Critchlow V. Anterior pituitary luteinizing hormone secretion during continuous perifusion in aging male rats. Mech Ageing Dev. 1984;25:103–115. doi: 10.1016/0047-6374(84)90133-7. [DOI] [PubMed] [Google Scholar]
- 65.Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab. 2001;86:529–535. doi: 10.1210/jcem.86.2.7200. [DOI] [PubMed] [Google Scholar]
- 66.Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metabolism. 1984;33:1052–1059. doi: 10.1016/0026-0495(84)90237-3. [DOI] [PubMed] [Google Scholar]
- 67.Reubens R, Dhondt M, Vermeulen A. Further studies on Leydig cell response to human choriogonadotropin. J Clin Endocrinol Metab. 1976;39:40–45. doi: 10.1210/jcem-39-1-40. [DOI] [PubMed] [Google Scholar]
- 68.Longcope C. The effect of human chorionic gonadotropin on plasma steroid levels in young and old men. Steroids. 1973;21:583–592. doi: 10.1016/0039-128x(73)90046-9. [DOI] [PubMed] [Google Scholar]
- 69.Simpkins JW, Mueller GP, Huang HH, Meites J. Evidence for depressed catecholamine and enhanced serotonin metabolism in aging male rats: possible relation to gonadotropin secretion. Endocrinology. 1977;100:1672–1678. doi: 10.1210/endo-100-6-1672. [DOI] [PubMed] [Google Scholar]
- 70.Sortino MA, Aleppo G, Scapagnini U, Canonico PL. Different responses of gonadotropin-releasing hormone (GnRH) release to glutamate receptor agonists during aging. Brain Res Bull. 1996;41:359–362. doi: 10.1016/s0361-9230(96)00199-2. [DOI] [PubMed] [Google Scholar]
- 71.Sohn EH, Wolden-Hanson T, Matsumoto AM. Testosterone (T)-induced changes in arcuate nucleus cocaine-amphetamine-regulated transcript and NPY mRNA are attenuated in old compared to young male brown Norway rats: contribution of T to age-related changes in cocaine-amphetamine-regulated transcript and NPY gene expression. Endocrinology. 2002;143:954–963. doi: 10.1210/endo.143.3.8670. [DOI] [PubMed] [Google Scholar]
- 72.Chen H, Zirkin BR. Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci USA. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]


